SANITATION ESSENTIALS FOR HUMAN FOOD MANUFACTURING FDA FSMA





























































- Slides: 71

SANITATION ESSENTIALS FOR HUMAN FOOD MANUFACTURING FDA FSMA Readiness Training 25 Aug 2016

Welcome Brenda Stahl, Ph. D CFSAN/OFS Division of Plant Products and Beverages

Agenda Brenda Stahl, CFSAN/OFS Ø Introduction Joe Stout, Commercial Food Sanitation Ø Industry Perspective Brenda Stahl, CFSAN/OFS ØFDA Perspective – PC Rule Requirements ØQ&A and Wrap Up

Objectives / Overview • Appreciate the importance of sanitation efficacy, management and verification in the food industry • Outline the modernized GMP’s requirements regarding sanitation, including training • Recognize the necessity for sanitation control • Describe current industry sanitation practices

Sanitation Essentials Industry Perspective Joseph Stout RS President Commercial Food Sanitation (CFS)

FDA Sanitation Essentials 1. The Big Picture 2. Hygienic Design 3. A Risk Based Approach to Design 4. The Process and Science of Cleaning 5. Seven Steps of Cleaning 6. Industry Partnership

#1 THE BIG PICTURE

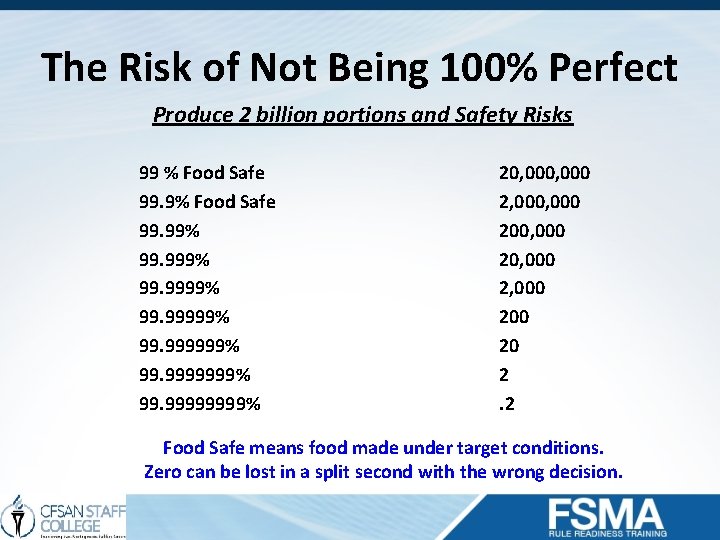
The Risk of Not Being 100% Perfect Produce 2 billion portions and Safety Risks 99 % Food Safe 99. 99% 99. 9999% 99. 999999% 99. 99999999% 20, 000 2, 000 200, 000 2, 000 20 2. 2 Food Safe means food made under target conditions. Zero can be lost in a split second with the wrong decision.

Food Safety Effective Management at “the Cliff” Influences that can push things to the edge; Change New people w/o knowledge Commercialization projects Old Plants / equipment Limited Investment Capital Labor cut backs w/o controls New products Expectation Food Safety Controls Assuring Food Safety; Hygienic Zoning Sanitation Programs Environmental Monitoring Sanitary Design Training Audits GMPs Documentation Shared knowledge Listeria Buffer Zone Lost Consumer Confidence Regulatory Salmonella Citations Illness / Death Lost Sales Special Situations Complaints Recalls

Safe food is all about Preventive Controls

CFS Preventive Plan for Environmental Pathogen Control & Sanitation Excellence in Food Manufacturing Plants #1 #2 Separation of Raw from RTE + #3 GMP Programs and Execution #4 Hygienic Design & Maintenance + for Sanitation + Sanitation Programs and Execution #5 #6 Environmental Monitoring Pest Control + = Zero Resident Pathogens +

#2 HYGIENIC DESIGN

Common Sense Approach to Sanitary Design If you cannot see it AND You cannot reach it YOU CANNOT CLEAN IT! Or sample it © 2016 Commercial Food Sanitation LLC. All rights reserved.

