Chemical Reactions I Chemical Changes in Matter Chemical










- Slides: 31

Chemical Reactions I. Chemical Changes in Matter Chemical Reaction n Law of Conservation of Mass n Chemical Equations n

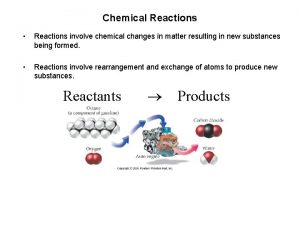
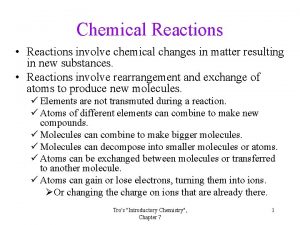
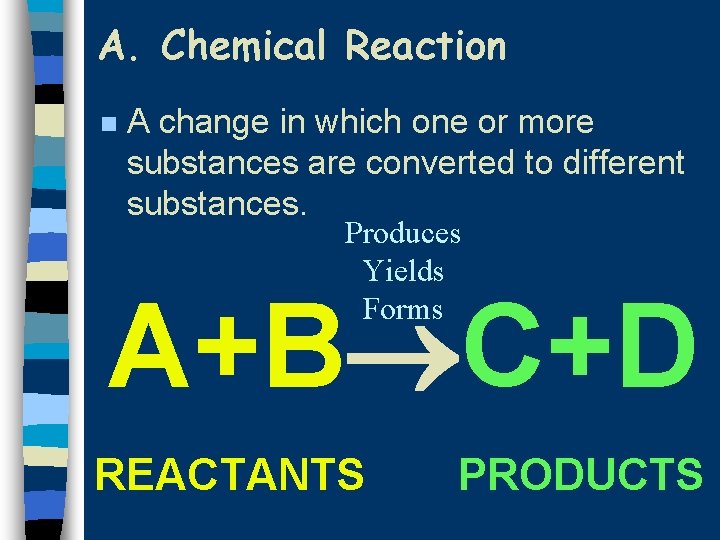
A. Chemical Reaction n A change in which one or more substances are converted to different substances. Produces Yields Forms A+B C+D REACTANTS PRODUCTS

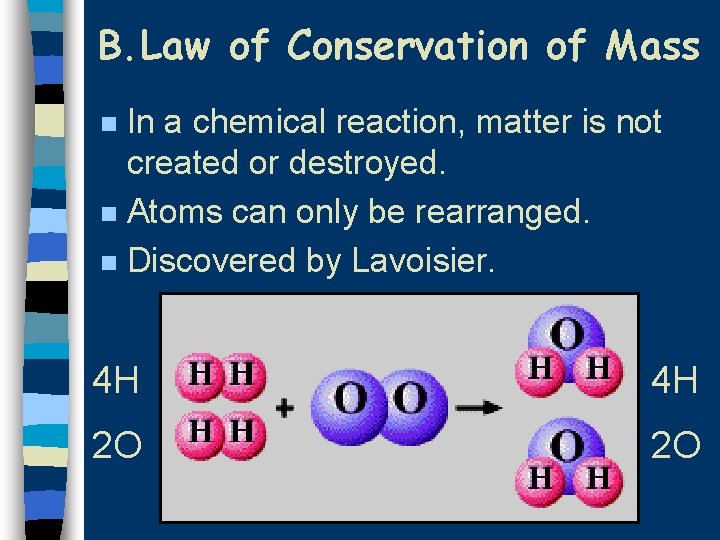
B. Law of Conservation of Mass In a chemical reaction, matter is not created or destroyed. n Atoms can only be rearranged. n Discovered by Lavoisier. n 4 H 4 H 2 O 2 O

