Renal Arteries and Hypertension Stenting and Energy Does













- Slides: 40

Renal Arteries and Hypertension: Stenting and Energy – Does Anything Work? Michael R. Jaff, DO Paul and Phyllis Fireman Endowed Chair of Vascular Medicine Massachusetts General Hospital Professor of Medicine Harvard Medical School Boston, Massachusetts

Michael R. Jaff, D. O. Conflicts of • Interest • Consultant – Abbott Vascular (non-compensated) – AOPA – Boston Scientific (non-compensated) – Cardinal Health – Cordis Corporation (non-compensated) – Janacare, Inc – Medtronic (non-compensated) – Micell, Inc – Novella (DSMB) – Primacea – Valiant – Volcano • 2 Board Member -VIVA Physicians (Not For Profit 501(c) 3 Organization) • www. vivapvd. com -Intersocietal Accreditation Commission 2 -CBSET Equity – Access Closure, Inc – Embolitech – I. C. Sciences, Inc – Janacare, Inc – MC 10 – Northwind Medical, Inc. – PQ Bypass, Inc – Primacea – Sano V, Inc. – Vascular Therapies, Inc February 2016

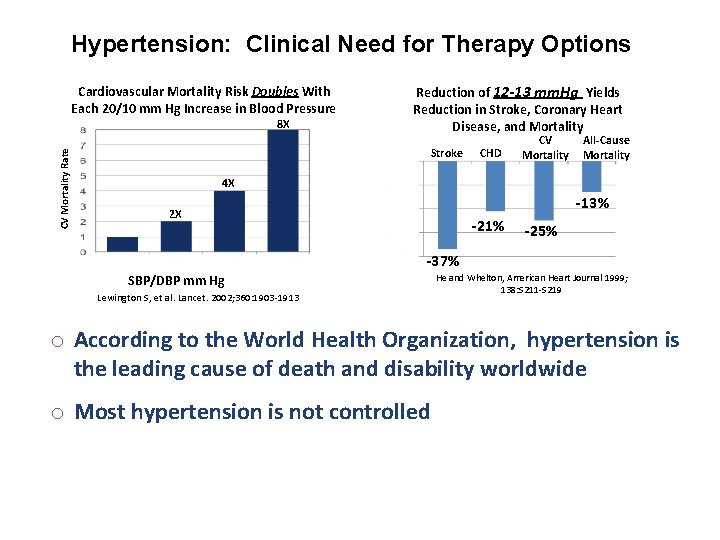
Hypertension: Clinical Need for Therapy Options Cardiovascular Mortality Risk Doubles With Each 20/10 mm Hg Increase in Blood Pressure CV Mortality Rate 8 X Reduction of 12 -13 mm. Hg Yields Reduction in Stroke, Coronary Heart Disease, and Mortality Stroke CHD CV Mortality All-Cause Mortality 4 X -13% 2 X -21% -25% -37% SBP/DBP mm Hg Lewington S, et al. Lancet. 2002; 360: 1903 -1913 He and Whelton, American Heart Journal 1999; 138: S 211 -S 219 o According to the World Health Organization, hypertension is the leading cause of death and disability worldwide o Most hypertension is not controlled

…and This Isn’t Going to Get Any Easier November 9, 2015 4

SPRINT Background • 9361 patients with HTN and SBP >130 mm. Hg • Randomized to – Intensive Therapy (SBP < 120 mm. Hg) – Standard Therapy (SBP <140 mm. Hg) 5

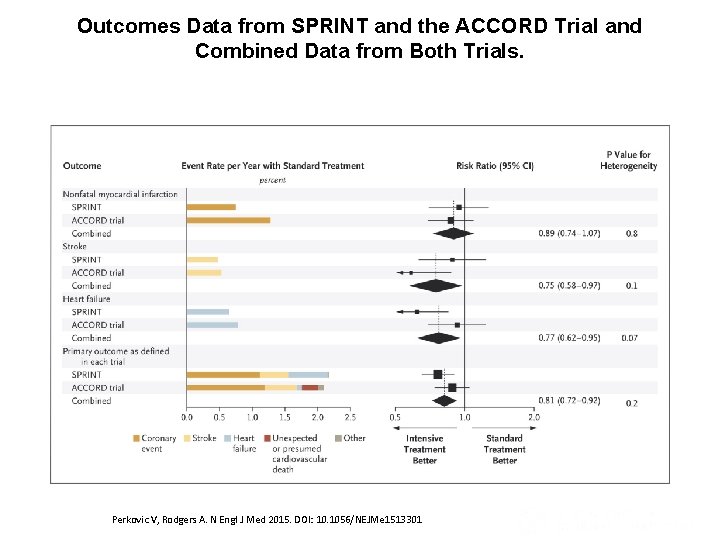
Outcomes Data from SPRINT and the ACCORD Trial and Combined Data from Both Trials. Perkovic V, Rodgers A. N Engl J Med 2015. DOI: 10. 1056/NEJMe 1513301