A few examples of poor design © 2016 Commercial Food Sanitation LLC. All rights reserved.

Sanitary Design Hard to Clean

Sanitary Design Easy To Clean




This poor design is protected by pasteurization discovered more than 150 years ago.

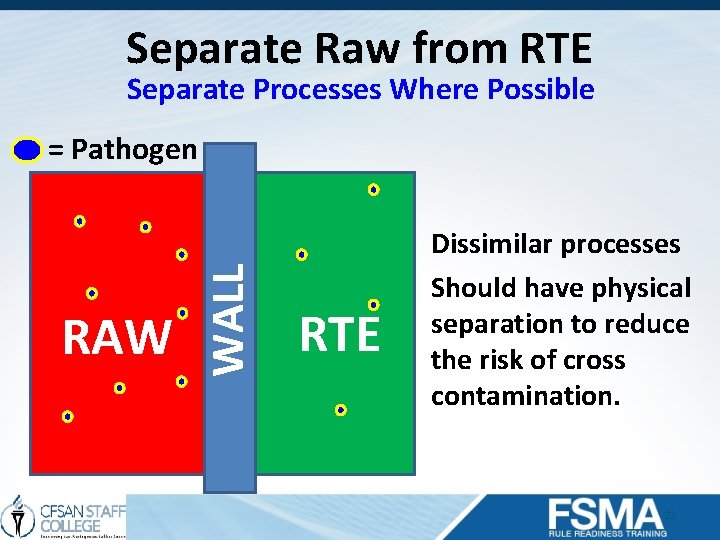
Separate Raw from RTE Separate Processes Where Possible RAW WALL = Pathogen RTE Dissimilar processes Should have physical separation to reduce the risk of cross contamination. 21


There are more than a billion of these connectors in use today. Even if a billion people make this mistake once and lose 1 second correcting the mistake, the lost time = 31 years.

International Journal of Food Microbiology 145 (2011) 1– 8 Review — Persistence of Listeria monocytogenes in food industry equipment and premises Brigitte Carpentier (a), Olivier Cerf (b) a) Laboratory of Food Safety, French Agency for Food, Environmental and Occupational Health Safety (ANSES), Maisons-Alfort, France b) Alfort Veterinary School, Maisons-Alfort, France We conclude by proposing that there are no strains of L. monocytogenes with unique properties that lead to persistence, but harborage sites in food industry premises and equipment where L. monocytogenes can persist. © 2011 Elsevier B. V.

Principles of Sanitary Design 1. Cleanable to a microbiological level 2. Made of compatible materials 3. Accessible for inspection, maintenance, sanitation 4. No liquid collection 5. Hollow areas hermetically sealed 6. No niches 7. Sanitary operation performance 8. Hygienic design of maintenance enclosures 9. Hygienic compatibility with other systems 10. Validated cleaning and sanitizing protocols 25

#3 A RISK BASED APPROACH TO HYGIENIC DESIGN © 2016 Commercial Food Sanitation LLC. All rights reserved. 26

