Percutaneous Mitral Valve Repair Coronary Sinus Approach Maurice







- Slides: 54

Percutaneous Mitral Valve Repair Coronary Sinus Approach Maurice Buchbinder, MD Foundation for Cardiovascular Medicine La Jolla, CA Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

DISCLOSURES Maurice Buchbinder, MD Honoraria – Boston Scientific Corporation, Cordis, a Johnson & Johnson company, Abbott Vascular

Disclosure. Stents? Why Degradable Speaker’s name: Maurice Buchbinder, MD I have the following potential conflicts of interest to report: Consulting Employment in industry Stockholder of a healthcare company Owner of a healthcare company I do not have any potential conflict of interest Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

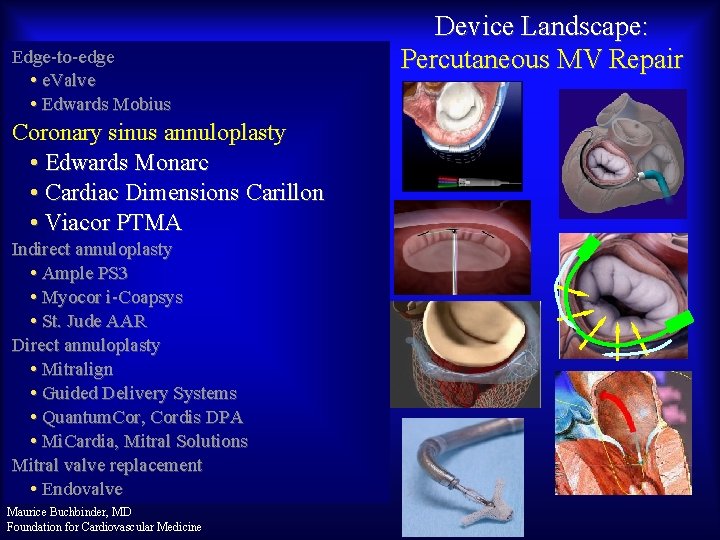
Edge-to-edge • e. Valve • Edwards Mobius Coronary sinus annuloplasty • Edwards Monarc • Cardiac Dimensions Carillon • Viacor PTMA Indirect annuloplasty • Ample PS 3 • Myocor i-Coapsys • St. Jude AAR Direct annuloplasty • Mitralign • Guided Delivery Systems • Quantum. Cor, Cordis DPA • Mi. Cardia, Mitral Solutions Mitral valve replacement • Endovalve Maurice Buchbinder, MD Foundation for Cardiovascular Medicine Device Landscape: Percutaneous MV Repair

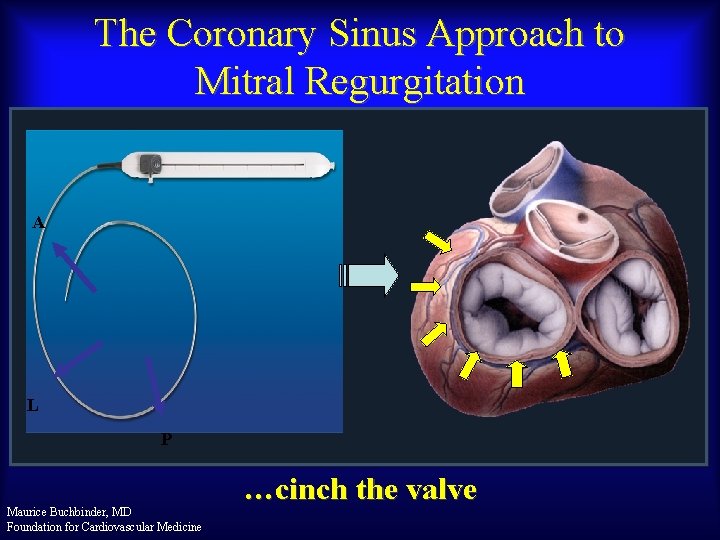
The Coronary Sinus Approach to Mitral Regurgitation A L P Maurice Buchbinder, MD Foundation for Cardiovascular Medicine …cinch the valve

