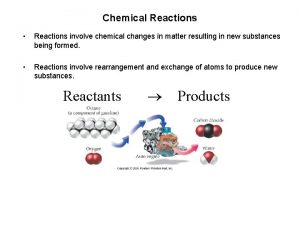
Chemical Reactions Reactions involve chemical changes in matter









- Slides: 36

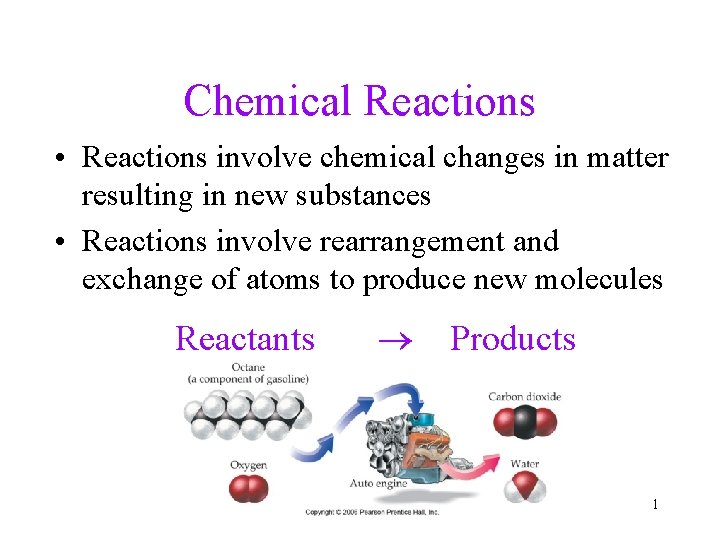
Chemical Reactions • Reactions involve chemical changes in matter resulting in new substances • Reactions involve rearrangement and exchange of atoms to produce new molecules Reactants Products 1

Evidence of Chemical Change Formation of a Gas Color Change 2 Emission of Light

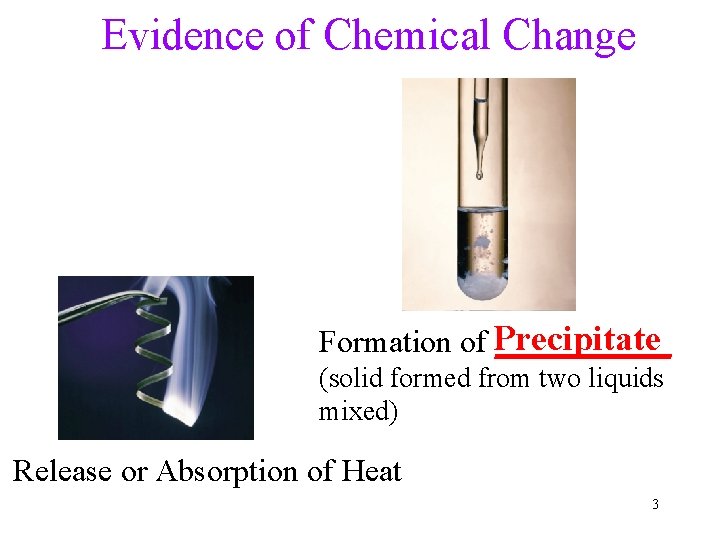
Evidence of Chemical Change Formation of Precipitate ______ (solid formed from two liquids mixed) Release or Absorption of Heat 3

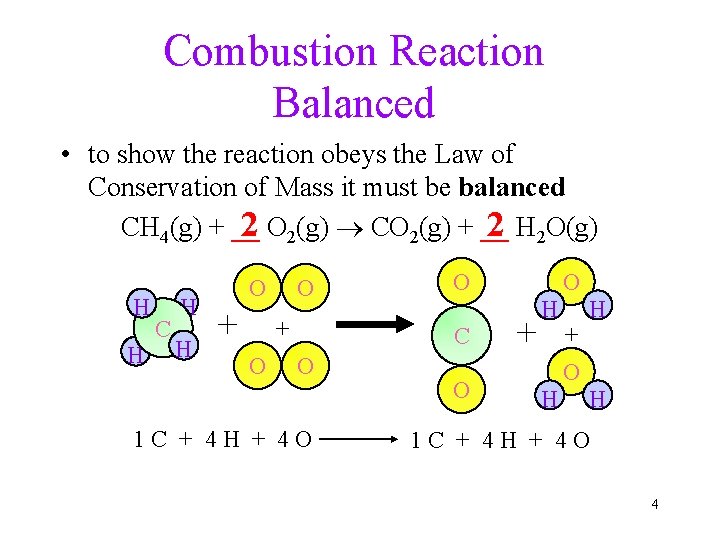
Combustion Reaction Balanced • to show the reaction obeys the Law of Conservation of Mass it must be balanced 2 O 2(g) CO 2(g) + __ 2 H 2 O(g) CH 4(g) + __ H H C H H O + O O C O 1 C + 4 H + 4 O O + H H O + O H H 1 C + 4 H + 4 O 4

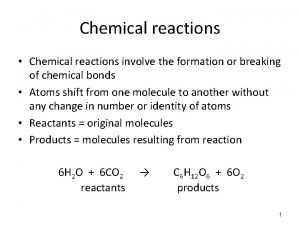
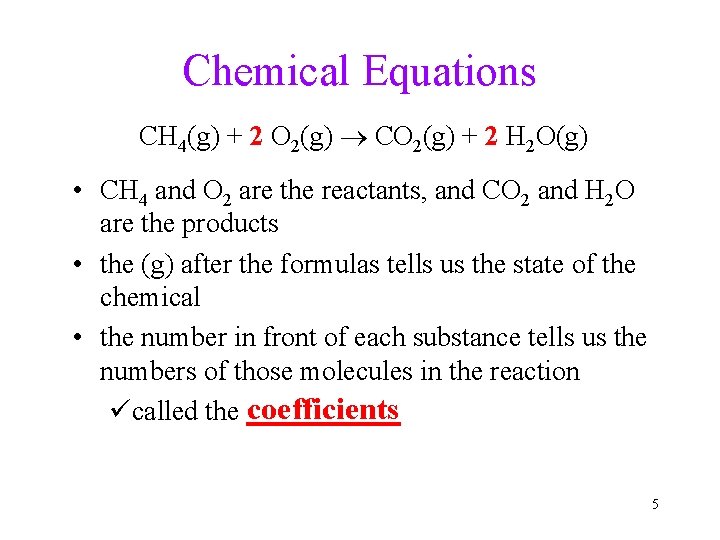
Chemical Equations CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) • CH 4 and O 2 are the reactants, and CO 2 and H 2 O are the products • the (g) after the formulas tells us the state of the chemical • the number in front of each substance tells us the numbers of those molecules in the reaction coefficients ücalled the ______ 5