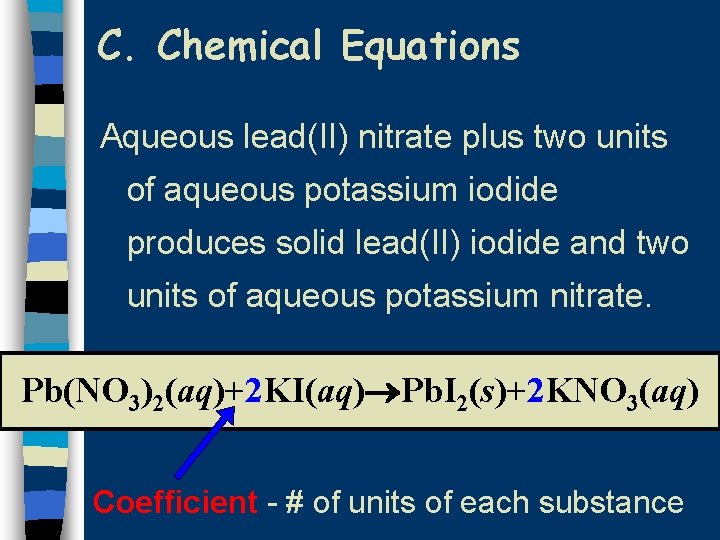
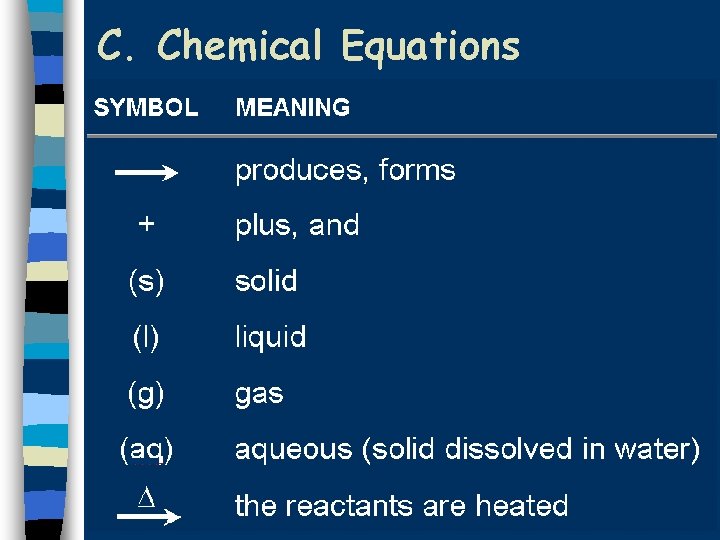
C. Chemical Equations Aqueous lead(II) nitrate plus two units of aqueous potassium iodide produces solid lead(II) iodide and two units of aqueous potassium nitrate. Pb(NO 3)2(aq)+2 KI(aq) Pb. I 2(s)+2 KNO 3(aq) Coefficient - # of units of each substance

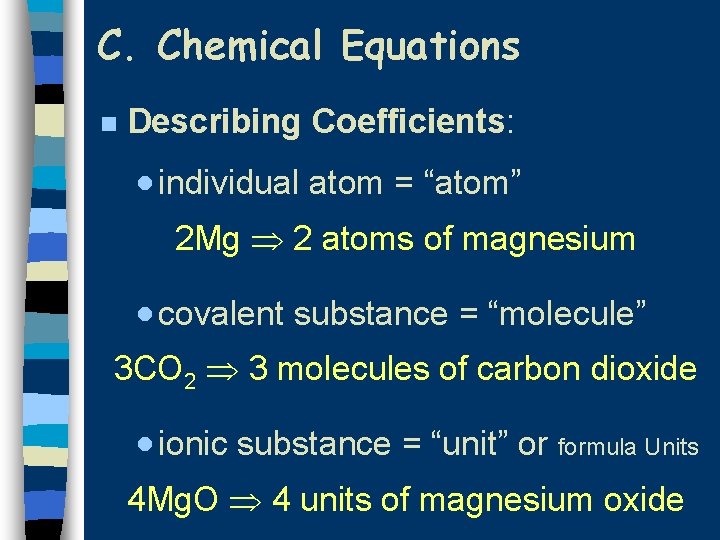
C. Chemical Equations n Describing Coefficients: · individual atom = “atom” 2 Mg 2 atoms of magnesium · covalent substance = “molecule” 3 CO 2 3 molecules of carbon dioxide · ionic substance = “unit” or formula Units 4 Mg. O 4 units of magnesium oxide