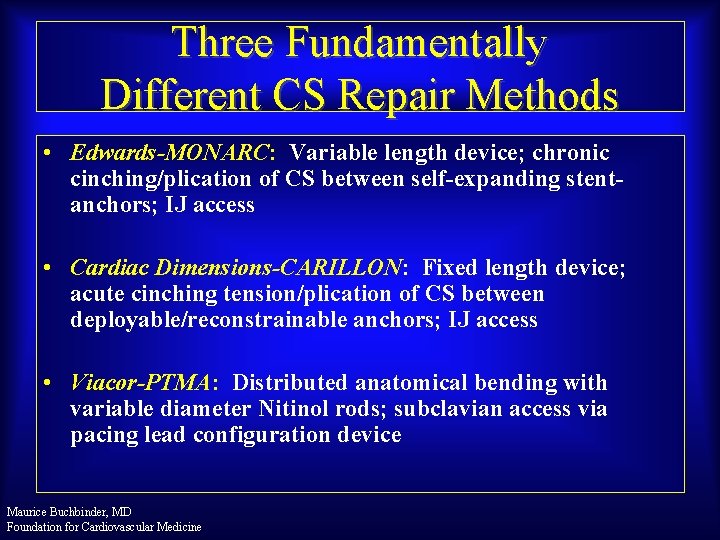
Three Fundamentally Different CS Repair Methods • Edwards-MONARC: Variable length device; chronic cinching/plication of CS between self-expanding stentanchors; IJ access • Cardiac Dimensions-CARILLON: Fixed length device; acute cinching tension/plication of CS between deployable/reconstrainable anchors; IJ access • Viacor-PTMA: Distributed anatomical bending with variable diameter Nitinol rods; subclavian access via pacing lead configuration device Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

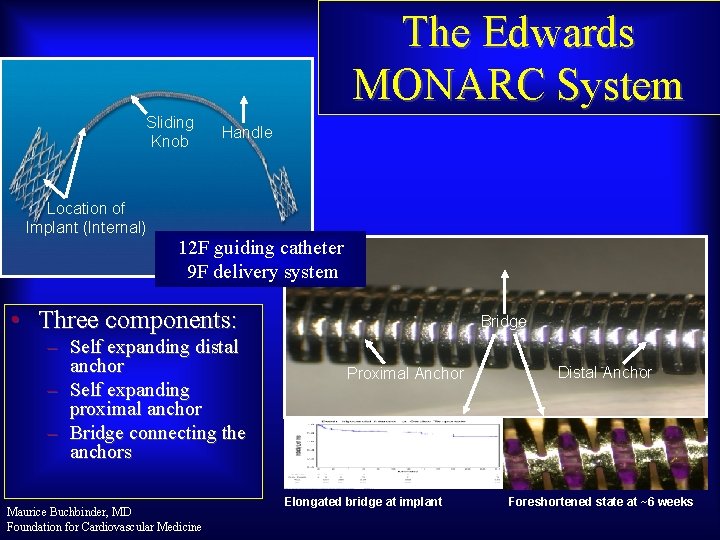
The Edwards MONARC System Sliding Knob Location of Implant (Internal) Handle 12 F guiding catheter 9 F delivery system • Three components: – Self expanding distal anchor – Self expanding proximal anchor – Bridge connecting the anchors Maurice Buchbinder, MD Foundation for Cardiovascular Medicine Bridge Proximal Anchor Elongated bridge at implant Distal Anchor Foreshortened state at ~6 weeks