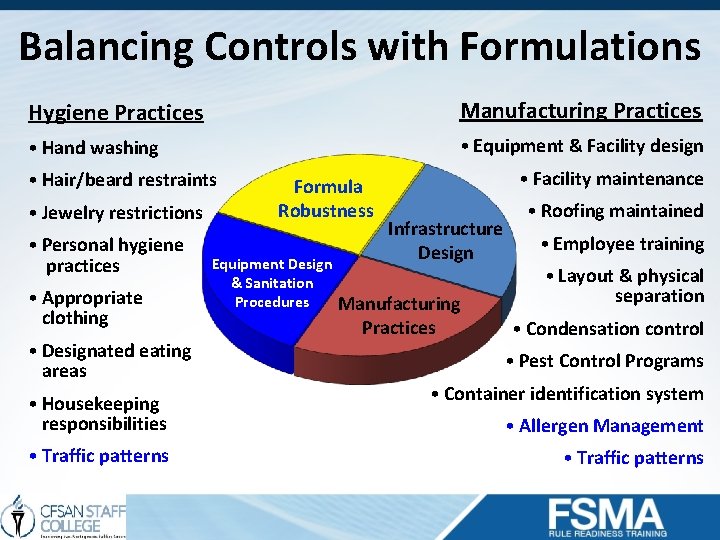
Balancing Controls with Formulations Hygiene Practices Manufacturing Practices • Hand washing • Equipment & Facility design • Hair/beard restraints • Jewelry restrictions • Personal hygiene practices • Appropriate clothing • Designated eating areas Formula Robustness Equipment Design & Sanitation Procedures • Facility maintenance Infrastructure Design Manufacturing Practices • Roofing maintained • Employee training • Layout & physical separation • Condensation control • Pest Control Programs • Housekeeping responsibilities • Container identification system • Traffic patterns • Allergen Management

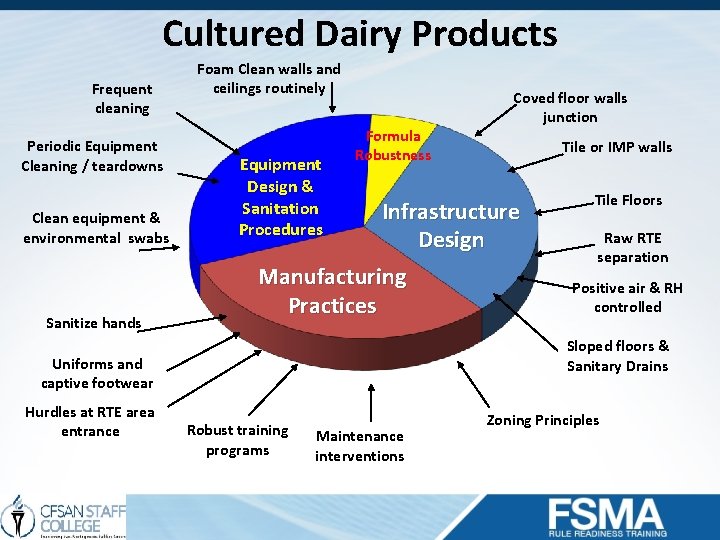
Cultured Dairy Products Frequent cleaning Periodic Equipment Cleaning / teardowns Clean equipment & environmental swabs Sanitize hands Foam Clean walls and ceilings routinely Equipment Design & Sanitation Procedures Formula Robustness Tile or IMP walls Infrastructure Design Manufacturing Practices Tile Floors Raw RTE separation Positive air & RH controlled Sloped floors & Sanitary Drains Uniforms and captive footwear Hurdles at RTE area entrance Coved floor walls junction Robust training programs Maintenance interventions Zoning Principles

#4 THE PROCESS AND SCIENCE OF CLEANING © 2016 Commercial Food Sanitation LLC. All rights reserved.

Cleaning Defined Cleaning in a food processing plant = • A proactive activity not a treatment • Protection for public health • Established procedures to control equipment and environmental conditions to protect food safety and quality. Target: – Unwanted microbial activity – Foreign Material – Chemical contaminants.

Classifications of Cleaning PIC Infrastructure cleaning – ceiling, walls, overheads PEC Equipment deep cleaning (typically micro sensitive) Routine Non-Routine Happens when line goes down equipment, facility Unscheduled cleaning – a spill of cheese. Seasonal Snow removal, pick up leaves in fall. Janitorial Clean offices and rest rooms.