Let’s Start with Atherosclerotic Renal Artery Stenosis

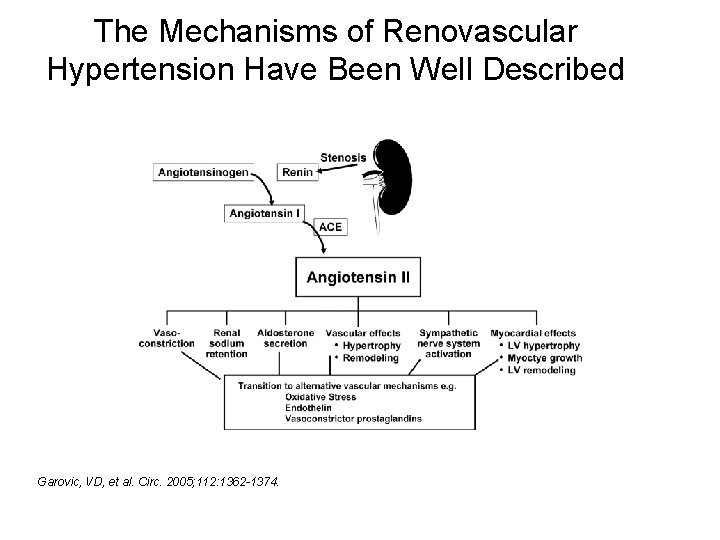
The Mechanisms of Renovascular Hypertension Have Been Well Described Garovic, VD, et al. Circ. 2005; 112: 1362 -1374.

Do You Think These Patients with ARAS and These Scenarios Warrant Intervention? • • • Dialysis-dependent renal failure Chronic renal insufficiency Refractory/resistant hypertension Cardiac disturbance syndrome Need for use of ACEI/ARB Unilateral renal artery stenosis

N Engl J Med. 2014; 370: 13 -22.

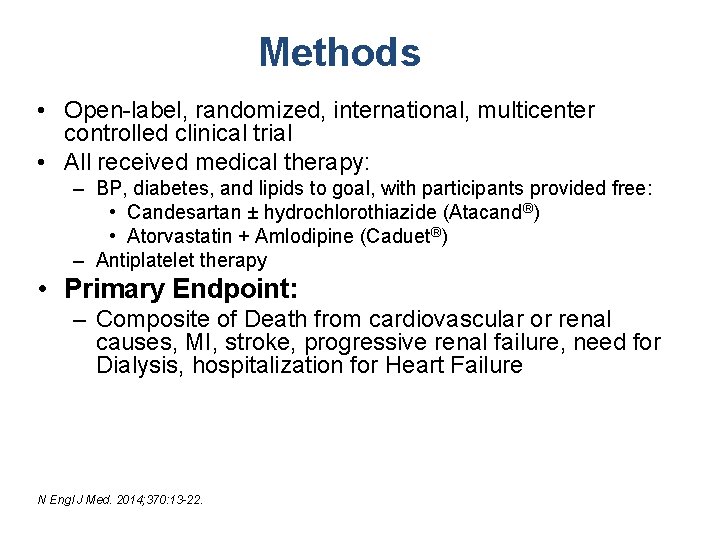
Methods • Open-label, randomized, international, multicenter controlled clinical trial • All received medical therapy: – BP, diabetes, and lipids to goal, with participants provided free: • Candesartan ± hydrochlorothiazide (Atacand®) • Atorvastatin + Amlodipine (Caduet®) – Antiplatelet therapy • Primary Endpoint: – Composite of Death from cardiovascular or renal causes, MI, stroke, progressive renal failure, need for Dialysis, hospitalization for Heart Failure N Engl J Med. 2014; 370: 13 -22.

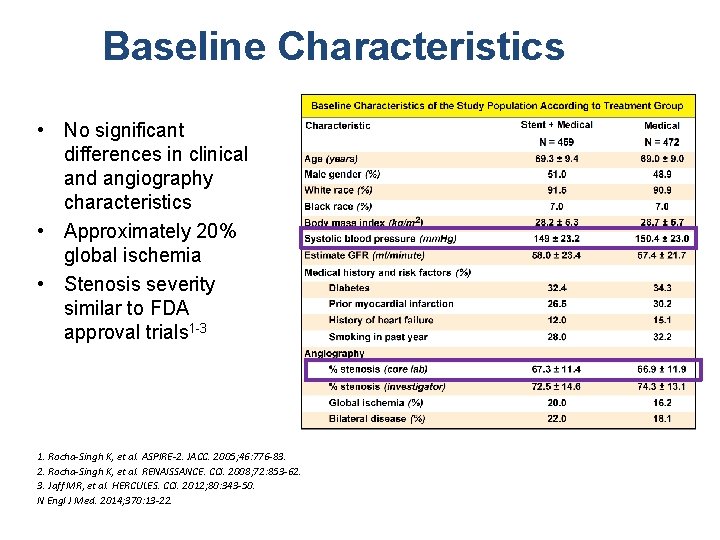
Baseline Characteristics • No significant differences in clinical and angiography characteristics • Approximately 20% global ischemia • Stenosis severity similar to FDA approval trials 1 -3 1. Rocha-Singh K, et al. ASPIRE-2. JACC. 2005; 46: 776 -83. 2. Rocha-Singh K, et al. RENAISSANCE. CCI. 2008; 72: 853 -62. 3. Jaff MR, et al. HERCULES. CCI. 2012; 80: 343 -50. N Engl J Med. 2014; 370: 13 -22.

Results: Periprocedural Clinical Complications … s r e t n e C • No participant required dialysis withinp 30 days of e l lti u randomization M n i n • 1/459 (0. 2%) in-stent + medical therapy initiated e v E , e dialysis between 30 uand 90 days after r d randomization roce P e f a S y l g in • 1 azstroke resulting in death, day of randomization, Am. Medical Therapy Only group. N Engl J Med. 2014; 370: 13 -22.

