States of Matter 8 1 Solids Liquids Gases

















- Slides: 38

States of Matter

8. 1 Solids, Liquids, Gases States of Matter- the physical forms in which a substance can exist Solid – ex. ice Liquid Gas Plasma – positively and negatively charged particles Most common in universe

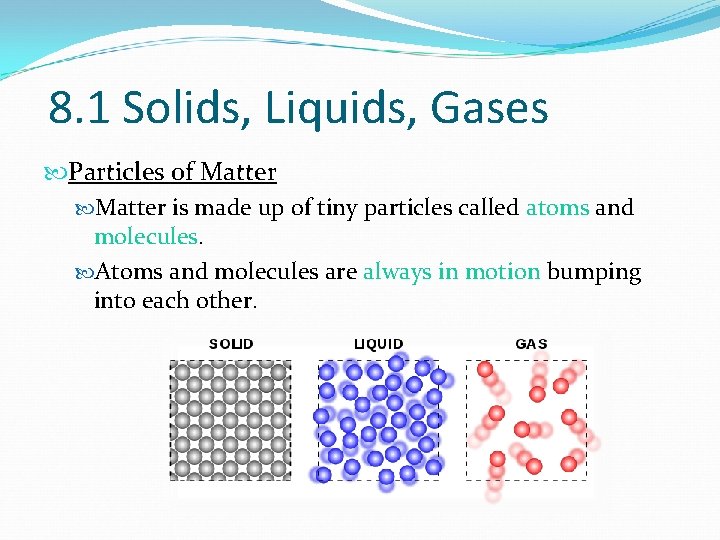
8. 1 Solids, Liquids, Gases Particles of Matter is made up of tiny particles called atoms and molecules. Atoms and molecules are always in motion bumping into each other.

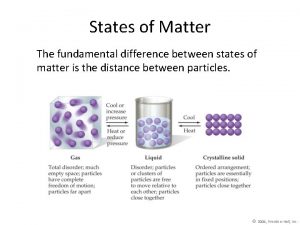
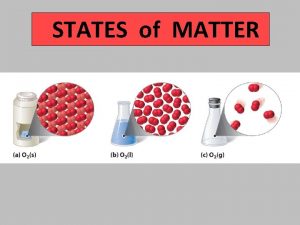
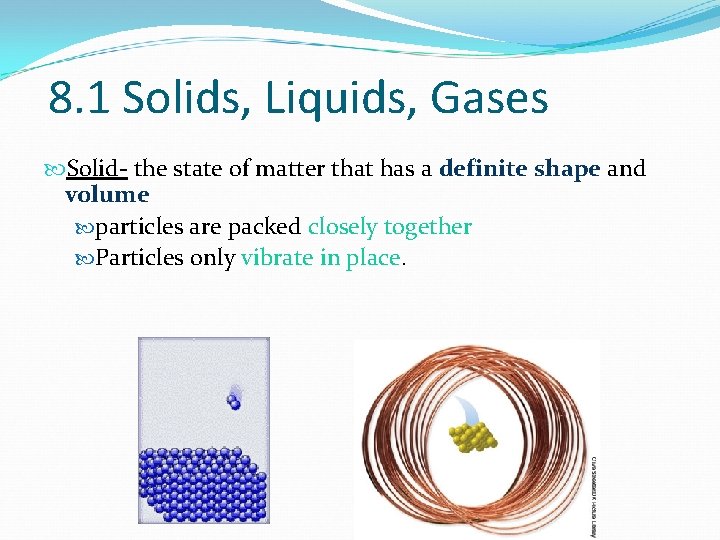
8. 1 Solids, Liquids, Gases Solid- the state of matter that has a definite shape and volume particles are packed closely together Particles only vibrate in place.

8. 1 Solids, Liquids, Gases Two types of solids: Crystalline- These solids have an orderly arrangement of particles in a repeating pattern. Examples include iron, diamond, ice, crystal Amorphous- These solids do not have a special arrangement. Examples include glass, rubber, wax.

8. 1 Solids, Liquids, Gases Liquid- the state of matter that has a definite volume but takes the shape of its container particles are close together Particles slide past one another. .