C. Chemical Equations

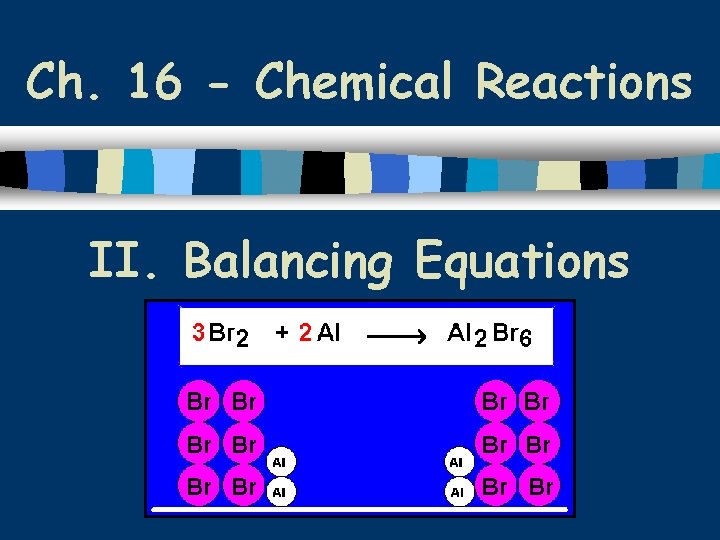
Ch. 16 - Chemical Reactions II. Balancing Equations

A. Steps for Balancing Equations 1. Write the unbalanced equation. 2. Count atoms on each side. 3. Add coefficients to make #s equal. Coefficient Subscript = # Atoms 4. Reduce coefficients to lowest possible ratio, if necessary. 5. Double check atom balance!!!

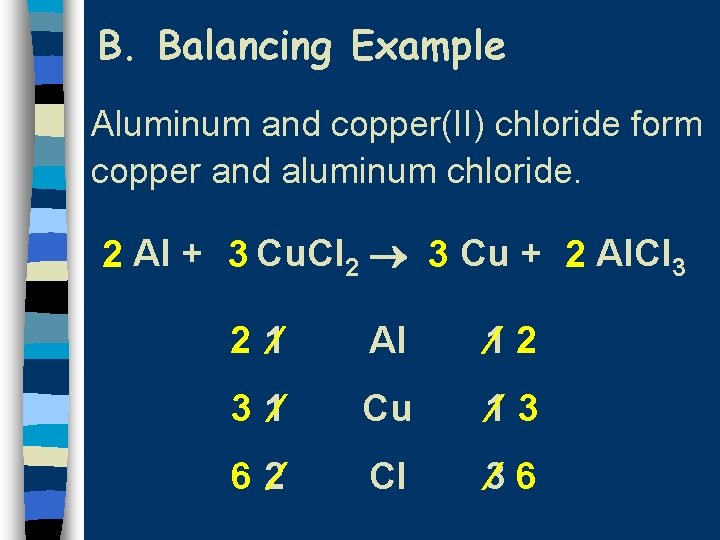
B. Balancing Example Aluminum and copper(II) chloride form copper and aluminum chloride. 2 Al + 3 Cu. Cl 2 3 Cu + 2 Al. Cl 3 2 1 Al 1 2 3 1 Cu 1 3 6 2 Cl 3 6