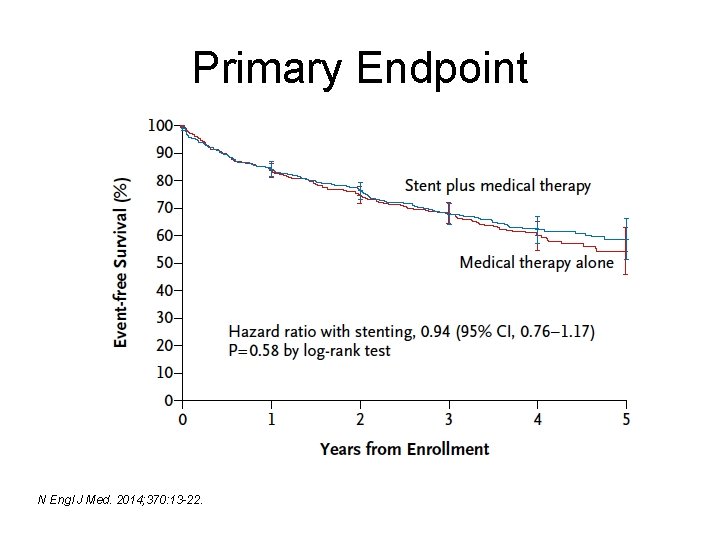
Primary Endpoint N Engl J Med. 2014; 370: 13 -22.

Results: Subgroups P Value for Interaction Stent vs Medical Therapy

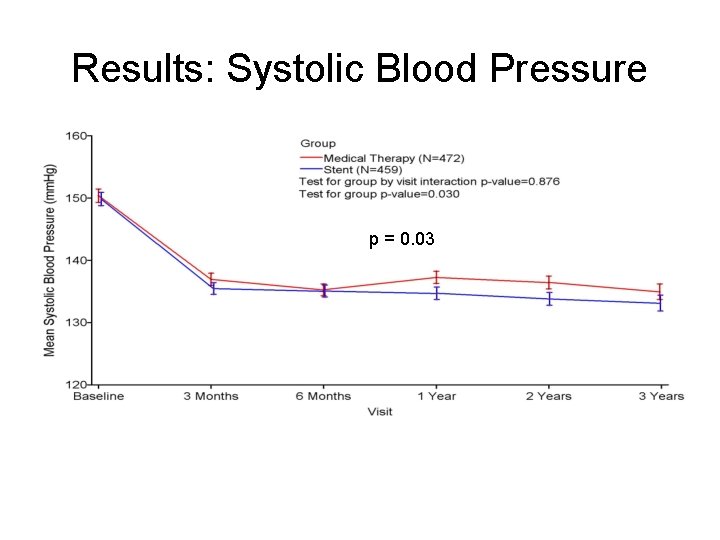
Results: Systolic Blood Pressure p = 0. 03

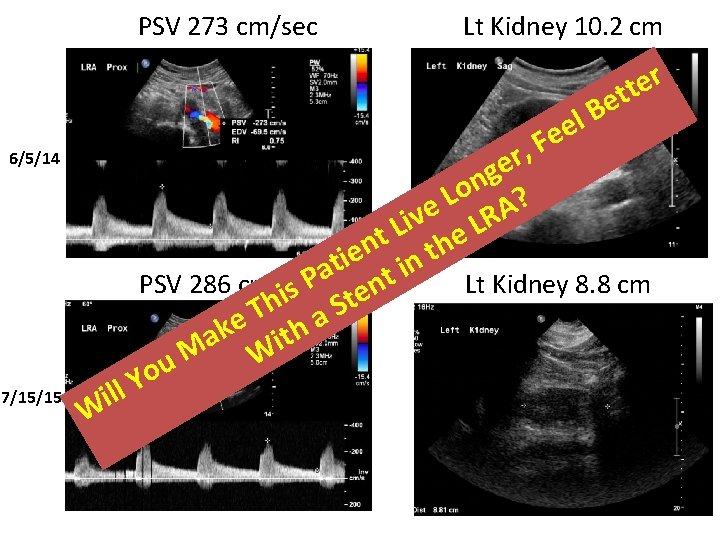
PSV 273 cm/sec Lt Kidney 10. 2 cm r e t t e B l e e F r, e g n o L A? e iv LR L t the n e in i t a nt P PSV 286 cm/sec Lt Kidney 8. 8 cm s i e t h S T a e k h t a i M W u o Y ill 6/5/14 7/15/15 W

What About Renal Denervation?

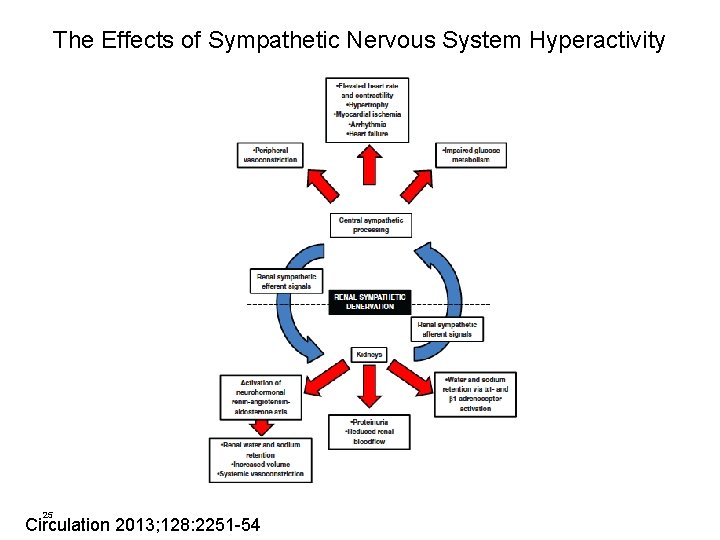
The Effects of Sympathetic Nervous System Hyperactivity 25 Circulation 2013; 128: 2251 -54