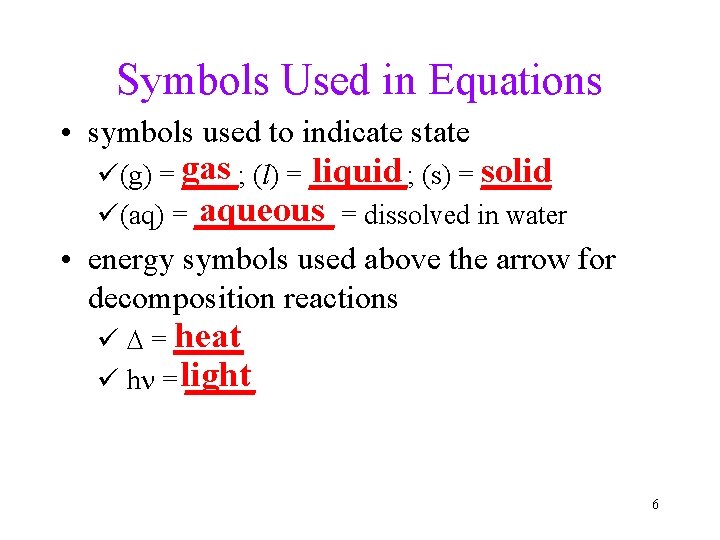
Symbols Used in Equations • symbols used to indicate state liquid (s) = _____ solid ü(g) = gas ____; (l) = _______; aqueous = dissolved in water ü(aq) = _____ • energy symbols used above the arrow for decomposition reactions heat ü D = _____ ü hn = light _____ 6

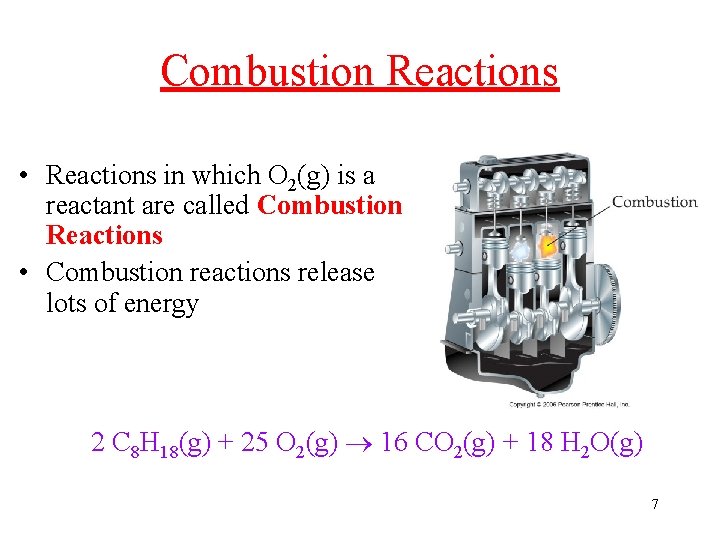
Combustion Reactions • Reactions in which O 2(g) is a reactant are called Combustion Reactions • Combustion reactions release lots of energy 2 C 8 H 18(g) + 25 O 2(g) 16 CO 2(g) + 18 H 2 O(g) 7

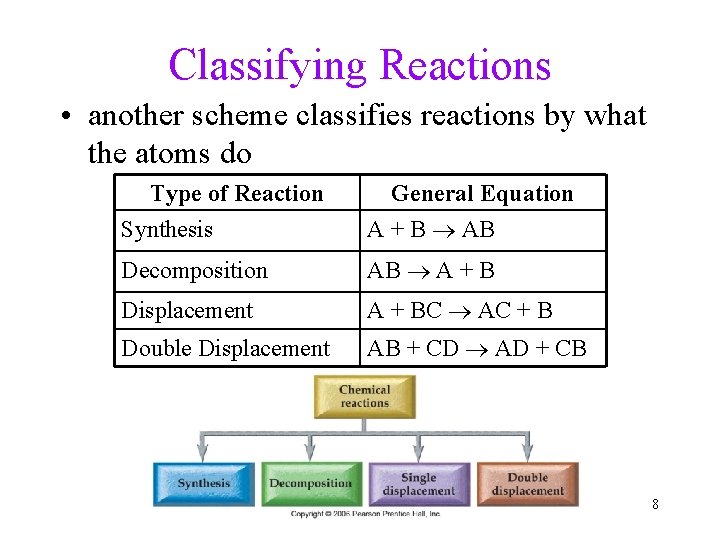
Classifying Reactions • another scheme classifies reactions by what the atoms do Type of Reaction Synthesis General Equation A + B AB Decomposition AB A + B Displacement A + BC AC + B Double Displacement AB + CD AD + CB 8

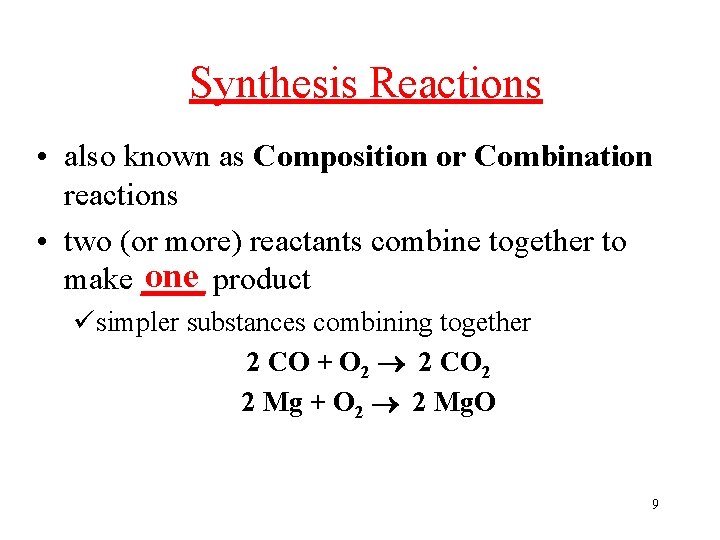
Synthesis Reactions • also known as Composition or Combination reactions • two (or more) reactants combine together to one product make ____ üsimpler substances combining together 2 CO + O 2 2 CO 2 2 Mg + O 2 2 Mg. O 9