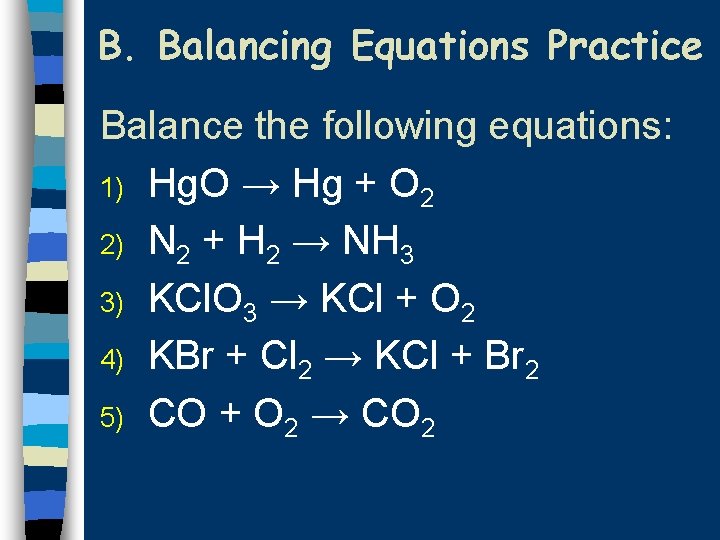
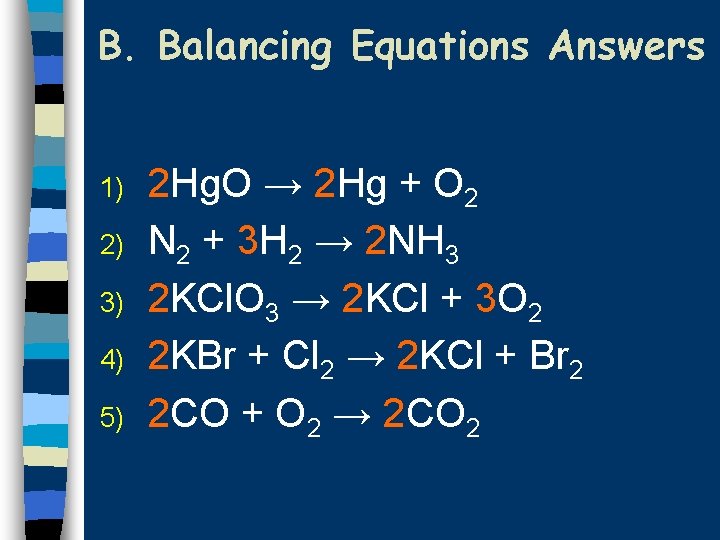
B. Balancing Equations Practice Balance the following equations: 1) Hg. O → Hg + O 2 2) N 2 + H 2 → NH 3 3) KCl. O 3 → KCl + O 2 4) KBr + Cl 2 → KCl + Br 2 5) CO + O 2 → CO 2

B. Balancing Equations Answers 1) 2) 3) 4) 5) 2 Hg. O → 2 Hg + O 2 N 2 + 3 H 2 → 2 NH 3 2 KCl. O 3 → 2 KCl + 3 O 2 2 KBr + Cl 2 → 2 KCl + Br 2 2 CO + O 2 → 2 CO 2

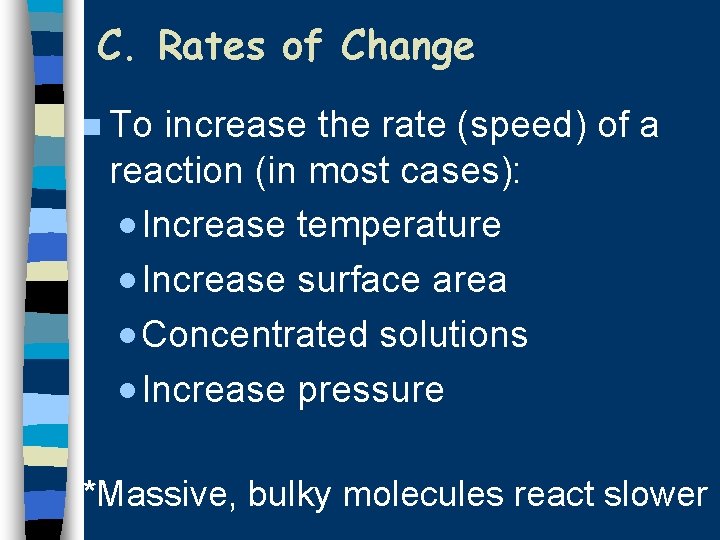
C. Rates of Change n To increase the rate (speed) of a reaction (in most cases): · Increase temperature · Increase surface area · Concentrated solutions · Increase pressure *Massive, bulky molecules react slower

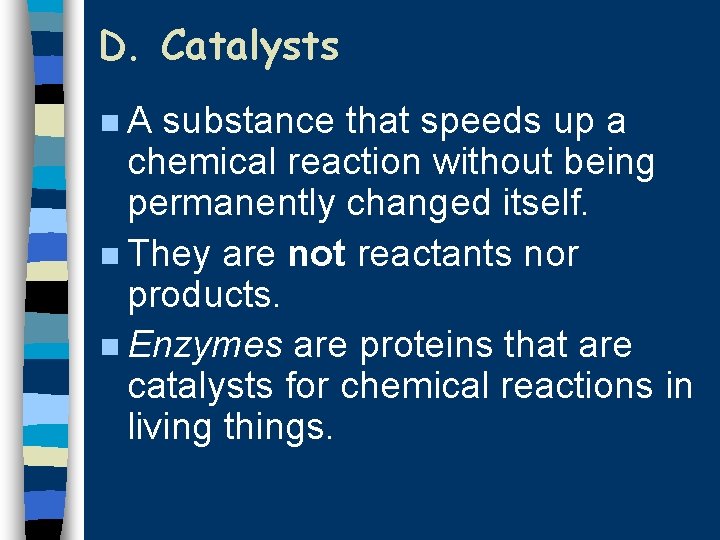
D. Catalysts n. A substance that speeds up a chemical reaction without being permanently changed itself. n They are not reactants nor products. n Enzymes are proteins that are catalysts for chemical reactions in living things.