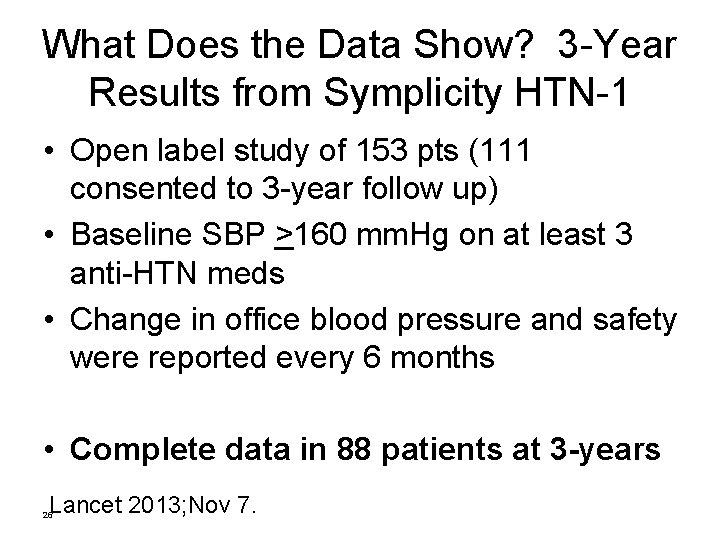
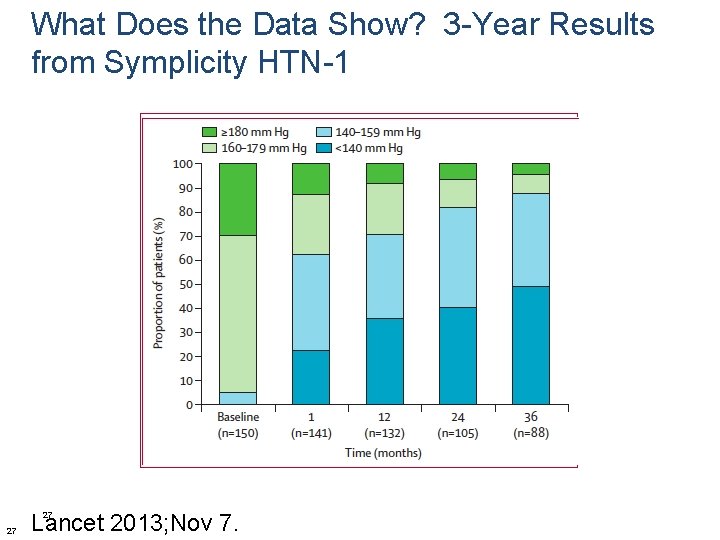
What Does the Data Show? 3 -Year Results from Symplicity HTN-1 • Open label study of 153 pts (111 consented to 3 -year follow up) • Baseline SBP >160 mm. Hg on at least 3 anti-HTN meds • Change in office blood pressure and safety were reported every 6 months • Complete data in 88 patients at 3 -years Lancet 2013; Nov 7. 26

What Does the Data Show? 3 -Year Results from Symplicity HTN-1 Lancet 2013; Nov 7. 27 27

So Clearly, Radiofrequency Ablation Works! Right? 28

Oh How The World Changes…

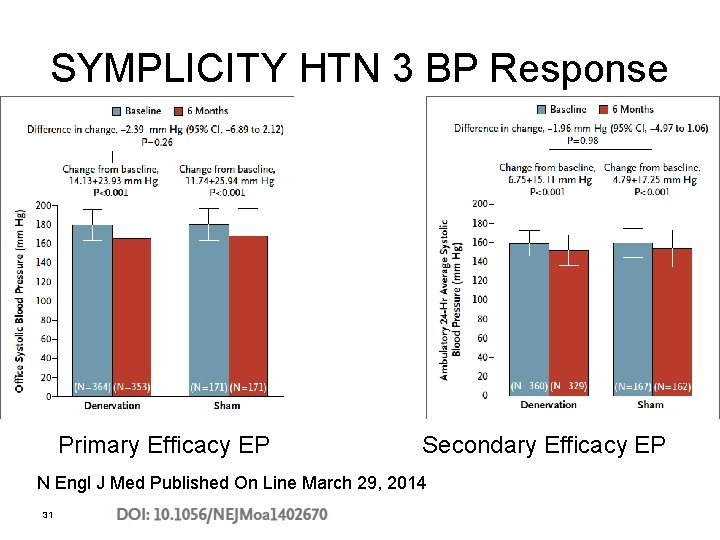
N Engl J Med Published On Line March 29, 2014 30

SYMPLICITY HTN 3 BP Response Primary Efficacy EP Secondary Efficacy EP N Engl J Med Published On Line March 29, 2014 31