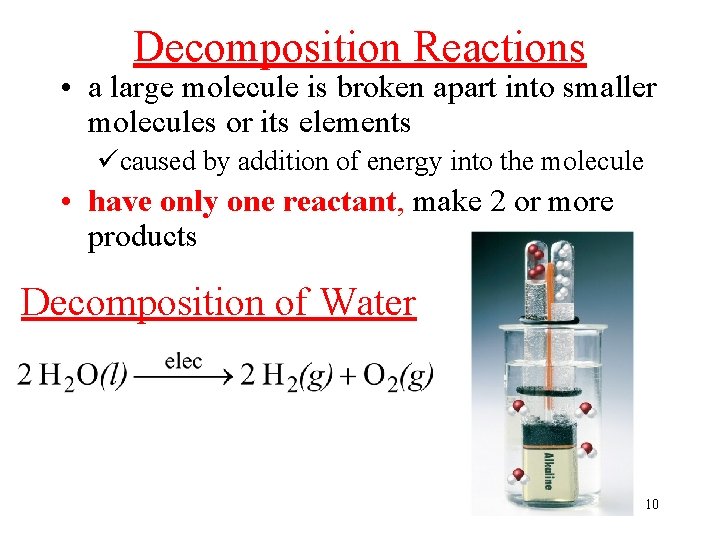
Decomposition Reactions • a large molecule is broken apart into smaller molecules or its elements ücaused by addition of energy into the molecule • have only one reactant, make 2 or more products Decomposition of Water 10

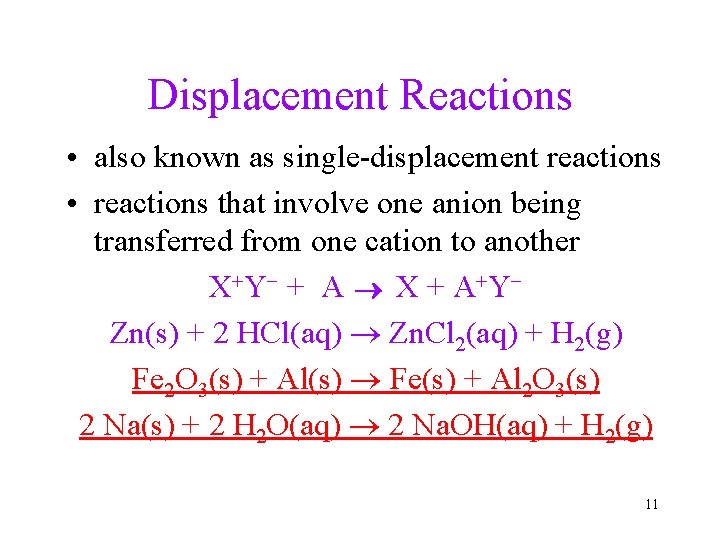
Displacement Reactions • also known as single-displacement reactions • reactions that involve one anion being transferred from one cation to another X+ Y- + A X + A + YZn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) Fe 2 O 3(s) + Al(s) Fe(s) + Al 2 O 3(s) 2 Na(s) + 2 H 2 O(aq) 2 Na. OH(aq) + H 2(g) 11

Double Displacement Reactions • two ionic compounds exchange ions • may be followed by decomposition of one of the products to make a gas • X +Y- (aq) + A+B- (aq) XB + AY • precipitation, acid-base and gas-evolving reactions are also double displacement reactions 12

Dissociation • when ionic compounds dissolve in water, the anions and cations are separated from each other - this is called _______ dissociation ü however not all ionic compounds are soluble in water! • when compounds containing polyatomic ions dissociate, the polyatomic group stays together as one ion 13

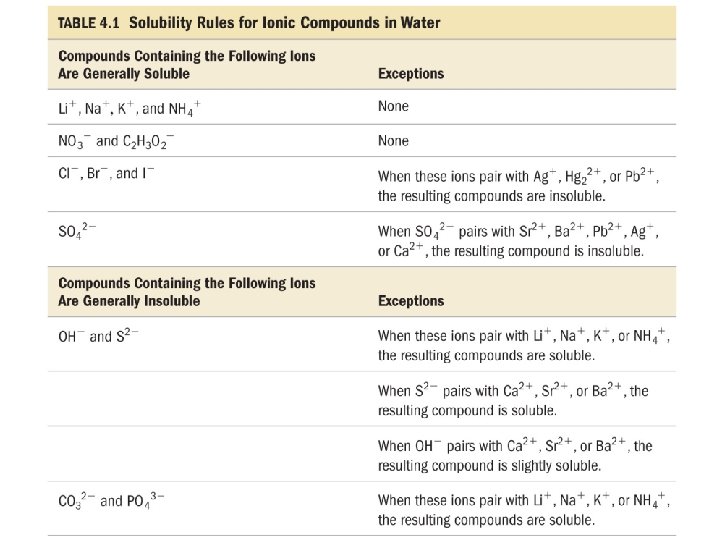
When will a Salt Dissolve? • a compound is soluble in a liquid if it dissolves in that liquid ü Na. Cl is soluble in water, but Ag. Cl is not • a compound is insoluble if a significant amount does not dissolve in that liquid ü Ag. Cl is insoluble in water Ø though there is a very small amount dissolved, but not enough to be significant 14


Determine if Each of the Following is Soluble in Water • • • KOH ______ soluble Ag. Br insoluble _______ soluble Ca. Cl 2 ______ soluble Pb(NO 3)2 ______ Pb. SO 4 insoluble _______ 16