Plant areas to be cleaned Plant Infrastructure Equipment Offices Rest Rooms / Locker Rooms Seasonal Activities Roofs / Building Exterior Our Focus Today

Periodic Infrastructure Cleaning What is PIC? All infrastructure has design flaws which create harborage / wear points for risks including, microbial, foreign material (pests) and chemical contamination prevention. Due to harborage and wear points, facilities must be routinely inspected, and swabbed (pathogens). The PIC cleaning (including risk points) should be listed in the MSS, have an SSOP and be scheduled at a frequency to maintain control. 33

Equipment Cleaning What is Routine Cleaning? Cleaning that occurs when lines shut down to proactively prevent microbial, foreign material or chemical contaminants. Procedures, chemicals and frequency should be captured in the SSOP. What is PEC? Periodic Equipment Cleaning is a tear down clean for hard to access areas. Equipment must be disassembled beyond what is done during normal routine cleaning. The PEC task with risk points should be listed in the MSS and scheduled at a frequency to maintain control and eliminate risk. PEC details and frequency should be captured in the equipment SSOP.

PEC and PIC are work arounds for poor equipment and facility sanitary design.

How do we clean it? Wet Full wet wash down Dry No water used at all – fully dry clean up Combination Waterless Limited use of water to remove soils and then dried The use of alcohol (with Quat) with wipers. Follow the 7 Steps of Cleaning

Periodic Equipment Cleaning PEC

Periodic Infrastructure Cleaning © 2016 Commercial Food Sanitation LLC. All rights reserved. PIC

Daily Scheduled Cleaning Routine

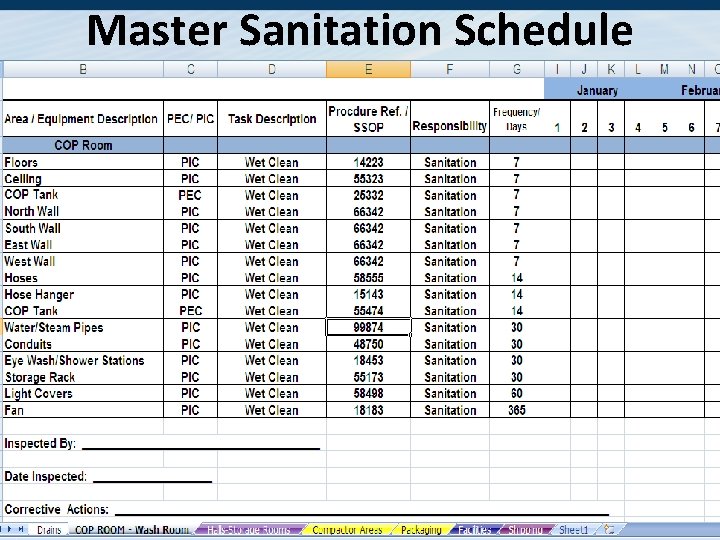
Master Sanitation Schedule (PEC, PIC, Routine) The Master Sanitation Schedule (MSS) contains • All items to be cleaned. • A reference # for the SSOP to use. • A SSOP should include – How the is equipment and facility is cleaned – Disassembly / Needed Utensils / Needed Equipment – Frequency of cleaning – The type of cleaning – wet or dry – Chemicals needed. – SSOPs should be guided by the Sanitary Design Checklist • A monthly report should show % completion of all non-routine cleaning tasks.

Master Sanitation Schedule 41

#5 THE 7 STEPS OF CLEANING

How do I clean it? Dry Vacuums Brushes, brooms, shovels, scrapers Dry towels Compressed air (Not advised – controlled) Accessibility – Scissor lifts – Ladders – Doors, ports • Dry Ice • Steam Cleaning • • • Follow the 7 Steps of Dry Cleaning

7 Steps of Effective Dry Cleaning 1. 2. 3. 4. 5. 6. 7. Pre-Sanitation Preparation Secure & Dismantle Pre-Clean Detail Cleaning Final Cleaning Sanitation Inspections Final Inspection and Documentation

How do I clean it? © 2016 Commercial Food Sanitation LLC. All rights reserved. Dry = no H 2 O

How do I clean it? Combination of dry and wet © 2016 Commercial Food Sanitation LLC. All rights reserved.