8. 1 Solids, Liquids, Gases Liquids have several properties that make them unique Surface tension- an uneven force that acts on the particles at the surface of a liquid. This causes some particles to form spherical drops like beads. Water has a high surface tension while rubbing alcohol has a low surface tension. Viscosity- a measurement of a liquid’s resistance to flow The stronger the attractions between the molecules, the more viscous the liquid is. Honey has a high viscosity. Water has a low viscosity.

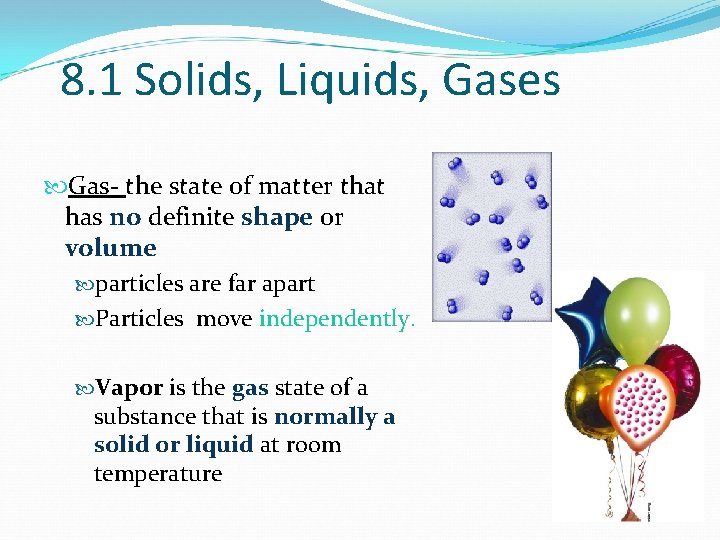
8. 1 Solids, Liquids, Gases Gas- the state of matter that has no definite shape or volume particles are far apart Particles move independently. Vapor is the gas state of a substance that is normally a solid or liquid at room temperature

8. 1 Solids, Liquids, Gases State of Matter Solid Liquid Gas Definite Shape? Definite Volume?

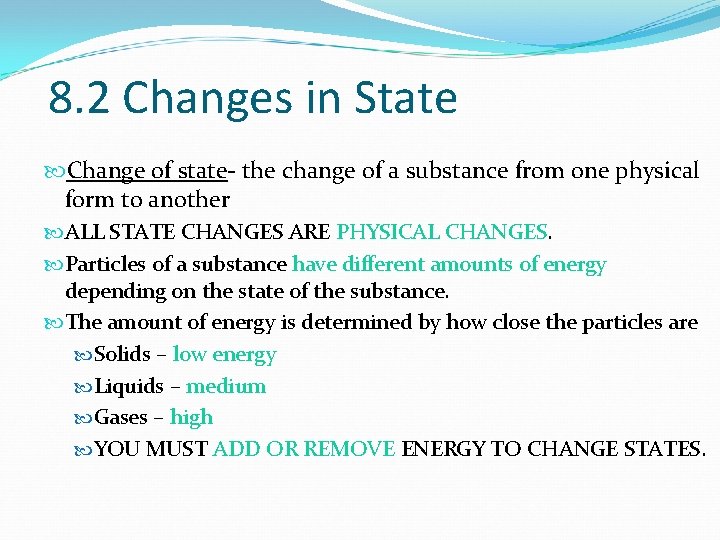
8. 2 Changes in State Change of state- the change of a substance from one physical form to another ALL STATE CHANGES ARE PHYSICAL CHANGES. Particles of a substance have different amounts of energy depending on the state of the substance. The amount of energy is determined by how close the particles are Solids – low energy Liquids – medium Gases – high YOU MUST ADD OR REMOVE ENERGY TO CHANGE STATES.