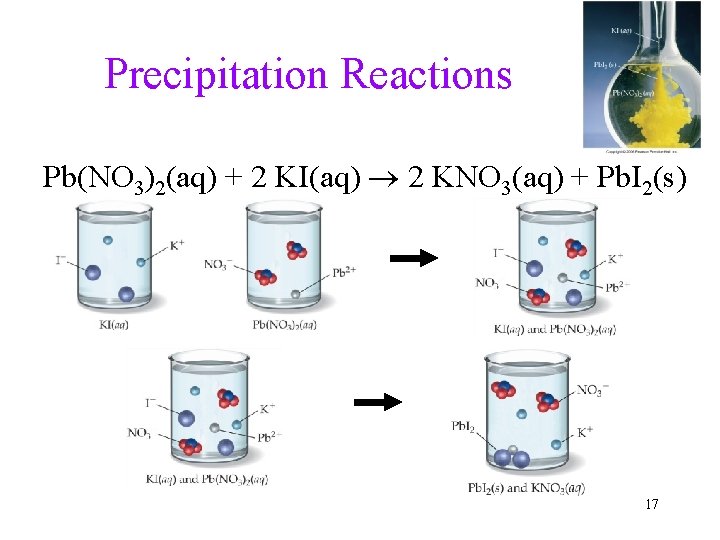
Precipitation Reactions Pb(NO 3)2(aq) + 2 KI(aq) 2 KNO 3(aq) + Pb. I 2(s) 17

No Precipitate Formation = No Reaction KI(aq) + Na. Cl(aq) KCl(aq) + Na. I(aq) all ions still present, no reaction (no rxn) 18

Ionic Equations • equations which describe the complete formulas of all equations reactant and products are called molecular __________ 2 KOH(aq) + Mg(NO 3)2(aq) 2 KNO 3(aq) + Mg(OH)2(s) • equations which describe the actual dissolved species are complete ionic equations called ____________ ü aqueous electrolytes are written as ions Ø soluble salts, strong acids, strong bases ü insoluble substances and nonelectrolytes written in molecule form Ø solids, liquids and gases are not dissolved, therefore molecule form 2 K+(aq) + 2 OH-(aq) + Mg 2+(aq) + 2 NO 3 -(aq) 2 K+(aq) + 2 NO 3 -(aq) + Mg(OH)2(s) 19

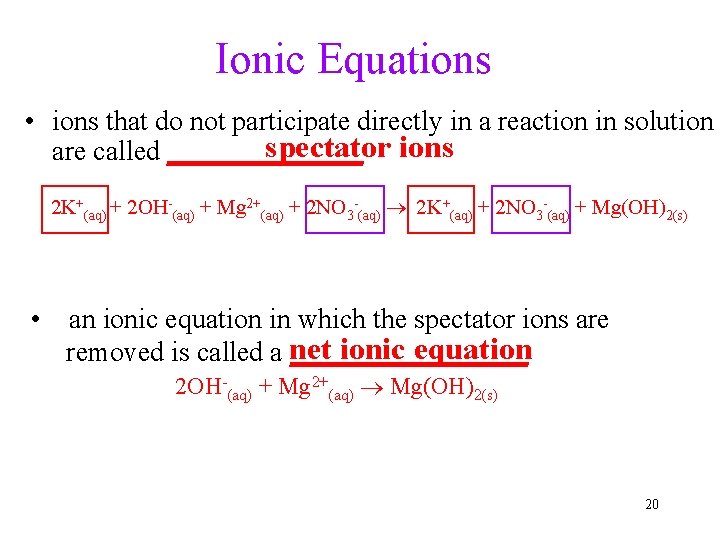
Ionic Equations • ions that do not participate directly in a reaction in solution spectator ions are called _______ 2 K+(aq) + 2 OH-(aq) + Mg 2+(aq) + 2 NO 3 -(aq) 2 K+(aq) + 2 NO 3 -(aq) + Mg(OH)2(s) • an ionic equation in which the spectator ions are ionic equation removed is called a net _________ 2 OH-(aq) + Mg 2+(aq) Mg(OH)2(s) 20

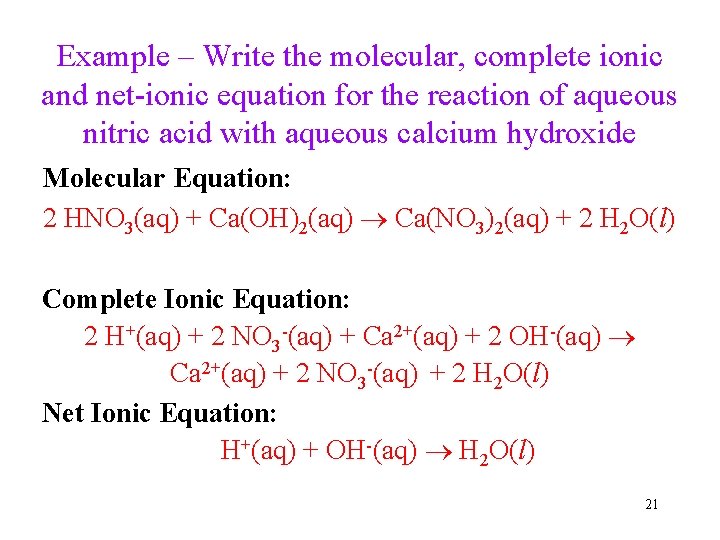
Example – Write the molecular, complete ionic and net-ionic equation for the reaction of aqueous nitric acid with aqueous calcium hydroxide Molecular Equation: 2 HNO 3(aq) + Ca(OH)2(aq) Ca(NO 3)2(aq) + 2 H 2 O(l) Complete Ionic Equation: 2 H+(aq) + 2 NO 3 -(aq) + Ca 2+(aq) + 2 OH-(aq) Ca 2+(aq) + 2 NO 3 -(aq) + 2 H 2 O(l) Net Ionic Equation: H+(aq) + OH-(aq) H 2 O(l) 21

Acid-Base Reactions • also called neutralization reactions because the acid and base neutralize each others properties • in the reaction of an acid with a base, the H+ from the acid combines with the OH- from the base to make water • the cation from the base combines with the anion from the acid to make the salt acid + base salt + water 2 HNO 3(aq) + Ca(OH)2(aq) Ca(NO 3)2(aq) + 2 H 2 O(l) 22