How do I clean it? Wet Follow the 7 Steps of Wet Cleaning

7 Steps of Effective Wet Cleaning 1. 2. 3. 4. 5. 6. 7. Dry Clean Pre-rinse Soap and Scrub Rinse and Inspect Assemble Pre-Op Sanitize © 2016 Commercial Food Sanitation LLC. All rights reserved.

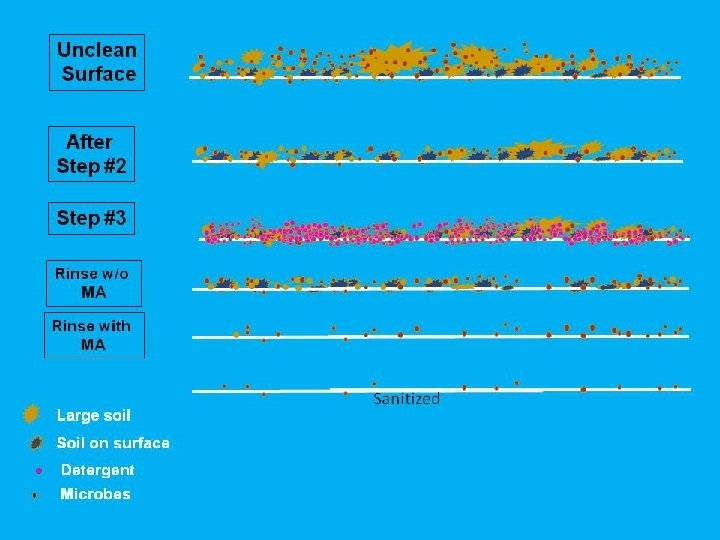
7 Steps of Effective Wet Sanitation One step at a time Enables Effective Sanitation • • • Step 7 GMP’s Continuous employee training Dedicated trainers & training tools Dedicated tool storage Single use cleaning aides Synchronized process Manual scrubbing Flood sanitizing Continuous inspection Flashlights ATP verification Sanitize & assemble Step 6 Post sanitation / pre-op inspection Step 5 Prepare formal inspection Step 4 Post rinse & self inspect Step 3 Apply detergent & scrub Step 2 In sync, top down pre-rinse Step 1 Secure, disassemble, dry clean Can lead to poor sanitation when not maintained • • • High pressure water or air Re-usable cleaning tools Congested work area Bearing Door seals Switches © 2016 Commercial Food Sanitation LLC. All rights reserved. Direct link to poor sanitation • • • Inaccessibility Hollow rollers Fibrous belting Bio films Aerosols Standing water Mops / foam squeegees Standing water Drain back-ups

© 2016 Commercial Food Sanitation LLC. All rights reserved.

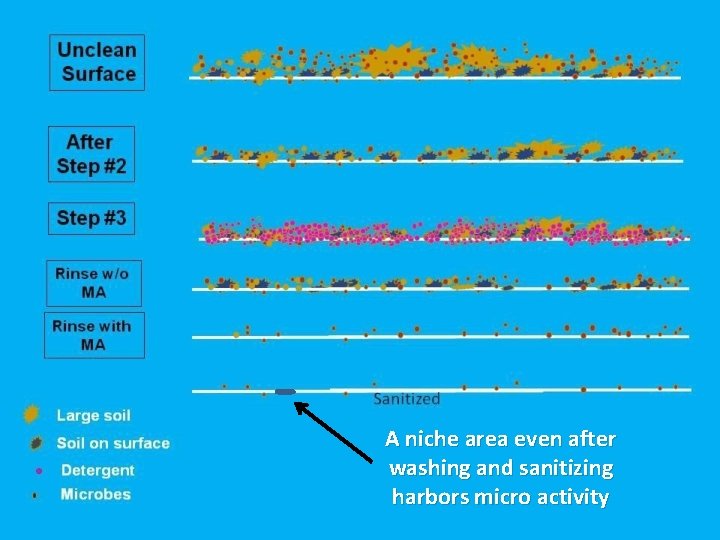
A niche area even after washing and sanitizing harbors micro activity

#6 INDUSTRY PARTNERSHIP © 2016 Commercial Food Sanitation LLC. All rights reserved.