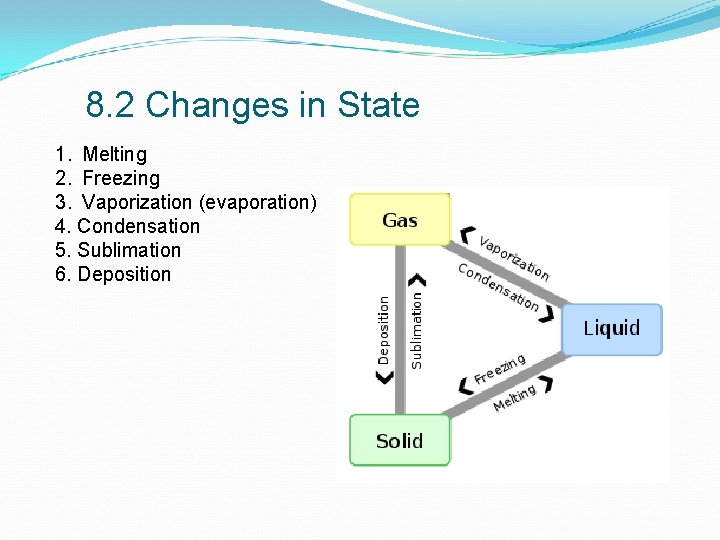
8. 2 Changes in State 1. Melting 2. Freezing 3. Vaporization (evaporation) 4. Condensation 5. Sublimation 6. Deposition

8. 2 Changes in State Melting: Solid to Liquid Melting- change in state from solid to liquid. Adding energy to ice raises its temperature. As the temperature increases, the particles energy increases When a certain temperature is reached the substance melts Ex. At 0°C ice melts Melting is endothermic- energy is gained by the substance

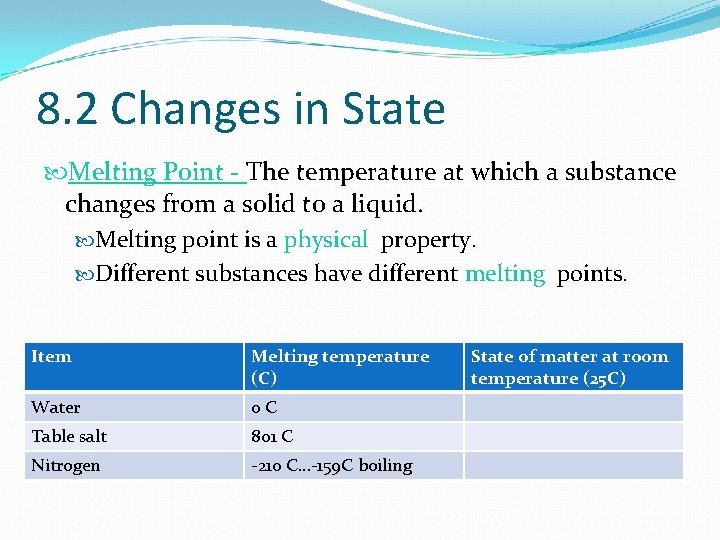
8. 2 Changes in State Melting Point - The temperature at which a substance changes from a solid to a liquid. Melting point is a physical property. Different substances have different melting points. Item Melting temperature (C) Water 0 C Table salt 801 C Nitrogen -210 C…-159 C boiling State of matter at room temperature (25 C)

8. 2 Changes in State Freezing: Liquid to Solid Freezing- change in state from a liquid to solid. Freezing point- The temperature at which liquid changes into a solid. Freezing is the reverse of melting, so they occur at the same temperature. Any solid is in the frozen state! Freezing is exothermic energy is removed from the substance

8. 2 Changes in State Vaporization: Liquid to Gas Vaporization- change in state form a liquid to gas. Evaporation is vaporization that occurs only at the surface of a liquid Boiling is the change of a liquid to a gas (vapor) throughout the whole liquid Boiling point- temperature at which a substance boils Vaporization requires adding energy so it is endothermic

8. 2 Changes in State Effects of Pressure on Boiling Point Water boils at 100ºC, but only at sea level because of atmospheric pressure. Atmospheric pressure is caused by the gases that make up the atmosphere. The higher you go above sea level, the fewer air particles there above you so atmospheric pressure is less. In Denver, the mile high city, water boils at 94 o C.

8. 2 Changes in State Condensation: Gas to Liquid Condensation- change of state from a gas to liquid. Energy must be removed - exothermic Condensation point- temperature at which a substance goes from a gas to a liquid. Condensation is the reverse of vaporization, so they occur at the same temperature.