32

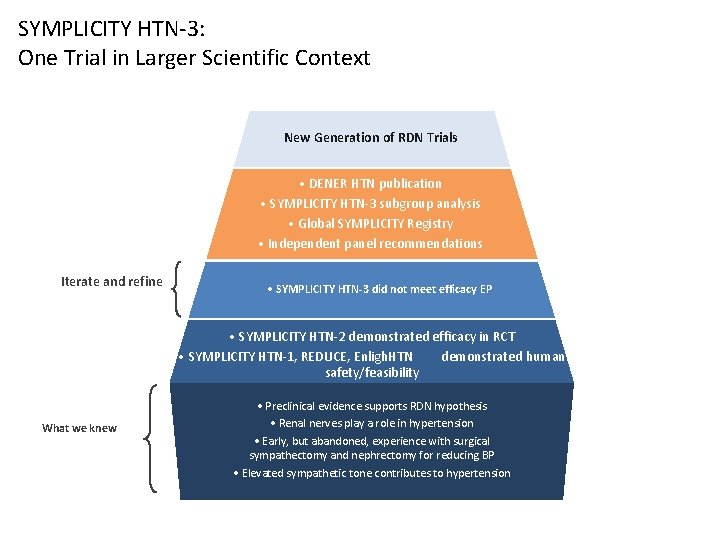
SYMPLICITY HTN-3: One Trial in Larger Scientific Context New Generation of RDN Trials • DENER HTN publication • SYMPLICITY HTN-3 subgroup analysis • Global SYMPLICITY Registry • Independent panel recommendations Iterate and refine • SYMPLICITY HTN-3 did not meet efficacy EP • SYMPLICITY HTN-2 demonstrated efficacy in RCT • SYMPLICITY HTN-1, REDUCE, Enligh. HTN demonstrated human safety/feasibility What we knew • Preclinical evidence supports RDN hypothesis • Renal nerves play a role in hypertension • Early, but abandoned, experience with surgical sympathectomy and nephrectomy for reducing BP • Elevated sympathetic tone contributes to hypertension

34

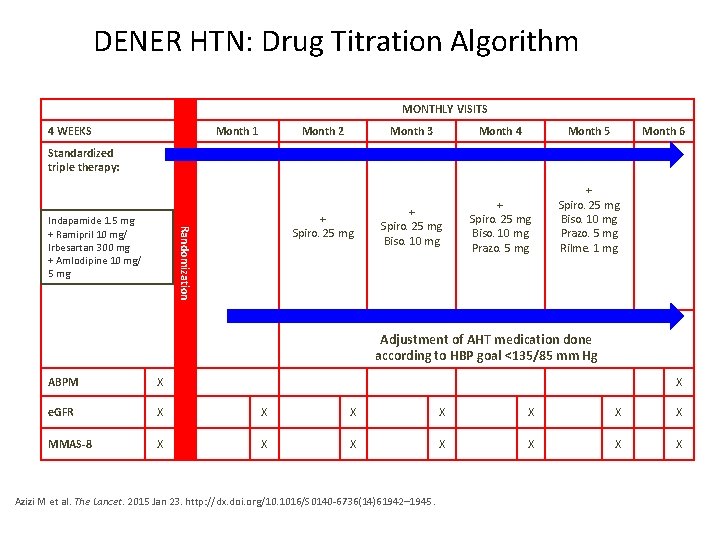
DENER HTN: Drug Titration Algorithm MONTHLY VISITS 4 WEEKS Month 1 Month 2 Month 3 Month 4 Month 5 Month 6 + Spiro. 25 mg Biso. 10 mg Prazo. 5 mg Rilme. 1 mg Standardized triple therapy: Randomization Indapamide 1. 5 mg + Ramipril 10 mg/ Irbesartan 300 mg + Amlodipine 10 mg/ 5 mg + Spiro. 25 mg Biso. 10 mg Adjustment of AHT medication done according to HBP goal <135/85 mm Hg ABPM X X e. GFR X X X X MMAS-8 X X X X Azizi M et al. The Lancet. 2015 Jan 23. http: //dx. doi. org/10. 1016/S 0140 -6736(14)61942– 1945.

DENER HTN Daytime and Nighttime ABPM From Randomization to 6 Months SBP Change from Baseline to 6 Months (mm Hg) 0 ∆: – 5. 9 mm Hg (95% CI: – 11. 3 to – 0. 5) p = 0. 0329 ∆: – 6. 3 mm Hg (95% CI: – 12. 0 to – 0. 6) p = 0. 0296 – 10 Denervation Control – 20 Daytime Primary endpoint Azizi M et al. The Lancet. 2015 Jan 23. http: //dx. doi. org/10. 1016/S 0140 -6736(14)61942– 1945. Nighttime

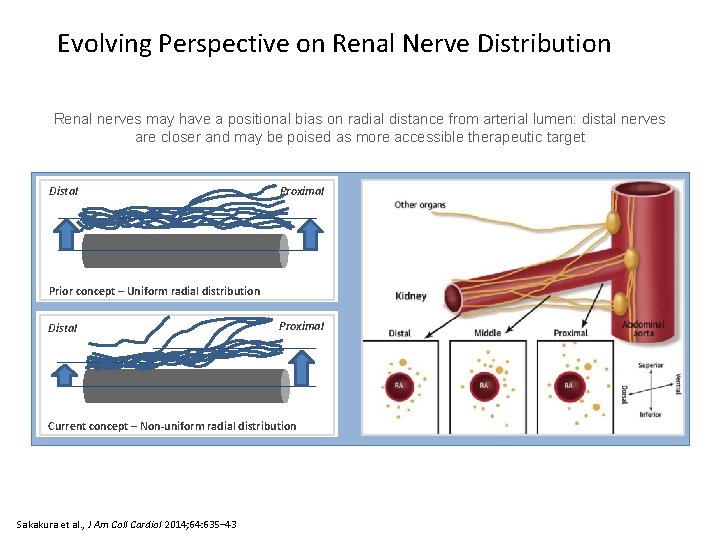
Evolving Perspective on Renal Nerve Distribution Renal nerves may have a positional bias on radial distance from arterial lumen: distal nerves are closer and may be poised as more accessible therapeutic target Distal Proximal Prior concept – Uniform radial distribution Distal Proximal Current concept – Non-uniform radial distribution Sakakura et al. , J Am Coll Cardiol 2014; 64: 635– 43

