Chemical reactions Chemical reactions involve the formation or







- Slides: 14

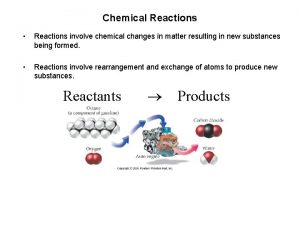
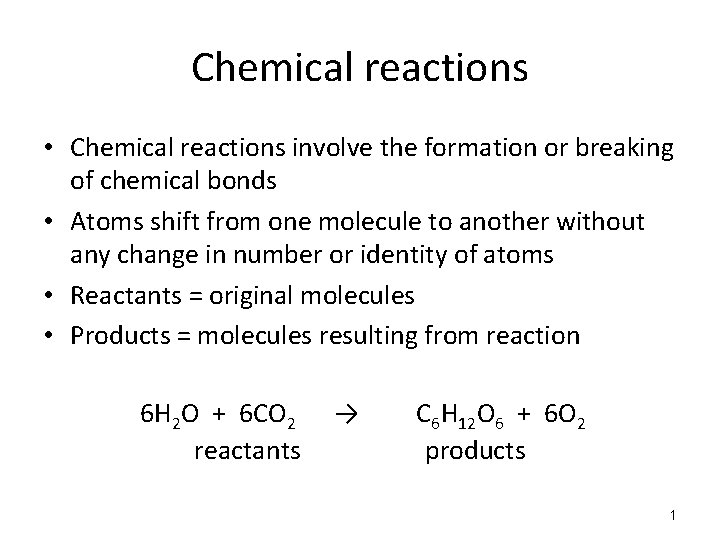
Chemical reactions • Chemical reactions involve the formation or breaking of chemical bonds • Atoms shift from one molecule to another without any change in number or identity of atoms • Reactants = original molecules • Products = molecules resulting from reaction 6 H 2 O + 6 CO 2 reactants → C 6 H 12 O 6 + 6 O 2 products 1

• • Extent of chemical reaction influenced by 1. 2. 3. Temperature Concentration of reactants and products Catalysts Many reactions are reversible – e. g. A + B C+D – The size of the arrows can indicate, in a given circumstance, in which direction the reaction is favored. For example, in the following, the reaction is favored to go to the right, but this does not mean that there will be zero reactants left at equilibrium, only that the concentration of products will be greater than the concentration of reactants at equilibrium. – e. g. A + B C+D

1. In an experiment described in a chemistry lab book, the directions state that after mixing the two chemicals (A and B) and waiting 5 minutes that B will be reduced. This means that A. chemical B has lost electrons to chemical A. B. chemical B has gained electrons from chemical A. C. chemical B has lost its ability to interact with chemical A. D. chemical B has become an isotope and can no longer interact with chemical A. E. chemical B has shared electrons from chemical A. 2. The First Law of Thermodynamics simply states that A. energy is constantly being created in the universe. B. disorder in the universe is continually increasing. C. energy can be created but not destroyed. D. energy cannot be created or destroyed, just changed from one form to another. E. energy can be recycled through the universe. 3. The Second Law of Thermodynamics simply states that A. energy can be recycled through the universe. B. energy cannot be created or destroyed, just changed from one form to another. C. disorder (or entropy) in the universe is continually increasing. D. energy is constantly being created in the universe. E. energy can be created but not destroyed. 4. In a chemical reaction in a living system, enzymes are used as catalysts. Which of the following statements about enzymes is incorrect? A. Enzymes enter reactions and can be reused. B. Enzymes speed up chemical reactions in living systems. C. Enzymes reduce the energy of activation necessary for a chemical reaction to go forward. D. Enzymes increase the energy of activation necessary for a chemical reaction to go forward. Enzymes are sometimes referred to as biological catalysts; however, not all biological catalysts are proteins.

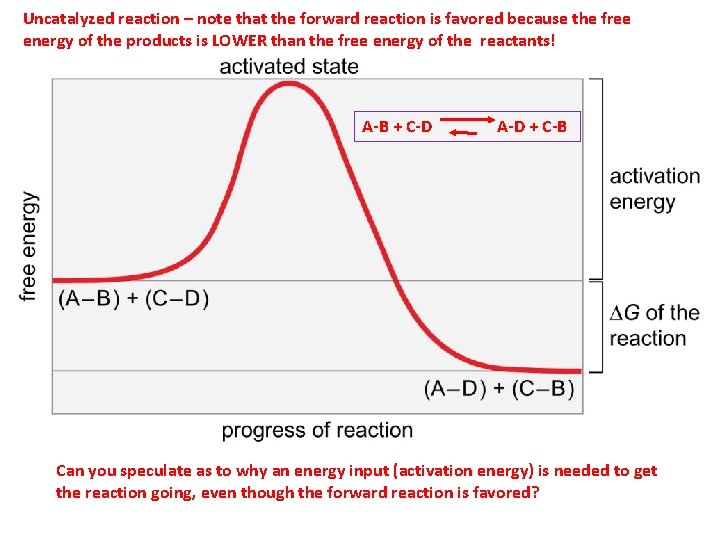
Figure 3 -12 Uncatalyzed reaction – note that the forward reaction is favored because the free energy of the products is LOWER than the free energy of the reactants! A-B + C-D A-D + C-B Can you speculate as to why an energy input (activation energy) is needed to get the reaction going, even though the forward reaction is favored?

