MATTER Ch 8 Solids Liquids Gases I States















- Slides: 23

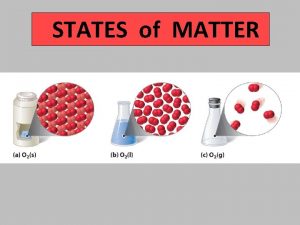
MATTER Ch. 8 - Solids, Liquids, & Gases I. States of Matter (p. 214 -220) w Kinetic Theory of Matter w Four States of Matter w Thermal Expansion

A. Kinetic Theory of Matter ö KTM w Tiny, constantly moving particles make up all matter. w The kinetic energy (motion) of these particles increases as temperature increases.

States of Matter ö Can by classified by their properties w Ability to flow w Ability to change shape w Ability to change volume w Movement of molecules

B. Four States of Matter ö Solids w low KE - particles vibrate but can’t move around w definite shape & volume w crystalline - repeating geometric pattern w amorphous - no pattern (e. g. glass, wax)

Water as a crystaline solid Ice

B. Four States of Matter ö Liquids w higher KE - particles can move around but are still close together w indefinite shape Ø Takes the shape of container w definite volume

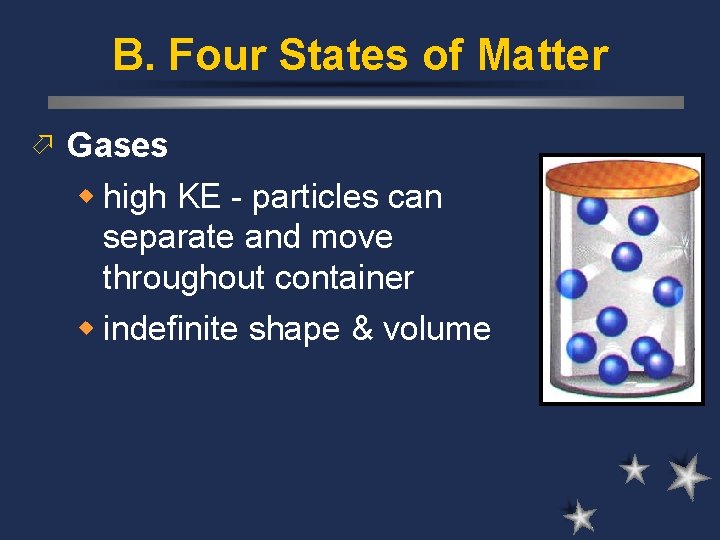
B. Four States of Matter ö Gases w high KE - particles can separate and move throughout container w indefinite shape & volume

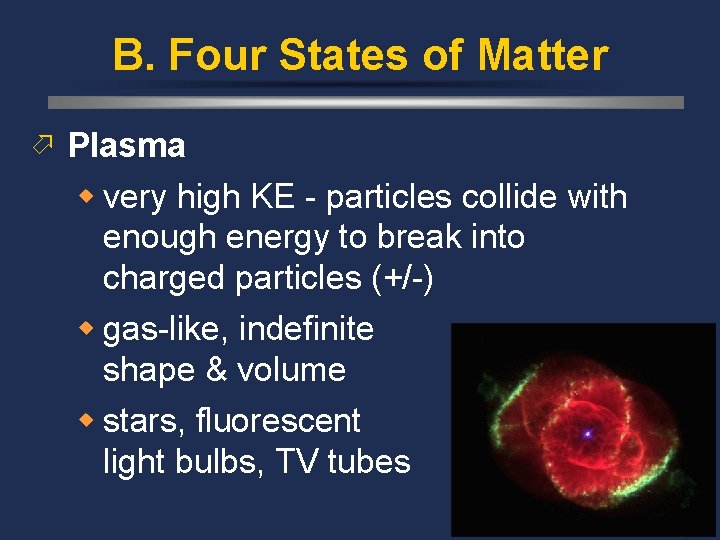
B. Four States of Matter ö Plasma w very high KE - particles collide with enough energy to break into charged particles (+/-) w gas-like, indefinite shape & volume w stars, fluorescent light bulbs, TV tubes