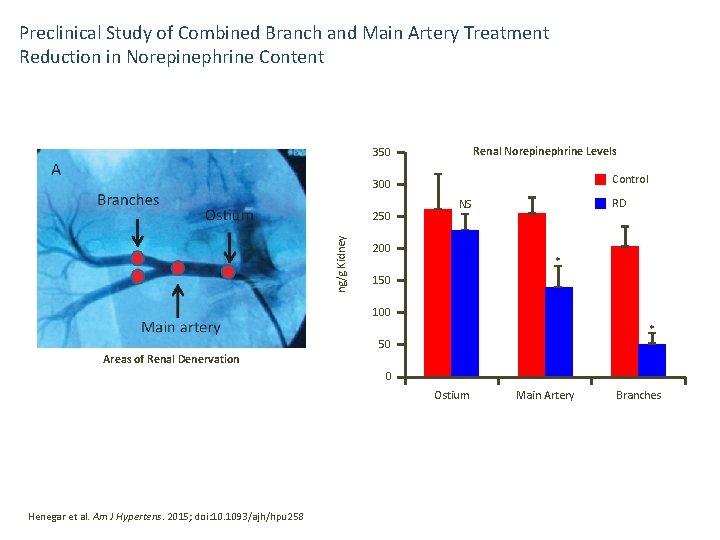
Preclinical Study of Combined Branch and Main Artery Treatment Reduction in Norepinephrine Content Renal Norepinephrine Levels 350 Control 300 ng/g Kidney 250 RD NS 200 * 150 100 * 50 Areas of Renal Denervation 0 Ostium Henegar et al. Am J Hypertens. 2015; doi: 10. 1093/ajh/hpu 258 Main Artery Branches

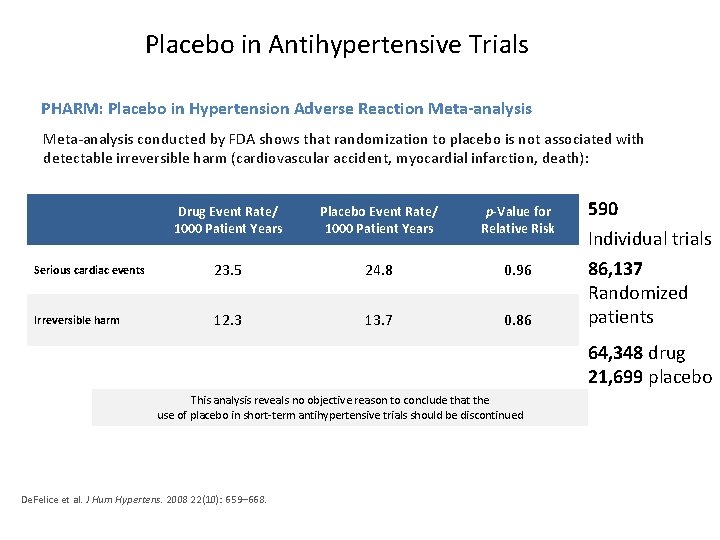
Placebo in Antihypertensive Trials PHARM: Placebo in Hypertension Adverse Reaction Meta-analysis conducted by FDA shows that randomization to placebo is not associated with detectable irreversible harm (cardiovascular accident, myocardial infarction, death): Drug Event Rate/ 1000 Patient Years Placebo Event Rate/ 1000 Patient Years p-Value for Relative Risk Serious cardiac events 23. 5 24. 8 0. 96 Irreversible harm 12. 3 13. 7 0. 86 590 Individual trials 86, 137 Randomized patients 64, 348 drug 21, 699 placebo This analysis reveals no objective reason to conclude that the use of placebo in short-term antihypertensive trials should be discontinued De. Felice et al. J Hum Hypertens. 2008 22(10): 659– 668.

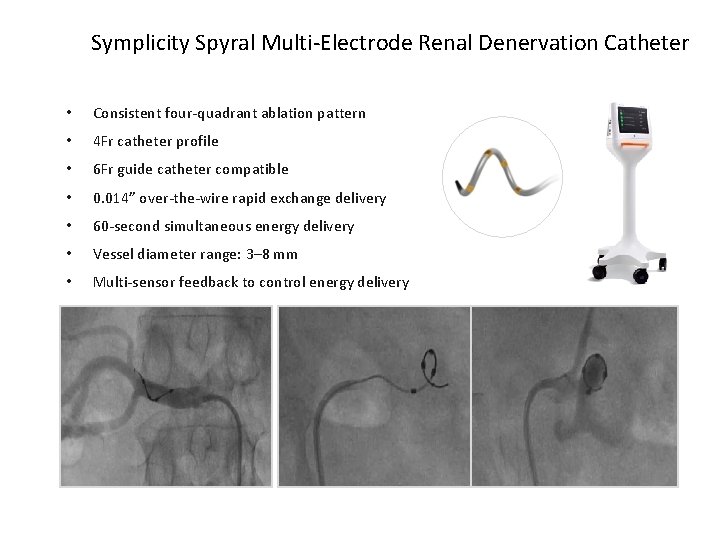
Symplicity Spyral Multi-Electrode Renal Denervation Catheter • Consistent four-quadrant ablation pattern • 4 Fr catheter profile • 6 Fr guide catheter compatible • 0. 014” over-the-wire rapid exchange delivery • 60 -second simultaneous energy delivery • Vessel diameter range: 3– 8 mm • Multi-sensor feedback to control energy delivery