E. Inhibitors n Substances that are used to combine with one of the reactants to prevent certain reactions from occurring. n Ex: Food preservatives; lemon juice on cut fruit to keep it from turning brown.

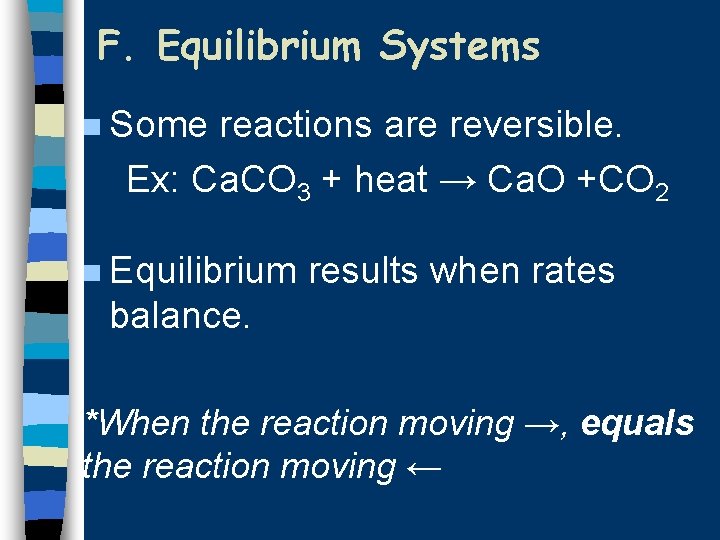
F. Equilibrium Systems n Some reactions are reversible. Ex: Ca. CO 3 + heat → Ca. O +CO 2 n Equilibrium results when rates balance. *When the reaction moving →, equals the reaction moving ←

Ch. 7 - Chemical Reactions III. Types of Reactions Synthesis n Decomposition n. Single-displacement n. Double-displacement n. Combustion n

Five (5) Main Types of Chemical Reactions: 1) Synthesis 2) Decomposition 3) Single-displacement (replacement) 4) Double-displacement (replacement) 5) Combustion

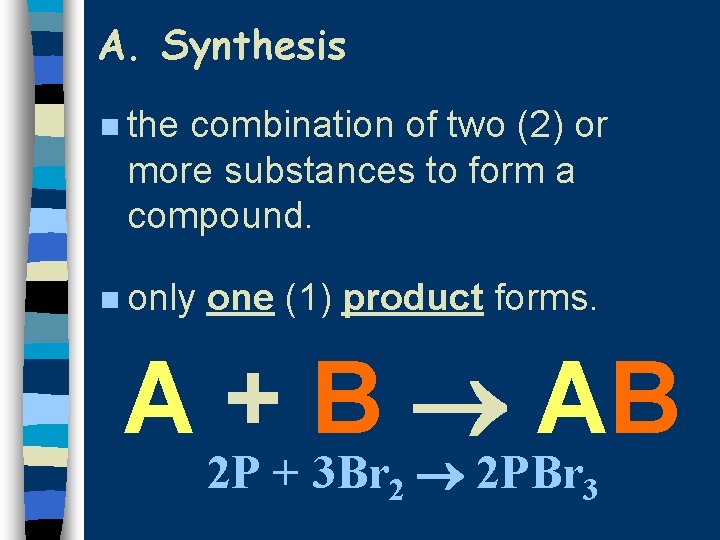
A. Synthesis n the combination of two (2) or more substances to form a compound. n only one (1) product forms. A + B AB 2 P + 3 Br 2 2 PBr 3