8. 2 Changes in State Sublimation: Solid to Gas Sublimation- the change from a solid to a gas without being a liquid Dry ice (carbon dioxide) The substance must gain a lot of energy for this to occur, therefore it is endothermic.

8. 2 Changes in State Deposition: Gas to Solid Deposition: the change from a gas to a solid without being a liquid Frost Deposition is an exothermic change because a lot energy must be removed.

8. 2 Changes in State Change in temperature vs. Change in state Change of temperature versus change of state When energy is added… Either the temperature changes OR the state changes Not both!

8. 2 Changes in State Change in temperature vs. Change in state When substances gain or lose energy, the temperature will change or remain the same. As the temperature rises, the particles gain energy. Once the particles have enough energy, the state will change. At this point the temperature will remain until the state change is complete. Boiling water will remain at 100 o. C until it all evaporates.

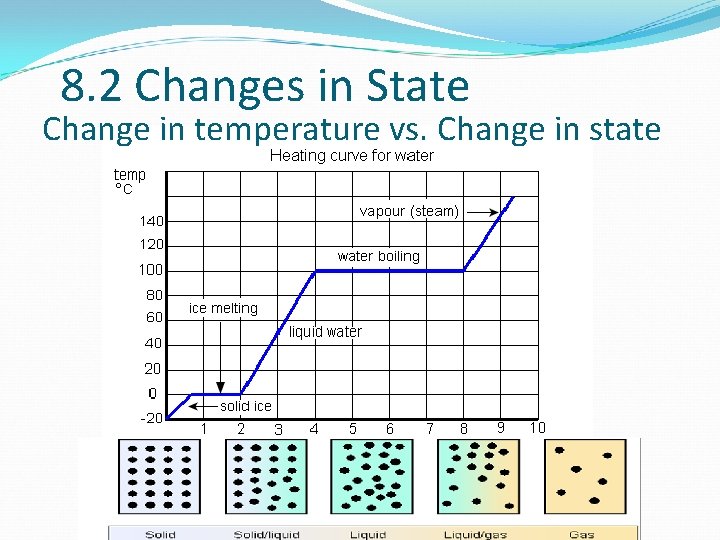
8. 2 Changes in State Change in temperature vs. Change in state

8. 2 Changes in State What’s happing in a phase change diagram? When you add heat to a solid… …the temperature increases until the melting point You still add heat… …the temperature stays while the solid melts You now add heat to a liquid… …the temperature increases until the boiling point You still add heat… …the temperature stays while the liquid vaporizes You now add heat to a gas… …the temperature will just keep increasing!

8. 2 Changes in State change Melting Freezing Evaporation Condensation Sublimation Deposition Starting state Ending state Energy added or removed Endothermic or exothermic

Partner Share Neatly and in your own words, Write at least 8 lines describing the difference between boiling and evaporation. Be sure to give an example of where there may be different boiling points for the same substance.

8. 3 Behavior of Gases The kinetic molecular theory is an explanation of how particles in matter behave Small particles make up all matter Particles are in constant, random motion Particles collide with other particles, other objects, an the walls of their container No energy is lost when particles collide Describing Gas Behavior Gases behave differently from solids and liquids. Gases have large amounts of empty space between molecules.

8. 3 Behavior of Gases What factors affect how gases behave? Temperature Volume Pressure

8. 3 Behavior of Gases Temperature is a measure of how fast the particles in an object are moving around. The faster the particles move, the more energy they have On a hot day, particles move faster and hit the inside walls of a balloon more often. Thus, increasing energy and pushing on the walls. On a cold day, particles have less energy They do not push very hard on the walls of the balloon.

8. 3 Behavior of Gases Volume is the amount of space that an object takes up Volume of a gas depends on its container Decrease volume…increase pressure Increase volume…decrease pressure

8. 3 Behaviorof of. Gases Pressure is the amount of force exerted per area on a surface • A basketball is very firm compared to a beach ball • It has a greater pressure. • The beach ball has a much lower pressure.