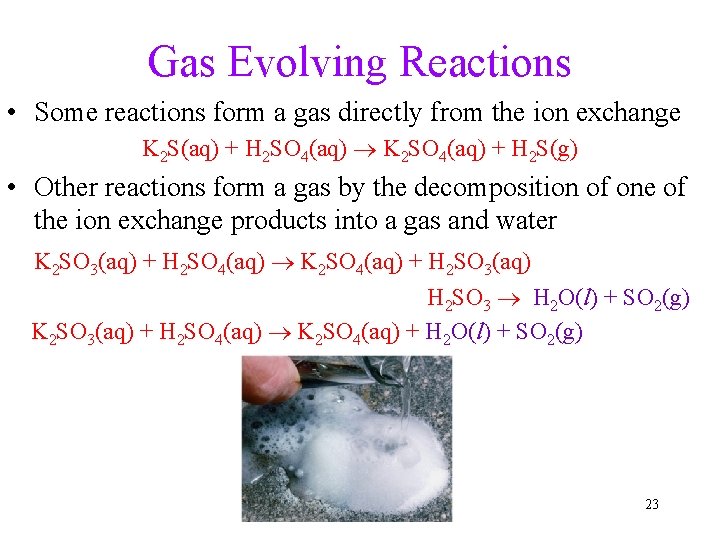
Gas Evolving Reactions • Some reactions form a gas directly from the ion exchange K 2 S(aq) + H 2 SO 4(aq) K 2 SO 4(aq) + H 2 S(g) • Other reactions form a gas by the decomposition of one of the ion exchange products into a gas and water K 2 SO 3(aq) + H 2 SO 4(aq) K 2 SO 4(aq) + H 2 SO 3(aq) H 2 SO 3 H 2 O(l) + SO 2(g) K 2 SO 3(aq) + H 2 SO 4(aq) K 2 SO 4(aq) + H 2 O(l) + SO 2(g) 23

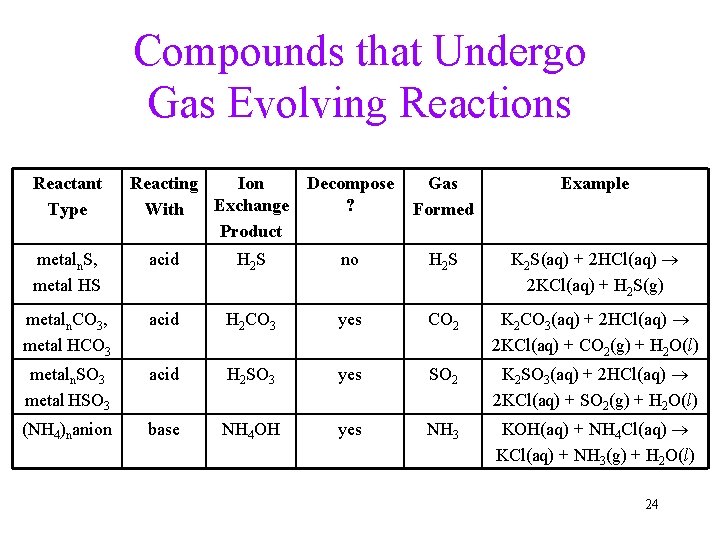
Compounds that Undergo Gas Evolving Reactions Reactant Type Reacting Ion Exchange With Product Decompose ? Gas Formed Example metaln. S, metal HS acid H 2 S no H 2 S K 2 S(aq) + 2 HCl(aq) 2 KCl(aq) + H 2 S(g) metaln. CO 3, metal HCO 3 acid H 2 CO 3 yes CO 2 K 2 CO 3(aq) + 2 HCl(aq) 2 KCl(aq) + CO 2(g) + H 2 O(l) metaln. SO 3 metal HSO 3 acid H 2 SO 3 yes SO 2 K 2 SO 3(aq) + 2 HCl(aq) 2 KCl(aq) + SO 2(g) + H 2 O(l) (NH 4)nanion base NH 4 OH yes NH 3 KOH(aq) + NH 4 Cl(aq) KCl(aq) + NH 3(g) + H 2 O(l) 24

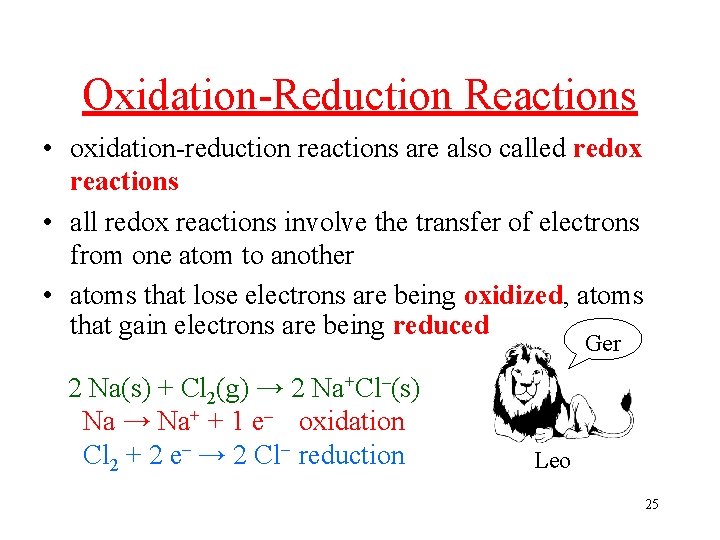
Oxidation-Reduction Reactions • oxidation-reduction reactions are also called redox reactions • all redox reactions involve the transfer of electrons from one atom to another • atoms that lose electrons are being oxidized, atoms that gain electrons are being reduced Ger 2 Na(s) + Cl 2(g) → 2 Na+Cl–(s) Na → Na+ + 1 e– oxidation Cl 2 + 2 e– → 2 Cl– reduction Leo 25