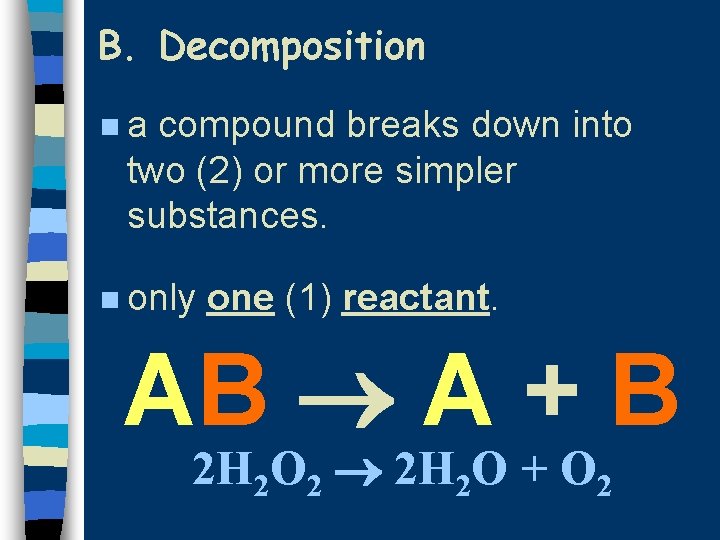
B. Decomposition na compound breaks down into two (2) or more simpler substances. n only one (1) reactant. AB A + B 2 H 2 O 2 2 H 2 O + O 2

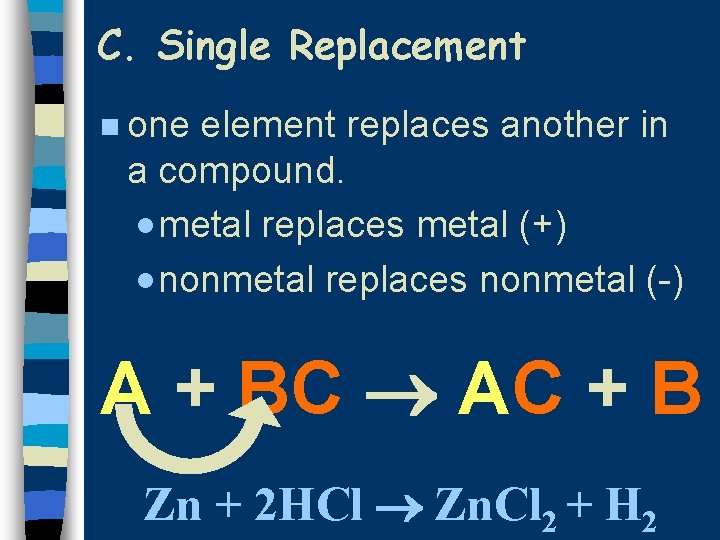
C. Single Replacement n one element replaces another in a compound. · metal replaces metal (+) · nonmetal replaces nonmetal (-) A + BC AC + B Zn + 2 HCl Zn. Cl 2 + H 2

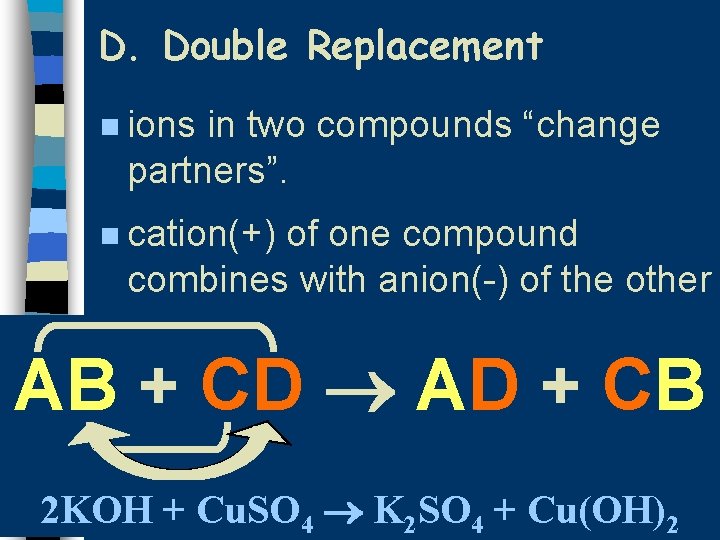
D. Double Replacement n ions in two compounds “change partners”. n cation(+) of one compound combines with anion(-) of the other AB + CD AD + CB 2 KOH + Cu. SO 4 K 2 SO 4 + Cu(OH)2