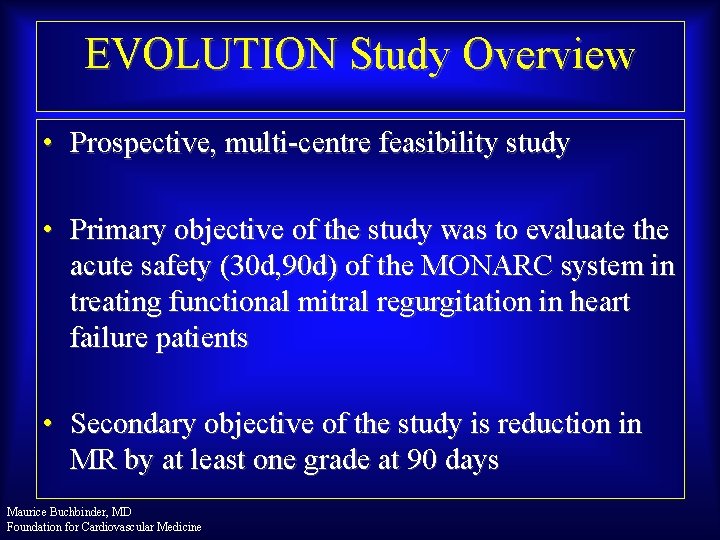
EVOLUTION Study Overview • Prospective, multi-centre feasibility study • Primary objective of the study was to evaluate the acute safety (30 d, 90 d) of the MONARC system in treating functional mitral regurgitation in heart failure patients • Secondary objective of the study is reduction in MR by at least one grade at 90 days Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

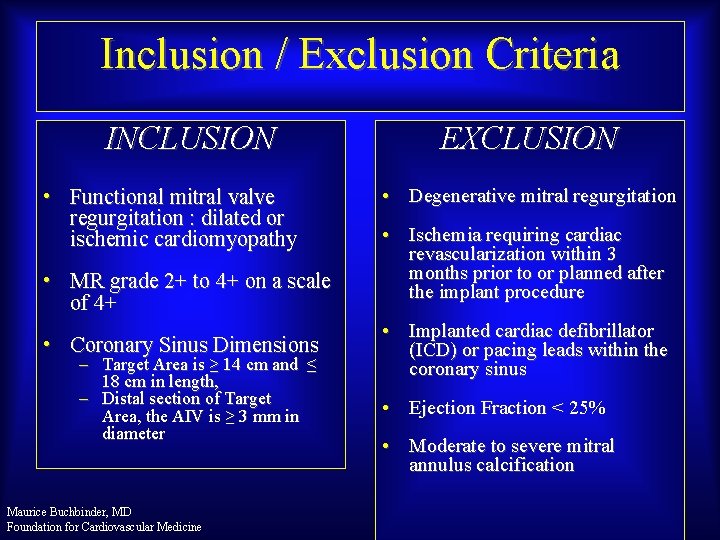
Inclusion / Exclusion Criteria INCLUSION • Functional mitral valve regurgitation : dilated or ischemic cardiomyopathy • MR grade 2+ to 4+ on a scale of 4+ • Coronary Sinus Dimensions – Target Area is ≥ 14 cm and ≤ 18 cm in length, – Distal section of Target Area, the AIV is ≥ 3 mm in diameter Maurice Buchbinder, MD Foundation for Cardiovascular Medicine EXCLUSION • Degenerative mitral regurgitation • Ischemia requiring cardiac revascularization within 3 months prior to or planned after the implant procedure • Implanted cardiac defibrillator (ICD) or pacing leads within the coronary sinus • Ejection Fraction < 25% • Moderate to severe mitral annulus calcification

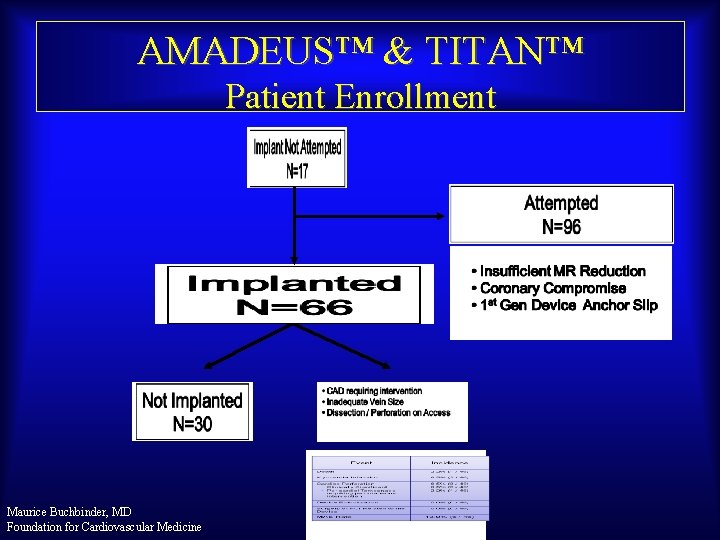
EVOLUTION Study Procedural Success Patients Enrolled n = 72 Device implanted n=59 (82%) • Procedural Time: 84 58 min Maurice Buchbinder, MD Foundation for Cardiovascular Medicine Device not implanted n=13 (18%) • Tortuous Anatomy • Size Outside of Offered Range