Things to promote for Continuous Improvement. The industry needs your partnership. • Encourage Hygienic Zoning to separate raw from RTE. • Imperfect facility or equipment designs should be deep cleaned at a frequency to maintain sanitary conditions. • Internal GMP audits should be thorough, identify risks and include action plans for remediation. • Encourage science based education and hands on training. Encourage ongoing training of new and existing employees. • Do not penalize facilities for finding positives. Encourage to find and eliminate. • Help educate consumers on the difference between raw and RTE foods and methods to control cross contamination in home kitchens. © 2016 Commercial Food Sanitation LLC. All rights reserved.

Sanitation Essentials Industry Perspective Joseph Stout RS joe. stout@cf-san. com (847)881 -6212 www. commercialfoodsanitation. com

Sanitation Essentials FDA Perspective Brenda Stahl, Ph. D CFSAN/OFS Division of Plant Products and Beverages

21 CFR Part 117 – Current Good Manufacturing Practice, Hazard Analysis, and Risk-based Preventive Control for Human Food • The preventive controls regulation (21 CFR Part 117) is divided into seven subparts: – 1. Subpart A: General Provisions – 2. Subpart B: Current Good Manufacturing Practices – 3. Subpart C: Hazard Analysis and Risk-Based Preventive Controls – 4. Subpart D: Modified Requirements – 5. Subpart E: Withdrawal of a Qualified Facility Exemption – 6. Subpart F: Requirements Applying to Records That Must Be Established and Maintained – 7. Subpart G: Supply-Chain Program

Subpart B GMP: 110 vs 117 • Clarification that require protection against contamination of food includes protection against allergen cross contact. • The term “shall” was replaced with the term “must. ” • “Manufacturing/processing” is used in place of “manufacturing” in order to be consistent with the definitions outlined in the Definitions section of Subpart A. • Certain provisions containing recommendations were deleted. For example, some provisions using the terms “should” or “compliance may be achieved by” are gone. • Cleaning of non-food contact surfaces at a frequency necessary to ensure protection against contamination of food is now required. • New requirements for holding and distributing human food by-products intended for use as animal food

Definitions • Preventive Control: risk-based procedures, practices, and processes that significantly minimize or prevent the hazards identified under the hazard analysis – consistent with the current scientific understanding of safe food manufacturing – “Knowledgeable Person” = Training

Definitions • Sanitation Practices: Can be a measure or procedure or activity that reduces or removes a microbial, chemical or physical hazard – Cleaning: removal of debris and food soils – Vacuuming – Clean-in-Place Systems – Sanitizing: adequately treat cleaned surfaces by a process that is effective in destroying vegetative cells of pathogens, and in substantially reducing numbers of other undesirable microorganisms, but without adversely affecting the product or its safety for the consumer.

Definitions • Sanitation Controls: procedures, practices, and processes to ensure that the facility is maintained in a sanitary condition adequate to significantly minimize or prevent hazards such as environmental pathogens, biological hazards due to employee handling, and food allergen hazards (§ 117. 135(c)(3)).

Sanitation Controls • Sanitation controls must include, as appropriate to the facility and the food, procedures, practices, and processes for the: • (i) Cleanliness of food-contact surfaces, including foodcontact surfaces of utensils and equipment; • (ii) Prevention of allergen cross-contact and crosscontamination from insanitary objects and from personnel to food, food packaging material, and other food-contact surfaces and from raw product to processed product.