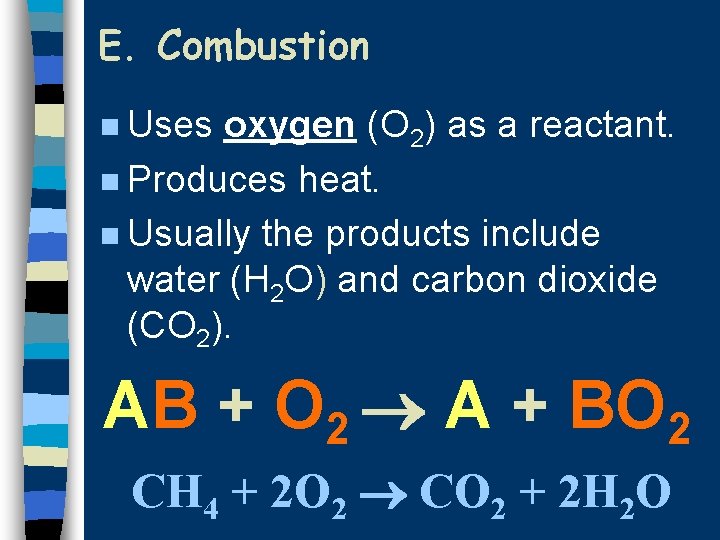
E. Combustion n Uses oxygen (O 2) as a reactant. n Produces heat. n Usually the products include water (H 2 O) and carbon dioxide (CO 2). AB + O 2 A + BO 2 CH 4 + 2 O 2 CO 2 + 2 H 2 O

Ch. 7 - Chemical Reactions IV. Energy & Chemical Reactions n. Signs of reactions n. Energy Changes n. Endothermic Reactions n Exothermic Reactions

5 Signs of a Chemical Reaction n Production of a gas n Production of a precipitant n Change in color n Change in odor n Production of light or heat

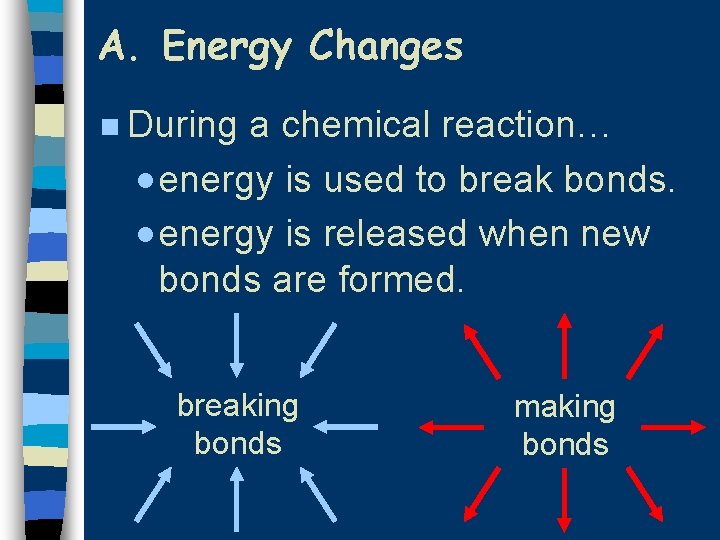
A. Energy Changes n During a chemical reaction… · energy is used to break bonds. · energy is released when new bonds are formed. breaking bonds making bonds

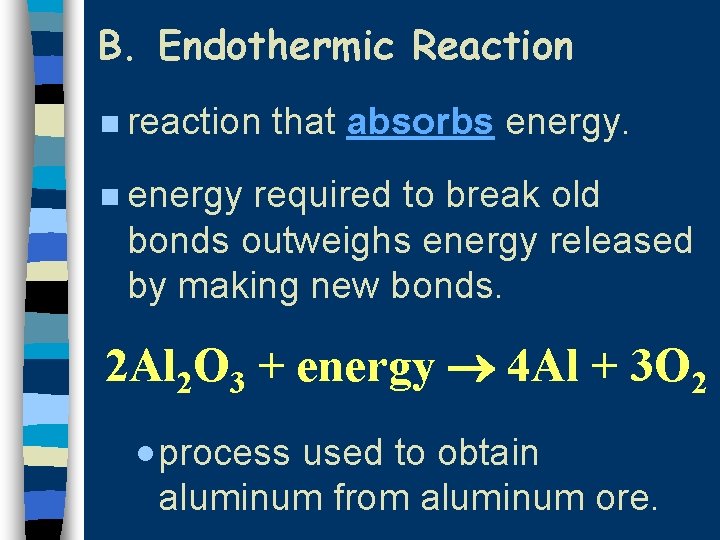
B. Endothermic Reaction n reaction that absorbs energy. n energy required to break old bonds outweighs energy released by making new bonds. 2 Al 2 O 3 + energy 4 Al + 3 O 2 · process used to obtain aluminum from aluminum ore.