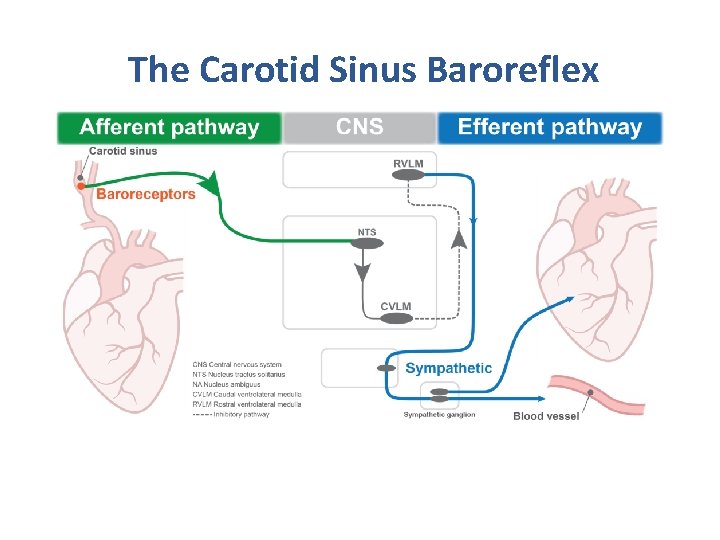
The Carotid Sinus Baroreflex

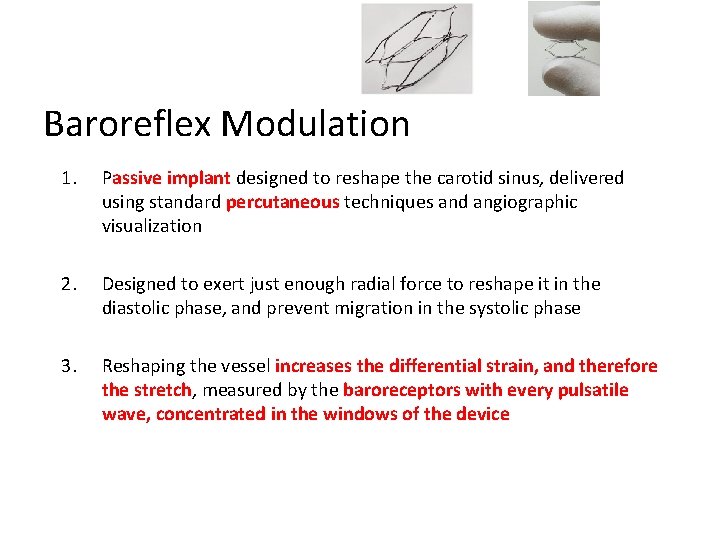
Baroreflex Modulation 1. Passive implant designed to reshape the carotid sinus, delivered using standard percutaneous techniques and angiographic visualization 2. Designed to exert just enough radial force to reshape it in the diastolic phase, and prevent migration in the systolic phase 3. Reshaping the vessel increases the differential strain, and therefore the stretch, measured by the baroreceptors with every pulsatile wave, concentrated in the windows of the device

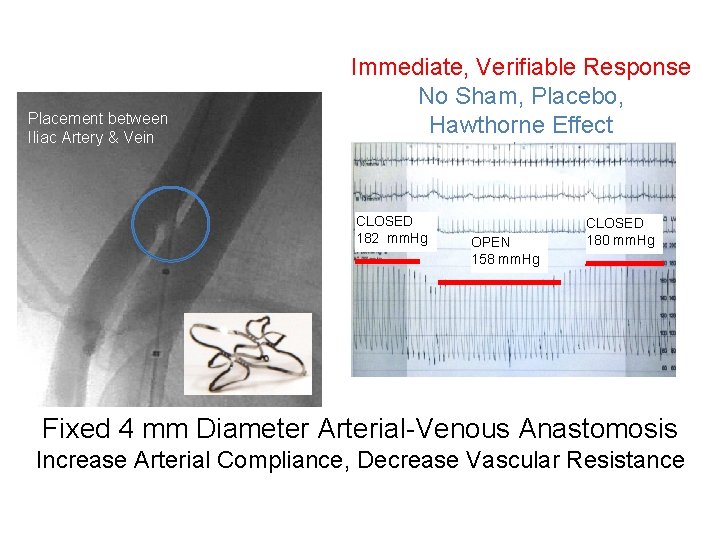
Placement between Iliac Artery & Vein Immediate, Verifiable Response No Sham, Placebo, Hawthorne Effect CLOSED 182 mm. Hg OPEN 158 mm. Hg CLOSED 180 mm. Hg Fixed 4 mm Diameter Arterial-Venous Anastomosis Increase Arterial Compliance, Decrease Vascular Resistance