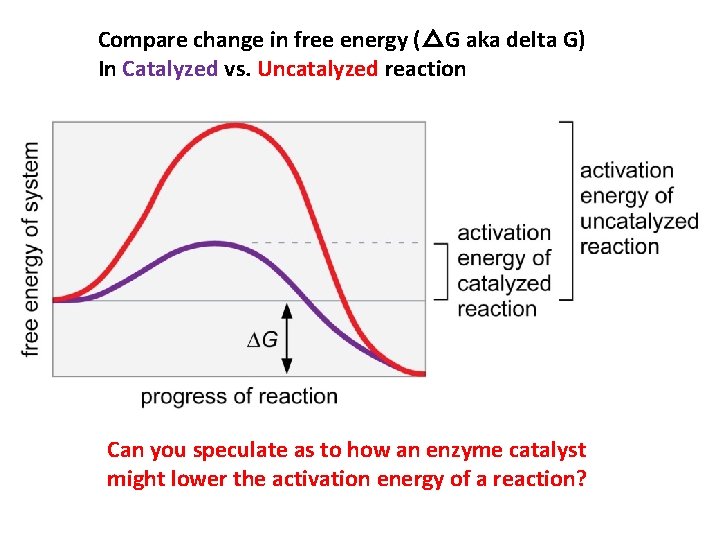
Figure 3 -13 Compare change in free energy (△G aka delta G) In Catalyzed vs. Uncatalyzed reaction Can you speculate as to how an enzyme catalyst might lower the activation energy of a reaction?

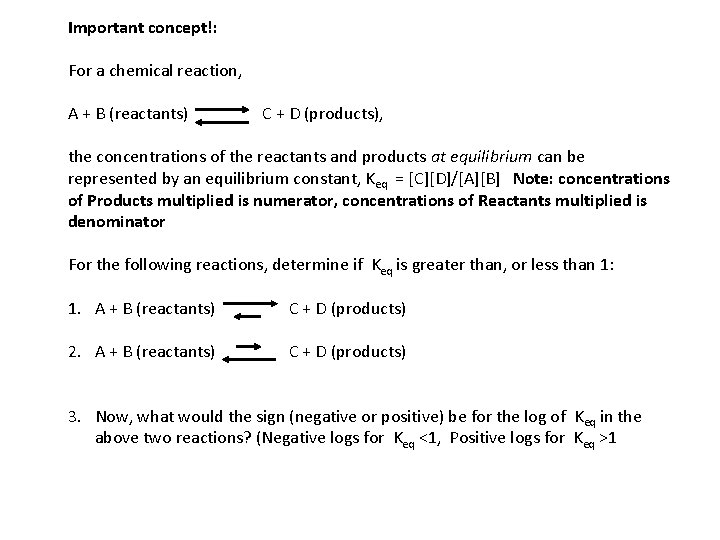
Important concept!: For a chemical reaction, A + B (reactants) C + D (products), the concentrations of the reactants and products at equilibrium can be represented by an equilibrium constant, Keq = [C][D]/[A][B] Note: concentrations of Products multiplied is numerator, concentrations of Reactants multiplied is denominator For the following reactions, determine if Keq is greater than, or less than 1: 1. A + B (reactants) C + D (products) 2. A + B (reactants) C + D (products) 3. Now, what would the sign (negative or positive) be for the log of Keq in the above two reactions? (Negative logs for Keq <1, Positive logs for Keq >1

5. Which of the following best depicts a diagram of enzymes and substrates when they react? Assume only forward reactions. Use the following to make your choice: E = enzyme, S = substrate, ES = enzyme-substrate complex, P = products. A. E + P ES B. E + S ES + P C. E + S E + P D. E + ES P + E E. E + P EP S + E 6. A researcher wants to slow down a particular cellular activity by controlling an enzyme that catalyzes that activity. All of the following choices are available except A. increasing the temperature of the cell's environment. B. decreasing the temperature of the cell's environment. C. reducing the p. H of the cell's environment. D. increasing the p. H of the cell's environment. E. adding substrate as it is depleted to the cell's environment. 7. A new antibiotic has been developed that will use competitive inhibitor enzyme inhibition. This means that the A. antibiotic will compete for substrate binding sites on the enzyme. B. antibiotic will compete for binding sites on the substrate. C. antibiotic will compete for binding sites on the enzyme-substrate complex. D. antibiotic will compete for binding sites on the product that is being produced. E. antibiotic will only compete for binding sites on the receptor proteins embedded in the phospholipid bilayer.