Example of Plasma


States of Matter Clip Show States of Matter. flv

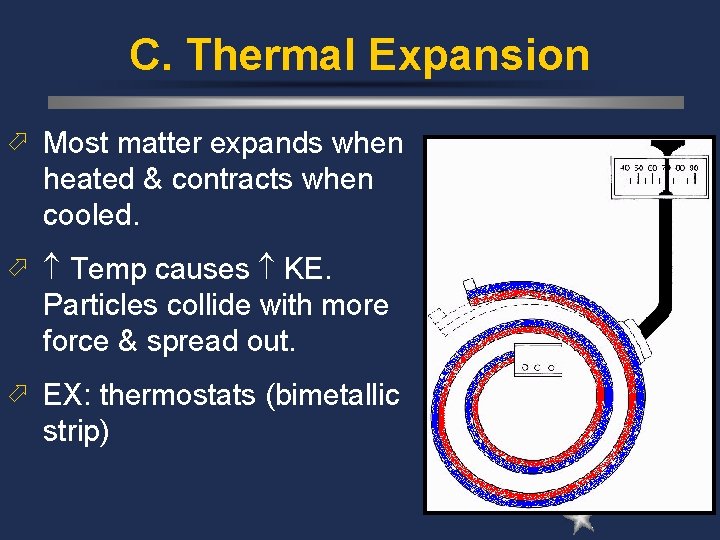
C. Thermal Expansion ö Most matter expands when heated & contracts when cooled. ö Temp causes KE. Particles collide with more force & spread out. ö EX: thermostats (bimetallic strip)

Question 1 Most _____ materials have a specific type of geometric arrangement. A. B. C. D. gaseous inert liquid solid

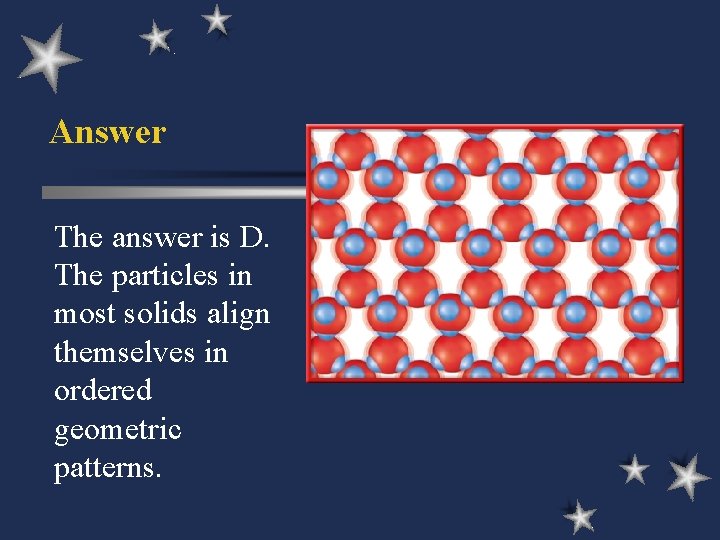
Answer The answer is D. The particles in most solids align themselves in ordered geometric patterns.

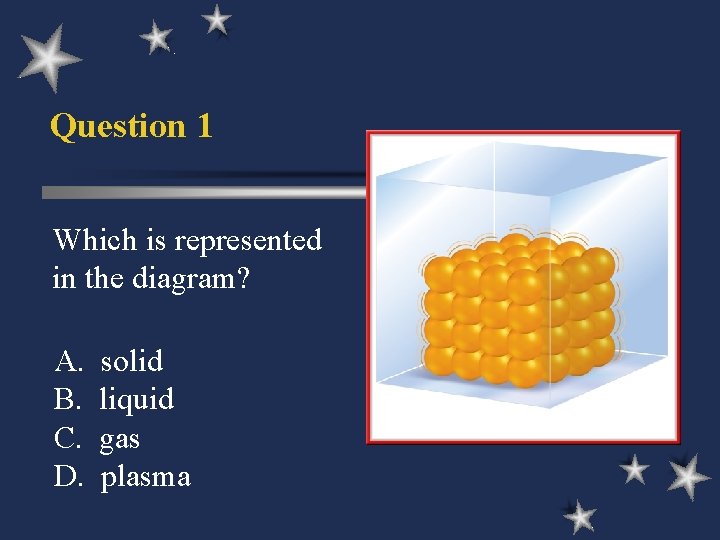
Question 1 Which is represented in the diagram? A. B. C. D. solid liquid gas plasma

Answer The answer is A. The particles in a solid are packed together tightly.