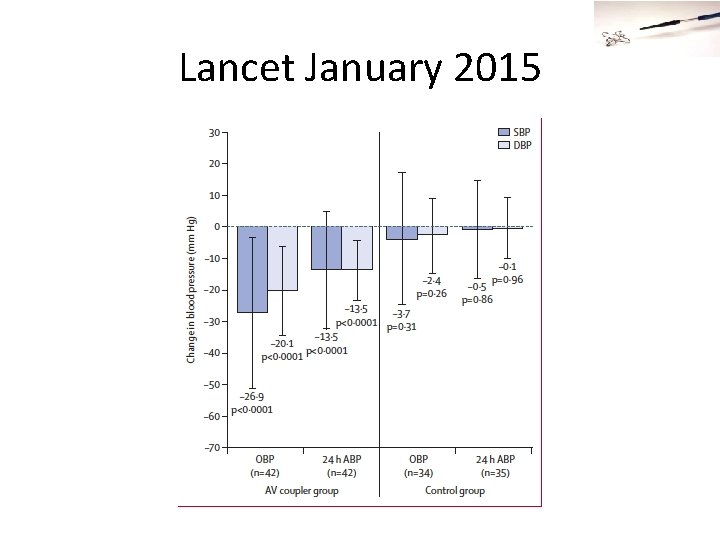
Think I’m Kidding? Lancet January 2015 46

Lancet January 2015

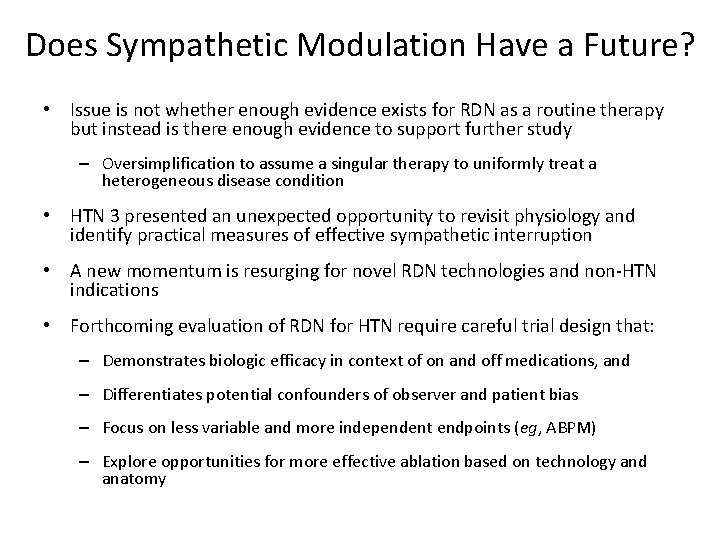
Does Sympathetic Modulation Have a Future? • Issue is not whether enough evidence exists for RDN as a routine therapy but instead is there enough evidence to support further study – Oversimplification to assume a singular therapy to uniformly treat a heterogeneous disease condition • HTN 3 presented an unexpected opportunity to revisit physiology and identify practical measures of effective sympathetic interruption • A new momentum is resurging for novel RDN technologies and non-HTN indications • Forthcoming evaluation of RDN for HTN require careful trial design that: – Demonstrates biologic efficacy in context of on and off medications, and – Differentiates potential confounders of observer and patient bias – Focus on less variable and more independent endpoints (eg, ABPM) – Explore opportunities for more effective ablation based on technology and anatomy
 Res extra commercium
Res extra commercium Teoria do nefron intacto
Teoria do nefron intacto Cortical and juxtamedullary nephrons difference
Cortical and juxtamedullary nephrons difference Reverse tap technique
Reverse tap technique Mini culotte stenting
Mini culotte stenting Inverted provisional stenting
Inverted provisional stenting Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Portal hypertension symptoms
Portal hypertension symptoms Lymphatic drainage of stomach
Lymphatic drainage of stomach Major arteries of the ascending aorta and aortic arch
Major arteries of the ascending aorta and aortic arch Medial striate artery
Medial striate artery Anterior choroidal artery
Anterior choroidal artery Cat dissection veins and arteries
Cat dissection veins and arteries Pulmonary circuit
Pulmonary circuit Lower extremity arteries and veins
Lower extremity arteries and veins Difference between arteries veins and capillaries
Difference between arteries veins and capillaries Difference between artery and vein in tabular form
Difference between artery and vein in tabular form Circulatory pathway
Circulatory pathway V
V Structure of arteries and veins
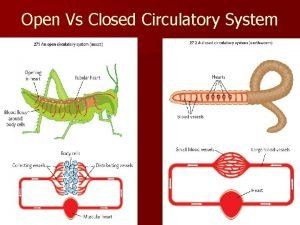
Structure of arteries and veins Open circulatory system vs closed
Open circulatory system vs closed Diueritique
Diueritique Vital signs cpr
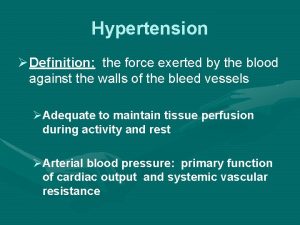
Vital signs cpr Hypertension
Hypertension Jnc7
Jnc7 Syndrome méningé
Syndrome méningé Pah vs pulmonary hypertension
Pah vs pulmonary hypertension Hypertension
Hypertension Equivalence hbpm
Equivalence hbpm Hypertension
Hypertension Endorine
Endorine Demadex
Demadex Htp segmentaire
Htp segmentaire Non pharmacological management of hypertension
Non pharmacological management of hypertension Rules of halves in hypertension
Rules of halves in hypertension Bp = co x svr
Bp = co x svr Definition of hypertension
Definition of hypertension Masked hypertension
Masked hypertension Rvsp calculation
Rvsp calculation Causes of secondary hypertension
Causes of secondary hypertension