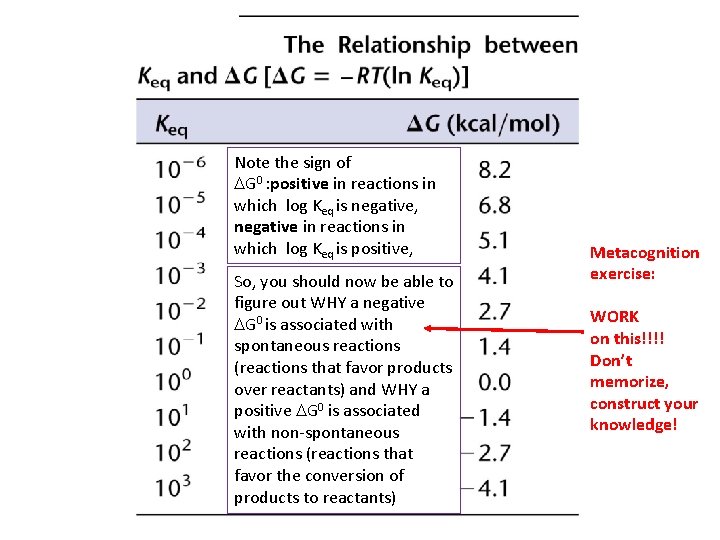
Note the sign of DG 0 : positive in reactions in which log Keq is negative, negative in reactions in which log Keq is positive, So, you should now be able to figure out WHY a negative DG 0 is associated with spontaneous reactions (reactions that favor products over reactants) and WHY a positive DG 0 is associated with non-spontaneous reactions (reactions that favor the conversion of products to reactants) Metacognition exercise: WORK on this!!!! Don’t memorize, construct your knowledge!

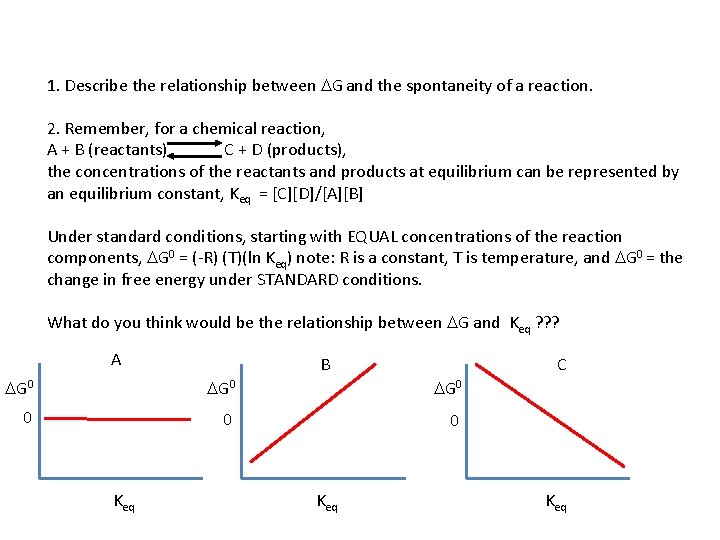
1. Describe the relationship between DG and the spontaneity of a reaction. 2. Remember, for a chemical reaction, A + B (reactants) C + D (products), the concentrations of the reactants and products at equilibrium can be represented by an equilibrium constant, Keq = [C][D]/[A][B] Under standard conditions, starting with EQUAL concentrations of the reaction components, DG 0 = (-R) (T)(ln Keq) note: R is a constant, T is temperature, and DG 0 = the change in free energy under STANDARD conditions. What do you think would be the relationship between DG and Keq ? ? ? A B C DG 0 0 Keq Keq

But, in biological systems, standard conditions do not exist, and DG = DG 0 + R x T x (ln [C][D]/[A][B] ) Note: DG is the ”REAL” change in free energy when standard conditions do not exist – we are not dealing with equilibrium: no Keq ! What can this tell us about why non-spontaneous reactions in a biochemical pathway can occur even without the input of energy (sometimes)? Another way to think about this: if concentrations of products of a reaction are kept low because they are used up quickly in subsequent reactions, what happens to DG? Discuss, with your small group the following equation (to the best of your ability): DG = DH – TDS. What are the variables and what is their relevance to chemical reactions? Which of the following scenarios would not result in a decrease in free energy? A. Enthalpy increases and entropy decreases. B. Enthalpy decreases and entropy increases. C. Enthalpy increases, but entropy increase outweighs increase in enthalpy. D. Enthalpy decreases enough to outweigh a slight decrease in entropy.