8. 3 Behavior of Gases Gas behavior laws Boyle’s Law Involves volume and pressure Charles’s Law Involves temperature and volume Guy-Lussac’s Law Involves temperature and pressure

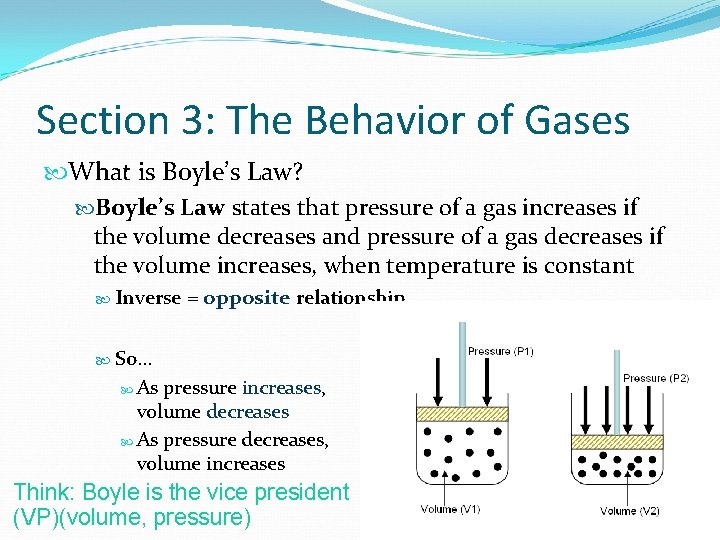
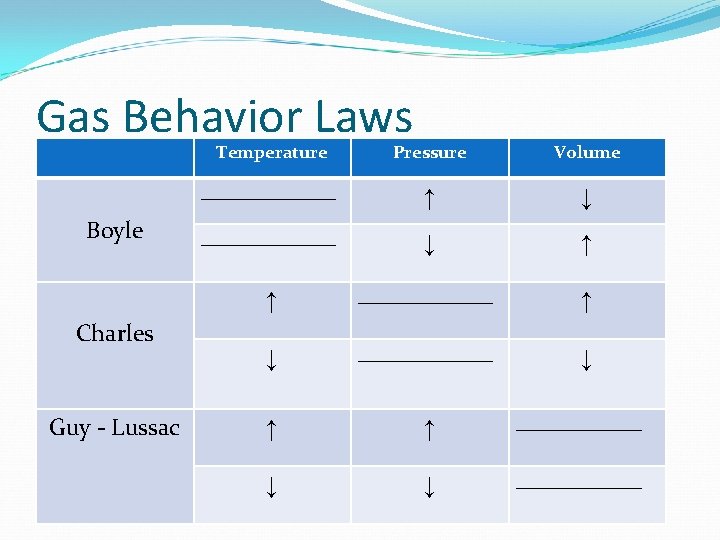
Section 3: The Behavior of Gases What is Boyle’s Law? Boyle’s Law states that pressure of a gas increases if the volume decreases and pressure of a gas decreases if the volume increases, when temperature is constant Inverse = opposite relationship So… As pressure increases, volume decreases As pressure decreases, volume increases Think: Boyle is the vice president (VP)(volume, pressure)

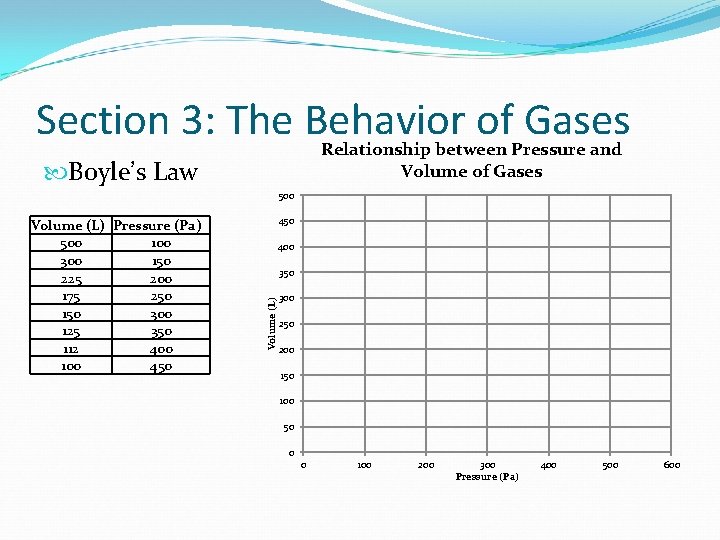
Section 3: The Behavior of Gases Relationship between Pressure and Volume of Gases Boyle’s Law 500 450 400 350 Volume (L) Pressure (Pa) 500 100 300 150 225 200 175 250 150 300 125 350 112 400 100 450 300 250 200 150 100 50 0 0 100 200 300 Pressure (Pa) 400 500 600