Definitions • Sanitation Controls: – Do not require validation (Supply Chain Controls, Allergen Controls) – Can be verified using environmental monitoring • Listeria swabbing in RTE facilities that have determined Listeria is a hazard that requires control – Not always directed to critical control points • “Appropriate for Food Safety” (21 CFR part 117. 135(a)(ii) • Preventive Controls can include Sanitation practices as Sanitation Controls, but not all Sanitation practices are Preventive Controls

Preventive Control or c. GMP? • Preventive controls are based on a hazard analysis performed by the facility • Example: RTE Facilities – If the facility manufactures an RTE food where environmental pathogens are a hazard that needs to be controlled, certain c. GMP programs elevate to preventive controls.

Preventive Control or c. GMP? • RTE Facilities – Elevated c. GMP’s – Sanitation of a specific food contact area • Utensils that are used directly prior to packaging in RTE foods – Sanitation of a specific non-food contact area • RTE facility that has a historical concern with Listeria in floor drains • Based on a hazard analysis of the food and the facility

Preventive Control or c. GMP? • Example of Sanitation Controls that can elevate to a preventive control – Use of a common line for different products with different allergens • Cross Contact/Allergen Control – Overall sanitation of a facility that manufactures both ready to eat and NRTE products within same manufacturing space

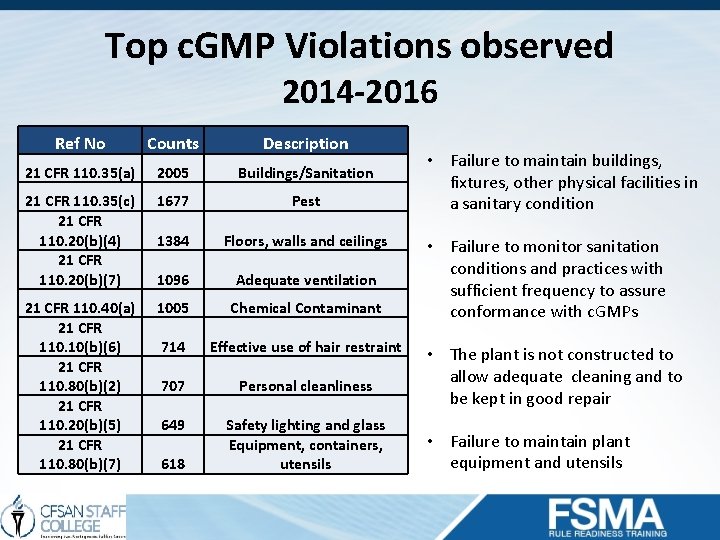
Top c. GMP Violations observed 2014 -2016 Ref No Counts Description 21 CFR 110. 35(a) 2005 Buildings/Sanitation 21 CFR 110. 35(c) 21 CFR 110. 20(b)(4) 21 CFR 110. 20(b)(7) 1677 Pest 1384 Floors, walls and ceilings 1096 Adequate ventilation 21 CFR 110. 40(a) 21 CFR 110. 10(b)(6) 21 CFR 110. 80(b)(2) 21 CFR 110. 20(b)(5) 21 CFR 110. 80(b)(7) 1005 Chemical Contaminant 714 Effective use of hair restraint 707 Personal cleanliness 649 Safety lighting and glass Equipment, containers, utensils 618 • Failure to maintain buildings, fixtures, other physical facilities in a sanitary condition • Failure to monitor sanitation conditions and practices with sufficient frequency to assure conformance with c. GMPs • The plant is not constructed to allow adequate cleaning and to be kept in good repair • Failure to maintain plant equipment and utensils

Subpart A (General Provisions): c. GMP Training Requirement • 117. 4(b)(2): All employees should receive training in the principles of food hygiene and food safety, including the importance of employee health and personal hygiene, as appropriate to the food, the facility and the individual's assigned duties.

Questions & Answers Brenda Stahl, CFSAN/OFS Brenda. Stahl@fda. hhs. gov Q & A – Use the Adobe Connect pod to type your questions

FSMA Rule Readiness Training Resources FDA Employees: http: //inside. fda. gov: 9003/CFS AN/CFSANStaff. College/Food. S afety. Modernazation. Act/ucm 449 582. htm State Employees: https: //www. foodshield. org/bl og-training-news/

Feedback To submit a question about FSMA, visit www. fda. gov/fsma and go to Contact Us Registered participants will receive an evaluation survey in two days.