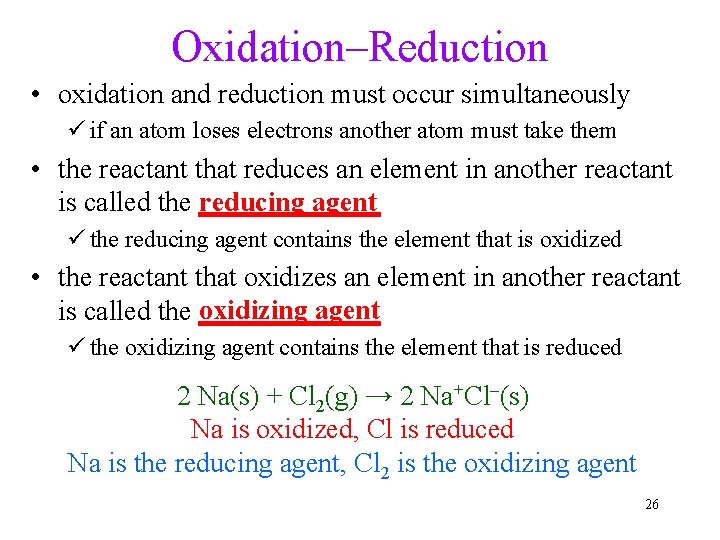
Oxidation–Reduction • oxidation and reduction must occur simultaneously ü if an atom loses electrons another atom must take them • the reactant that reduces an element in another reactant reducing agent is called the _______ ü the reducing agent contains the element that is oxidized • the reactant that oxidizes an element in another reactant oxidizing agent is called the _______ ü the oxidizing agent contains the element that is reduced 2 Na(s) + Cl 2(g) → 2 Na+Cl–(s) Na is oxidized, Cl is reduced Na is the reducing agent, Cl 2 is the oxidizing agent 26

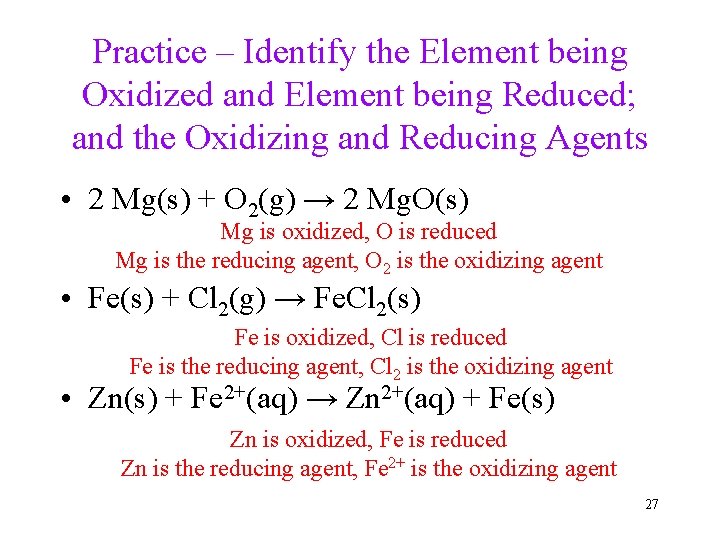
Practice – Identify the Element being Oxidized and Element being Reduced; and the Oxidizing and Reducing Agents • 2 Mg(s) + O 2(g) → 2 Mg. O(s) Mg is oxidized, O is reduced Mg is the reducing agent, O 2 is the oxidizing agent • Fe(s) + Cl 2(g) → Fe. Cl 2(s) Fe is oxidized, Cl is reduced Fe is the reducing agent, Cl 2 is the oxidizing agent • Zn(s) + Fe 2+(aq) → Zn 2+(aq) + Fe(s) Zn is oxidized, Fe is reduced Zn is the reducing agent, Fe 2+ is the oxidizing agent 27

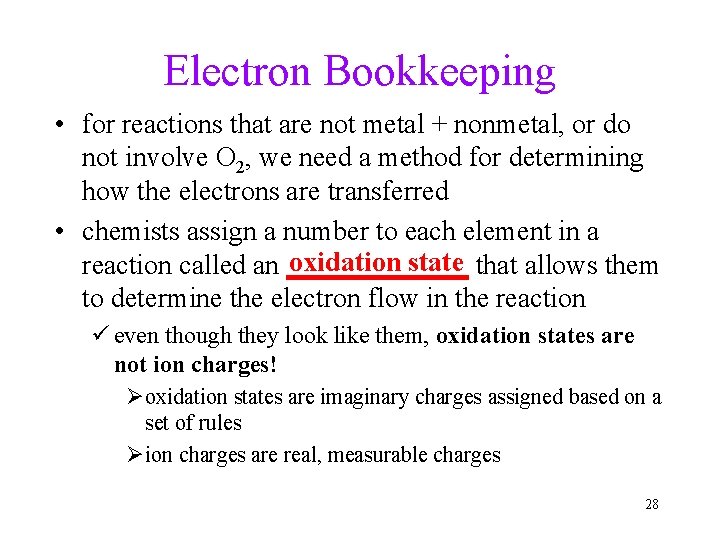
Electron Bookkeeping • for reactions that are not metal + nonmetal, or do not involve O 2, we need a method for determining how the electrons are transferred • chemists assign a number to each element in a oxidation state that allows them reaction called an _______ to determine the electron flow in the reaction ü even though they look like them, oxidation states are not ion charges! Øoxidation states are imaginary charges assigned based on a set of rules Øion charges are real, measurable charges 28

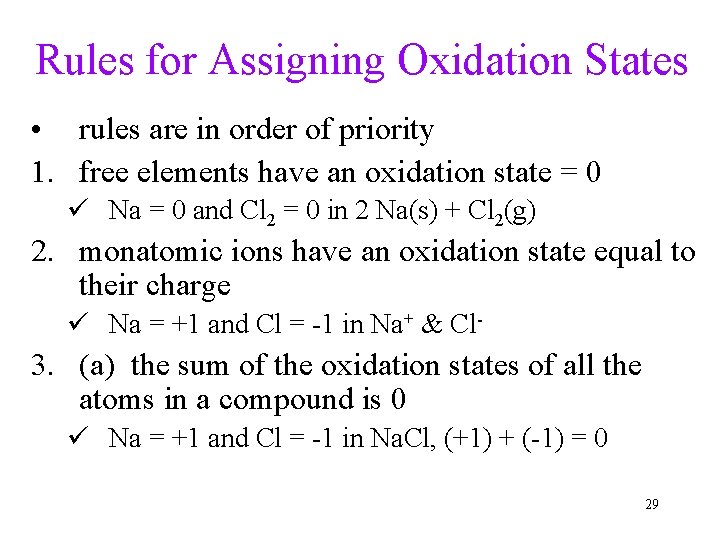
Rules for Assigning Oxidation States • rules are in order of priority 1. free elements have an oxidation state = 0 ü Na = 0 and Cl 2 = 0 in 2 Na(s) + Cl 2(g) 2. monatomic ions have an oxidation state equal to their charge ü Na = +1 and Cl = -1 in Na+ & Cl- 3. (a) the sum of the oxidation states of all the atoms in a compound is 0 ü Na = +1 and Cl = -1 in Na. Cl, (+1) + (-1) = 0 29