Solids, Liquids, and Gases The matter that surrounds you is either a solid, liquid, or gas. Make the following Foldable to help you organize information about solids, liquids, and gases.

Fold a sheet of paper in half lengthwise. Make the back edge about 5 cm longer than the front edge.

Turn the paper so the fold is on the bottom. Then fold it into thirds.

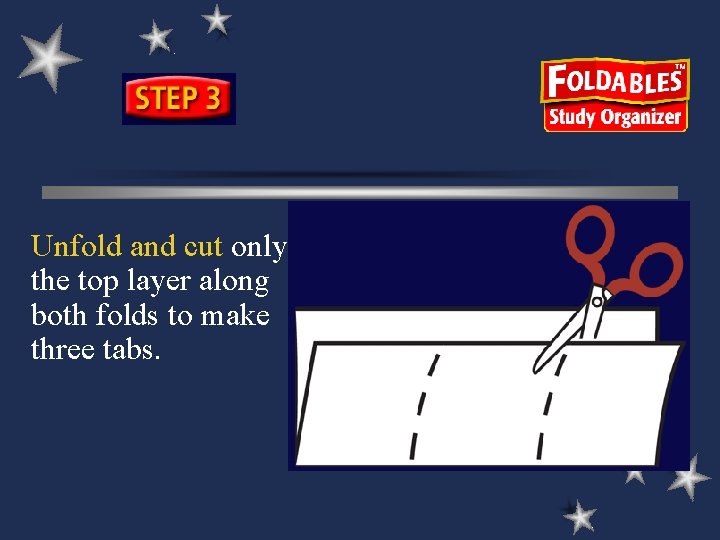
Unfold and cut only the top layer along both folds to make three tabs.

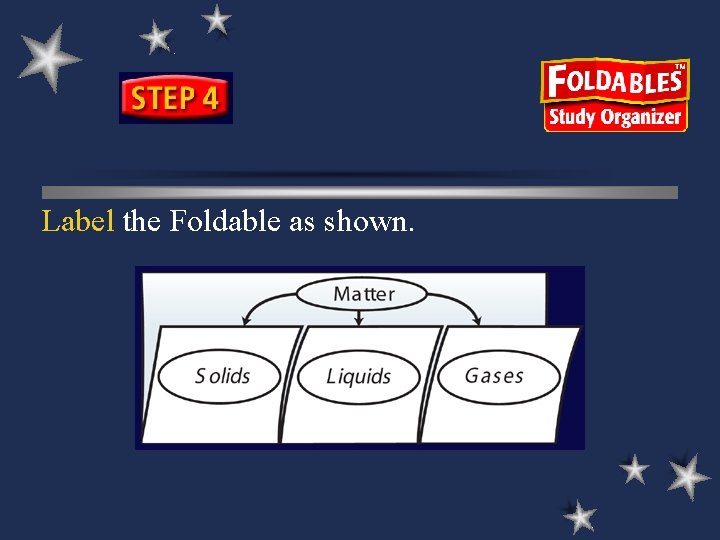
Label the Foldable as shown.

Read for Main Ideas As you read the chapter, list the characteristics of solids, liquids, and gases under the appropriate tab. List some examples of each under the tab also.
 Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Properties of solid, liquid and gas
Properties of solid, liquid and gas Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Thermal expansion and contraction examples
Thermal expansion and contraction examples Properties of solids liquids and gases
Properties of solids liquids and gases Why is gas easier to compress than a liquid
Why is gas easier to compress than a liquid Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Buoyancyability
Buoyancyability Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Process of liquid to gas
Process of liquid to gas Particle movement in solids liquids and gases
Particle movement in solids liquids and gases States of matter venn diagram
States of matter venn diagram Lesson outline lesson 1 solids liquids and gases answer key
Lesson outline lesson 1 solids liquids and gases answer key Constant rate filtration example
Constant rate filtration example Liquids and solids menu
Liquids and solids menu Kesler science.com
Kesler science.com Adhesive force
Adhesive force Solid liquid and gas molecules
Solid liquid and gas molecules Molecular theory of gases and liquids
Molecular theory of gases and liquids Matter
Matter Flow chart of pure substances and mixtures
Flow chart of pure substances and mixtures Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Solid
Solid