Chemical Reactions Reactions involve chemical changes in matter






- Slides: 8

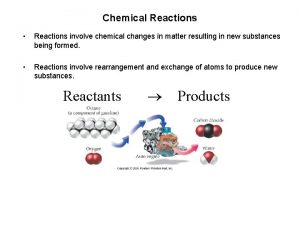
Chemical Reactions • Reactions involve chemical changes in matter resulting in new substances. • Reactions involve rearrangement and exchange of atoms to produce new molecules. ü Elements are not transmuted during a reaction. ü Atoms of different elements can combine to make new compounds. ü Molecules can combine to make bigger molecules. ü Molecules can decompose into smaller molecules or atoms. ü Atoms can be exchanged between molecules or transferred to another molecule. ü Atoms can gain or lose electrons, turning them into ions. ØOr changing the charge on ions that are already there. Tro's "Introductory Chemistry", Chapter 7 1

Evidence of Chemical Reactions • Look for evidence of a new substance. • Visual clues (permanent). üColor change. üPrecipitate formation. ØSolid that forms when liquid solutions are mixed. üGas bubbles. üLarge energy changes. ØContainer becomes very hot or cold. ØEmission of light. • Other clues. üNew odor. üWhooshing sound from a tube. üPermanent new state. Tro's "Introductory Chemistry", Chapter 7 2

Evidence of Chemical Change Release or Absorption of Heat Formation of a Gas Color Change Emission of Light Formation of Solid Precipitate Tro's "Introductory Chemistry", Chapter 7 3

Evidence of Chemical Change, Continued • In order to be absolutely sure that a chemical reaction has taken place, you need to go down to the molecular level and analyze the structures of the molecules at the beginning and end. Tro's "Introductory Chemistry", Chapter 7 Is boiling water a chemical change? 4

Practice—Decide Whether Each of the Following Involve a Chemical Reaction. • • • Photosynthesis Yes, CO 2 and H 2 O combine into carbohydrates Heating sugar until it turns black Yes, sugar decomposing Heating ice until it turns liquid No, molecules still same Yes, food decomposing and combining Digestion of food with stomach acid Dissolving sugar in water No, molecules still same Burning of alcohol in a flambé dessert Yes, alcohol combining with O 2 to make CO 2 and H 2 O Tro's "Introductory Chemistry", Chapter 7 5

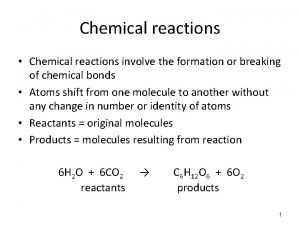
Chemical Equations • Short-hand way of describing a reaction. • Provides information about the reaction. üFormulas of reactants and products. üStates of reactants and products. üRelative numbers of reactant and product molecules that are required. üCan be used to determine masses of reactants used and products that can be made. Tro's "Introductory Chemistry", Chapter 7 6

Conservation of Mass • Matter cannot be created or destroyed. üTherefore, the total mass cannot change. üAnd the total mass of the reactants will be the same as the total mass of the products. • In a chemical reaction, all the atoms present at the beginning are still present at the end. üIf all the atoms are still there, then the mass will not change. Tro's "Introductory Chemistry", Chapter 7 7

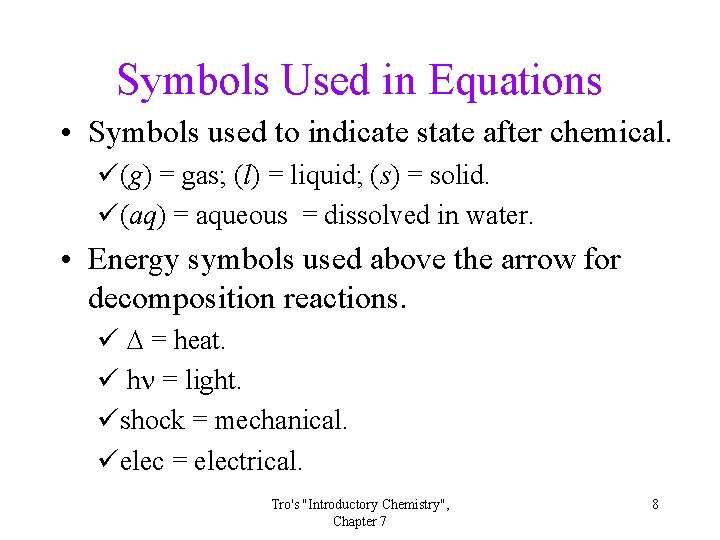
Symbols Used in Equations • Symbols used to indicate state after chemical. ü(g) = gas; (l) = liquid; (s) = solid. ü(aq) = aqueous = dissolved in water. • Energy symbols used above the arrow for decomposition reactions. ü D = heat. ü hn = light. üshock = mechanical. üelec = electrical. Tro's "Introductory Chemistry", Chapter 7 8
 Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical and physical changes
Chemical and physical changes True or false: chemical and physical changes alter matter.
True or false: chemical and physical changes alter matter. Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Elizabeth mulroney
Elizabeth mulroney Concept map matter
Concept map matter