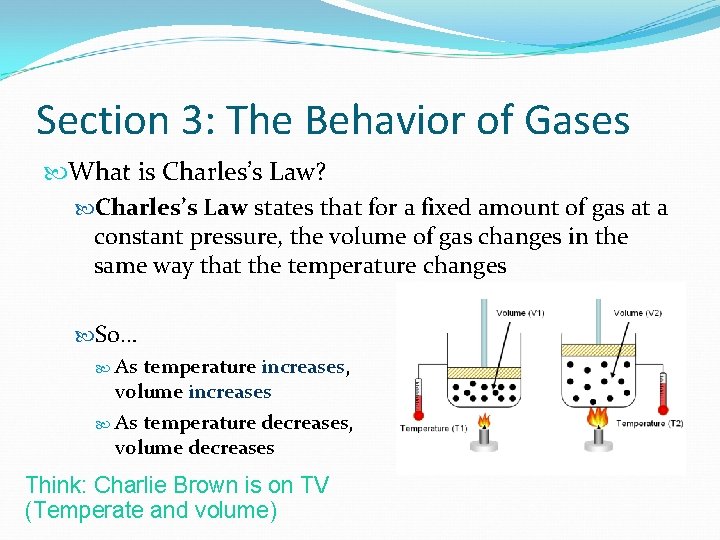
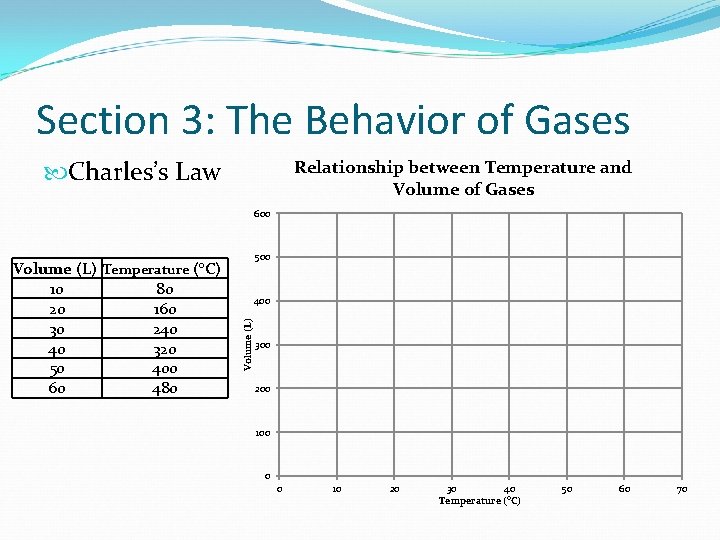
Section 3: The Behavior of Gases What is Charles’s Law? Charles’s Law states that for a fixed amount of gas at a constant pressure, the volume of gas changes in the same way that the temperature changes So… As temperature increases, volume increases As temperature decreases, volume decreases Think: Charlie Brown is on TV (Temperate and volume)

Section 3: The Behavior of Gases Charles’s Law Relationship between Temperature and Volume of Gases 600 500 400 Volume (L) Temperature (°C) 10 80 20 160 30 240 40 320 50 400 60 480 300 200 100 0 0 10 20 30 40 Temperature (°C) 50 60 70