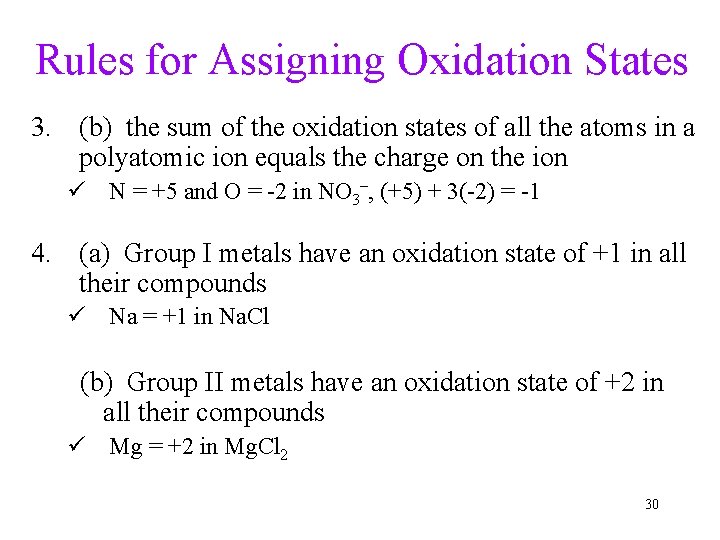
Rules for Assigning Oxidation States 3. (b) the sum of the oxidation states of all the atoms in a polyatomic ion equals the charge on the ion ü N = +5 and O = -2 in NO 3–, (+5) + 3(-2) = -1 4. (a) Group I metals have an oxidation state of +1 in all their compounds ü Na = +1 in Na. Cl (b) Group II metals have an oxidation state of +2 in all their compounds ü Mg = +2 in Mg. Cl 2 30

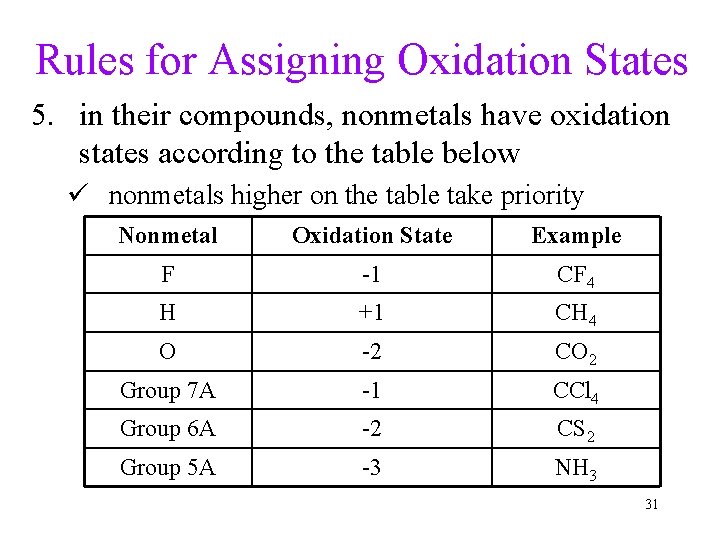
Rules for Assigning Oxidation States 5. in their compounds, nonmetals have oxidation states according to the table below ü nonmetals higher on the table take priority Nonmetal Oxidation State Example F -1 CF 4 H +1 CH 4 O -2 CO 2 Group 7 A -1 CCl 4 Group 6 A -2 CS 2 Group 5 A -3 NH 3 31

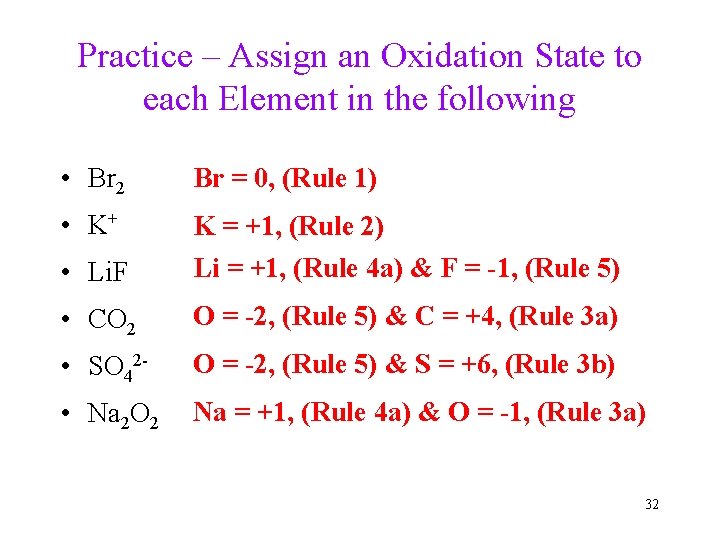
Practice – Assign an Oxidation State to each Element in the following • Br 2 Br = 0, (Rule 1) • K+ • Li. F K = +1, (Rule 2) Li = +1, (Rule 4 a) & F = -1, (Rule 5) • CO 2 O = -2, (Rule 5) & C = +4, (Rule 3 a) • SO 42 - O = -2, (Rule 5) & S = +6, (Rule 3 b) • Na 2 O 2 Na = +1, (Rule 4 a) & O = -1, (Rule 3 a) 32

Oxidation and Reduction • oxidation occurs when an atom’s oxidation increases during a reaction state ____ • reduction occurs when an atom’s oxidation state decreases ____ during a reaction CH 4 + 2 O 2 → CO 2 + 2 H 2 O -4 +1 0 +4 – 2 +1 -2 oxidation reduction 33