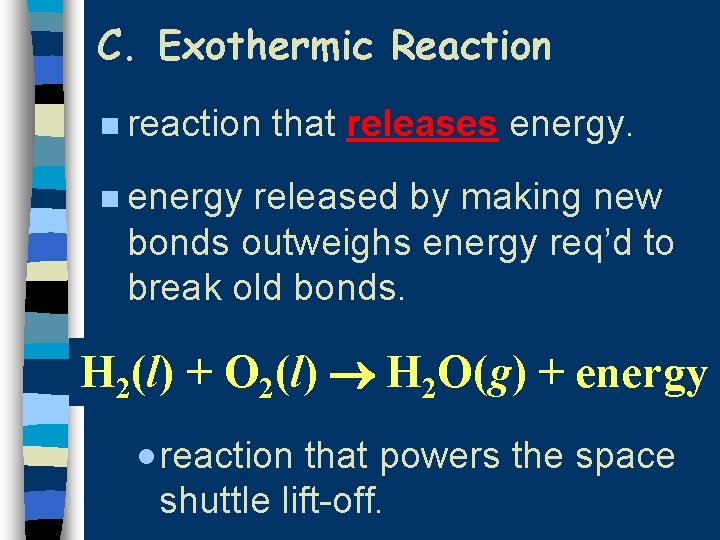
C. Exothermic Reaction n reaction that releases energy. n energy released by making new bonds outweighs energy req’d to break old bonds. H 2(l) + O 2(l) H 2 O(g) + energy · reaction that powers the space shuttle lift-off.

Identify each as endothermic or exothermic 1. 2. 3. 4. 5. 6. Container gets warm Container gets cold Ice forms Steam is released H 2 + CO 2 -> H 2 O + CO + 394 k. J N 2 O 4+ 57. 2 k. J -> 2 NO 2

Ch. 7 - Chemical Reactions V. Law of Conservation of Mass application nexamples n

Conservation of mass explained n In all chemical reactions mass is conserved n The mass of reactants MUST equal the mass of products. n This fact can be used to determine the amount of a missing reactant or product.

Conservation of mass explained Mass of reactants = mass of products 1) 14 g Al and 23 g O produces ____ grams of Aluminum oxide. 2) 25 g water breaks down into 19 grams of oxygen and ____ g Hydrogen 3) ____ g water break down into 82 g oxygen and 21 g hydrogen.
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 1 chemical changes
Section 1 chemical changes Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Physical change
Physical change Eating food physical or chemical change
Eating food physical or chemical change Example of oxidation reduction reaction
Example of oxidation reduction reaction Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Are kc and kp equal
Are kc and kp equal Elizabeth mulroney
Elizabeth mulroney Phases changes of matter
Phases changes of matter Which is a big idea for matter and change
Which is a big idea for matter and change Definition of substance
Definition of substance R constant
R constant Properties and changes of matter worksheet
Properties and changes of matter worksheet Change in state of matter
Change in state of matter Matter-properties and changes answer key
Matter-properties and changes answer key Phase changes
Phase changes Physical changes of matter
Physical changes of matter Types of change in matter
Types of change in matter Classification of matter concept map
Classification of matter concept map Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers What is gray matter
What is gray matter Section 1 composition of matter
Section 1 composition of matter Median and lateral apertures
Median and lateral apertures Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter White matter nervous system
White matter nervous system Section 1 composition of matter chapter 15 answer key
Section 1 composition of matter chapter 15 answer key Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Gray matter and white matter
Gray matter and white matter Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Types of reactions chemistry
Types of reactions chemistry