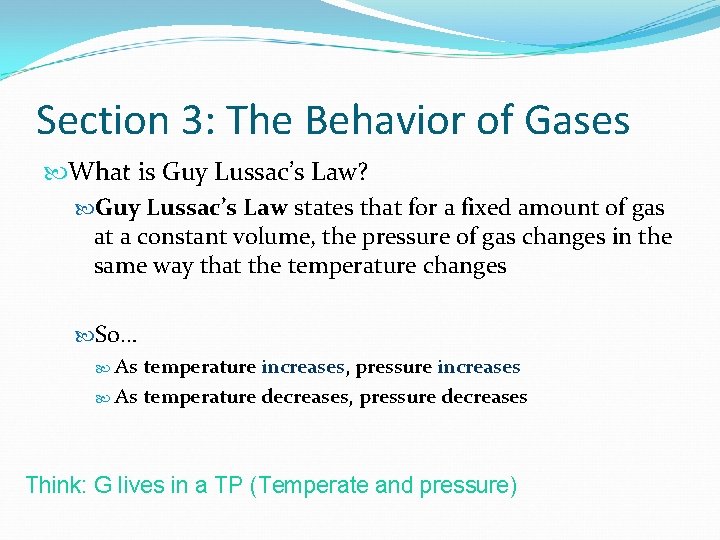
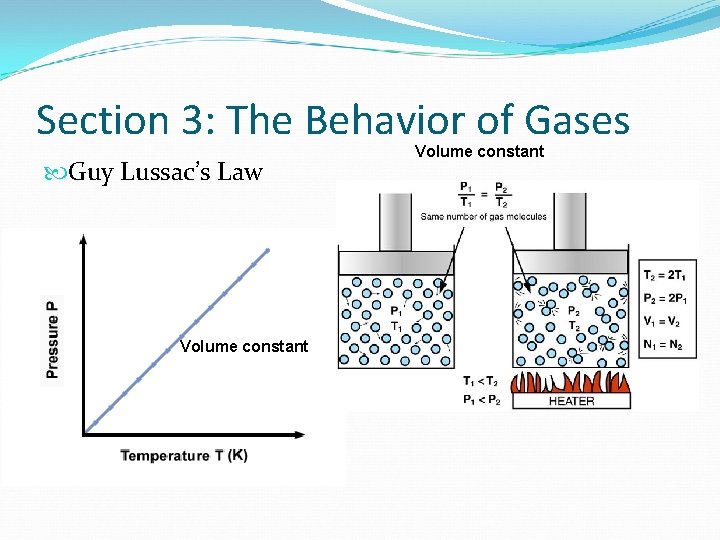
Section 3: The Behavior of Gases What is Guy Lussac’s Law? Guy Lussac’s Law states that for a fixed amount of gas at a constant volume, the pressure of gas changes in the same way that the temperature changes So… As temperature increases, pressure increases As temperature decreases, pressure decreases Think: G lives in a TP (Temperate and pressure)

Section 3: The Behavior of Gases Guy Lussac’s Law Volume constant

Gas Behavior Laws Boyle Charles Guy - Lussac Temperature Pressure Volume _______________ ↑ ↓ _______________ ↓ ↑ ↑ _______ ↓ ↓ _______
 Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Properties of solid liquid and gas
Properties of solid liquid and gas Why are gases eaiser to compress than solids or liquids?
Why are gases eaiser to compress than solids or liquids? Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Examples of solids liquids and gases pictures
Examples of solids liquids and gases pictures Red liquid element
Red liquid element Venn diagram states of matter
Venn diagram states of matter The science duo physical and chemical changes
The science duo physical and chemical changes How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases The properties of solids liquids and gases
The properties of solids liquids and gases Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Thermal expansion and contraction examples
Thermal expansion and contraction examples Liquids and solids menu
Liquids and solids menu Kesler science.com
Kesler science.com Adhesive force
Adhesive force Solid liquid and gas particles
Solid liquid and gas particles Constant rate filtration example
Constant rate filtration example Molecular theory of gases and liquids
Molecular theory of gases and liquids Gray matter and white matter
Gray matter and white matter Grey matter
Grey matter States of matter basics
States of matter basics 5 states of matter
5 states of matter Thermal energy states of matter
Thermal energy states of matter States of matter solid liquid gas
States of matter solid liquid gas Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key The fundamental difference between states of matter is the
The fundamental difference between states of matter is the Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter The kinetic theory of matter states that
The kinetic theory of matter states that Four phases of matter
Four phases of matter States of matter objectives
States of matter objectives States of matter
States of matter State of matter in chemical equations
State of matter in chemical equations Whats states of matter
Whats states of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter States of matter foldable
States of matter foldable Flow energy review
Flow energy review Changing states of matter
Changing states of matter