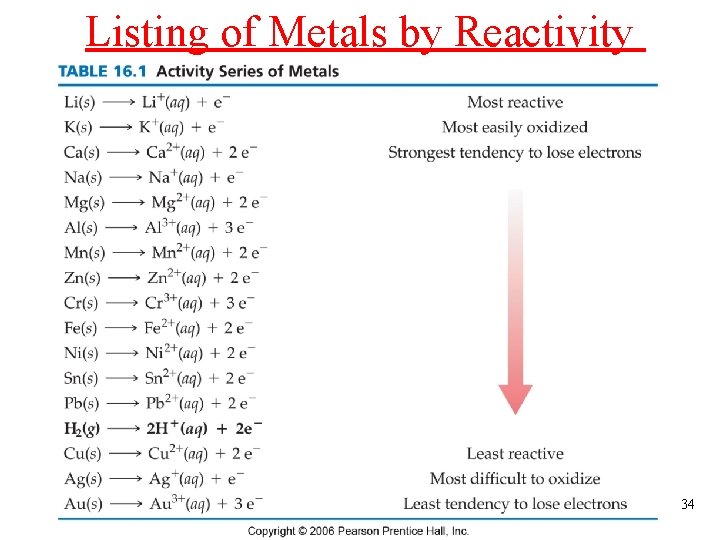
Listing of Metals by Reactivity 34

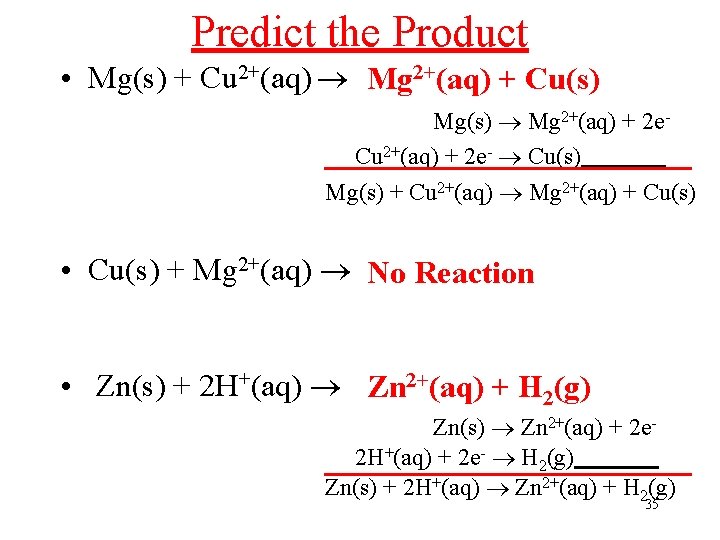
Predict the Product • Mg(s) + Cu 2+(aq) Mg 2+(aq) + Cu(s) Mg(s) Mg 2+(aq) + 2 e. Cu 2+(aq) + 2 e- Cu(s) Mg(s) + Cu 2+(aq) Mg 2+(aq) + Cu(s) • Cu(s) + Mg 2+(aq) No Reaction • Zn(s) + 2 H+(aq) Zn 2+(aq) + H 2(g) Zn(s) Zn 2+(aq) + 2 e 2 H+(aq) + 2 e- H 2(g) Zn(s) + 2 H+(aq) Zn 2+(aq) + H 2(g) 35

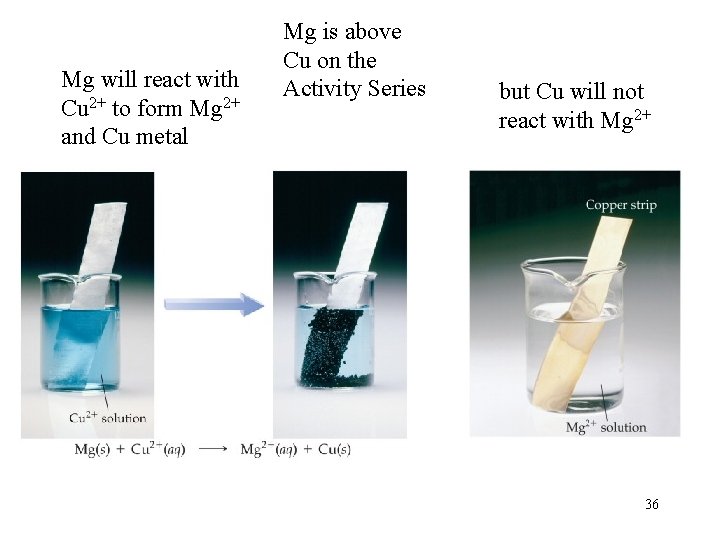
Mg will react with Cu 2+ to form Mg 2+ and Cu metal Mg is above Cu on the Activity Series but Cu will not react with Mg 2+ 36
 Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions What is a chemical change
What is a chemical change True or false: chemical and physical changes alter matter.
True or false: chemical and physical changes alter matter. Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Are kc and kp equal
Are kc and kp equal Elizabeth mulroney
Elizabeth mulroney Chemical properties of air
Chemical properties of air Change in state of matter
Change in state of matter Classification of matter concept map
Classification of matter concept map 5 phases of matter
5 phases of matter Which is a “big idea” for matter and change?
Which is a “big idea” for matter and change? Two types of changes in matter
Two types of changes in matter Definition of substance
Definition of substance Stp law
Stp law Properties and changes of matter worksheet
Properties and changes of matter worksheet Matter-properties and changes answer key
Matter-properties and changes answer key Melting freezing evaporation condensation sublimation
Melting freezing evaporation condensation sublimation How to write half reactions
How to write half reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet What is grey and white matter
What is grey and white matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Gray matter and white matter
Gray matter and white matter Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter White matter
White matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Gray matter and white matter
Gray matter and white matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers An example of a physical change
An example of a physical change Physical and chemical changes
Physical and chemical changes Whats physical change
Whats physical change Physical and chemical changes examples
Physical and chemical changes examples Physical and chemical changes generation genius
Physical and chemical changes generation genius The science duo physical and chemical changes
The science duo physical and chemical changes Difference between chemical and physical property
Difference between chemical and physical property