Don’t view the following until you’ve tried hard to answer all the questions on previous slides!

1. In an experiment described in a chemistry lab book, the directions state that after mixing the two chemicals (A and B) and waiting 5 minutes that B will be reduced. This means that A. chemical B has lost electrons to chemical A. B. chemical B has gained electrons from chemical A. C. chemical B has lost its ability to interact with chemical A. D. chemical B has become an isotope and can no longer interact with chemical A. E. chemical B has shared electrons from chemical A. 2. The First Law of Thermodynamics simply states that A. energy is constantly being created in the universe. B. disorder in the universe is continually increasing. C. energy can be created but not destroyed. D. energy cannot be created or destroyed, just changed from one form to another. E. energy can be recycled through the universe. 3. The Second Law of Thermodynamics simply states that A. energy can be recycled through the universe. B. energy cannot be created or destroyed, just changed from one form to another. C. disorder (or entropy) in the universe is continually increasing. D. energy is constantly being created in the universe. E. energy can be created but not destroyed. 4. In a chemical reaction in a living system, enzymes are used as catalysts. Which of the following statements about enzymes is incorrect? A. Enzymes enter reactions and can be reused. B. Enzymes speed up chemical reactions in living systems. C. Enzymes reduce the energy of activation necessary for a chemical reaction to go forward. D. Enzymes increase the energy of activation necessary for a chemical reaction to go forward. Enzymes are sometimes referred to as biological catalysts; however, not all biological catalysts are proteins.

5. Which of the following best depicts a diagram of enzymes and substrates when they react? Assume only forward reactions. Use the following to make your choice: E = enzyme, S = substrate (reactant/s), ES = enzyme-substrate complex, P = products. A. E + P ES B. E + S ES + P C. E + S E + P D. E + ES P + E E. E + P EP S + E 6. A researcher wants to slow down a particular cellular activity by controlling an enzyme that catalyzes that activity. All of the following choices are available except A. increasing the temperature of the cell's environment. B. decreasing the temperature of the cell's environment. C. reducing the p. H of the cell's environment. D. increasing the p. H of the cell's environment. E. adding substrate as it is depleted to the cell's environment. 7. A new antibiotic has been developed that will use competitive inhibitor enzyme inhibition. This means that the A. antibiotic will compete for substrate binding sites on the enzyme. B. antibiotic will compete for binding sites on the substrate. C. antibiotic will compete for binding sites on the enzyme-substrate complex. D. antibiotic will compete for binding sites on the product that is being produced. E. antibiotic will only compete for binding sites on the receptor proteins embedded in the phospholipid bilayer.

But, in biological systems, standard conditions do not exist, and DG = DG 0 + R x T x (ln [C][D]/[A][B] ) Note: DG is the ”REAL” change in free energy when standard conditions do not exist! What can this tell us about why non-spontaneous reactions in a biochemical pathway can occur even without the input of energy (sometimes)? Discuss, with your small group, or alone, the following equation (to the best of your ability): DG = DH – TDS. What are the variables and what is their relevance to chemical reactions? DG = change in free energy, = DH change in enthalpy, DS = change in entropy, T = temperature. Enthalpy is energy of chemical bonds (in our usage). Entropy is amount od disorder (randomness). Positive DH’s tend to make reactions less spontaneous, positive DS’s tend to make reactions more spontaneous Which of the following reaction scenarios would NOT result in a decrease in free energy? A. Enthalpy increases and entropy decreases. B. Enthalpy decreases and entropy increases. C. Enthalpy increases, but entropy increase outweighs increase in enthalpy. D. Enthalpy decreases enough to outweigh a slight decrease in entropy.
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Types of reactions
Types of reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Half redox reaction
Half redox reaction Formation initiale vs formation continue
Formation initiale vs formation continue Chemical reactions in soil
Chemical reactions in soil Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chapter 6 section 1 atoms elements and compounds answer key
Chapter 6 section 1 atoms elements and compounds answer key Chemical reactions classification
Chemical reactions classification Unit 5 chemical reactions
Unit 5 chemical reactions Chemical reactions reactants and products
Chemical reactions reactants and products Combustion chemical reaction
Combustion chemical reaction