THANK YOU FOR ATTENDING FSMA TRAINING SANITATION ESSENTIALS FOR HUMAN FOOD MANUFACTURING FDA FSMA Readiness Training
 Importance of sanitation
Importance of sanitation Fsma overview
Fsma overview Fsma pcp
Fsma pcp Manufacturing cost vs non manufacturing cost
Manufacturing cost vs non manufacturing cost Job costing and process costing
Job costing and process costing Controllable costs
Controllable costs Manufacturing cost vs non manufacturing cost
Manufacturing cost vs non manufacturing cost Additive manufacturing steps
Additive manufacturing steps Unit 2 food food food
Unit 2 food food food Eltonian pyramid
Eltonian pyramid Sanitation and hygiene
Sanitation and hygiene Sanitation and hygiene
Sanitation and hygiene Sanitation importance
Sanitation importance Delvic sanitation initiatives
Delvic sanitation initiatives Environmental sanitation clearance
Environmental sanitation clearance Wash fit
Wash fit Shellfish sanitation program
Shellfish sanitation program Moeller sanitation
Moeller sanitation Conclusion on sanitation
Conclusion on sanitation Conclusion for hygiene
Conclusion for hygiene Pdq sanitation
Pdq sanitation Sanitation system definition
Sanitation system definition Odf tracking
Odf tracking Chapter 8 sanitation procedures
Chapter 8 sanitation procedures Sanitation barrier psm
Sanitation barrier psm Chapter 1 safety and sanitation principles
Chapter 1 safety and sanitation principles Central rural sanitation programme 1986
Central rural sanitation programme 1986 Drawing of sanitation
Drawing of sanitation Stakeholders in water and sanitation
Stakeholders in water and sanitation Poultry hygiene and sanitation
Poultry hygiene and sanitation Sanitation and hygiene
Sanitation and hygiene Cleaning and sanitation manual for breweries
Cleaning and sanitation manual for breweries Sanitation items
Sanitation items Gmp sanitation and hygiene
Gmp sanitation and hygiene Gmp sanitation and hygiene
Gmp sanitation and hygiene Basic principles of personal hygiene
Basic principles of personal hygiene Why is sanitation important
Why is sanitation important Why is sanitation important
Why is sanitation important Why is sanitation important
Why is sanitation important Isafety app
Isafety app On-site sanitation
On-site sanitation Goof
Goof Ministry of sanitation
Ministry of sanitation Summary of hygiene
Summary of hygiene Why is sanitation important
Why is sanitation important Components of environmental sanitation
Components of environmental sanitation Elizabethan era hygiene
Elizabethan era hygiene Compendium of sanitation systems and technologies
Compendium of sanitation systems and technologies Formuö
Formuö Novell typiska drag
Novell typiska drag Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Ekologiskt fotavtryck
Ekologiskt fotavtryck Varför kallas perioden 1918-1939 för mellankrigstiden
Varför kallas perioden 1918-1939 för mellankrigstiden En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Underlag för särskild löneskatt på pensionskostnader
Underlag för särskild löneskatt på pensionskostnader Tidbok yrkesförare
Tidbok yrkesförare A gastrica
A gastrica Förklara densitet för barn
Förklara densitet för barn Datorkunskap för nybörjare
Datorkunskap för nybörjare Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Debatt mall
Debatt mall Delegerande ledarstil
Delegerande ledarstil Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Kraft per area
Kraft per area Svenskt ramverk för digital samverkan
Svenskt ramverk för digital samverkan I gullregnens månad
I gullregnens månad Presentera för publik crossboss
Presentera för publik crossboss Jiddisch
Jiddisch Kanaans land
Kanaans land Treserva lathund
Treserva lathund Luftstrupen för medicinare
Luftstrupen för medicinare