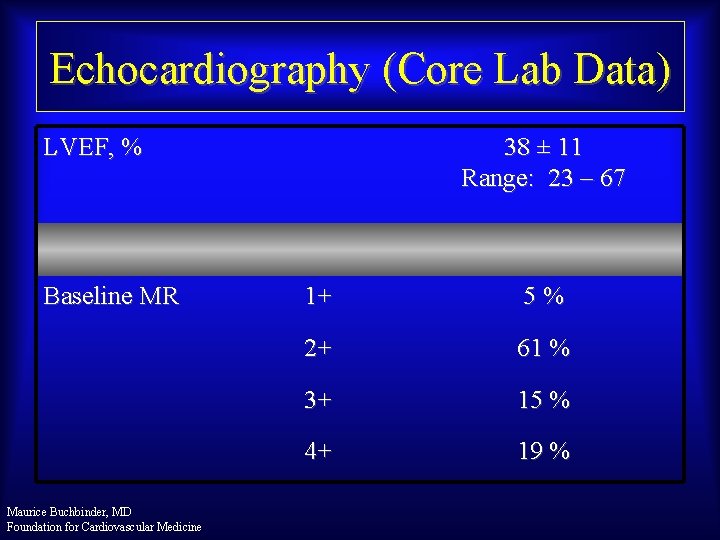
Echocardiography (Core Lab Data) LVEF, % Baseline MR Maurice Buchbinder, MD Foundation for Cardiovascular Medicine 38 ± 11 Range: 23 – 67 1+ 5% 2+ 61 % 3+ 15 % 4+ 19 %

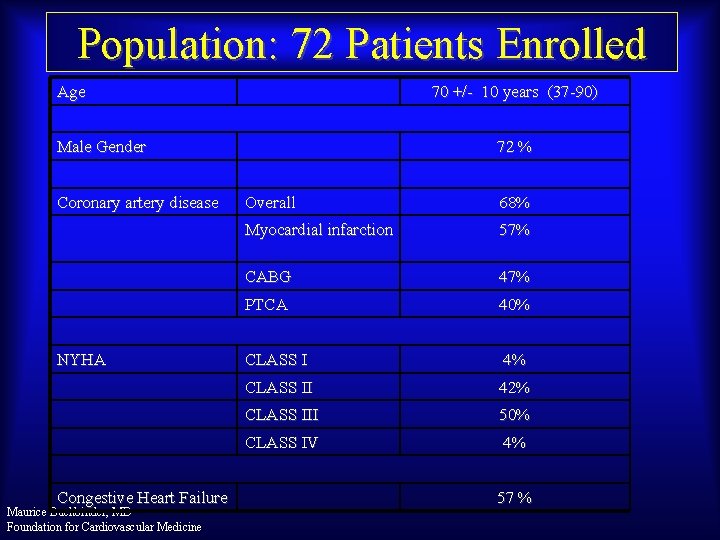
Population: 72 Patients Enrolled Age 70 +/- 10 years (37 -90) Male Gender Coronary artery disease NYHA Congestive Heart Failure Maurice Buchbinder, MD Foundation for Cardiovascular Medicine 72 % Overall 68% Myocardial infarction 57% CABG 47% PTCA 40% CLASS I 4% CLASS II 42% CLASS III 50% CLASS IV 4% 57 %

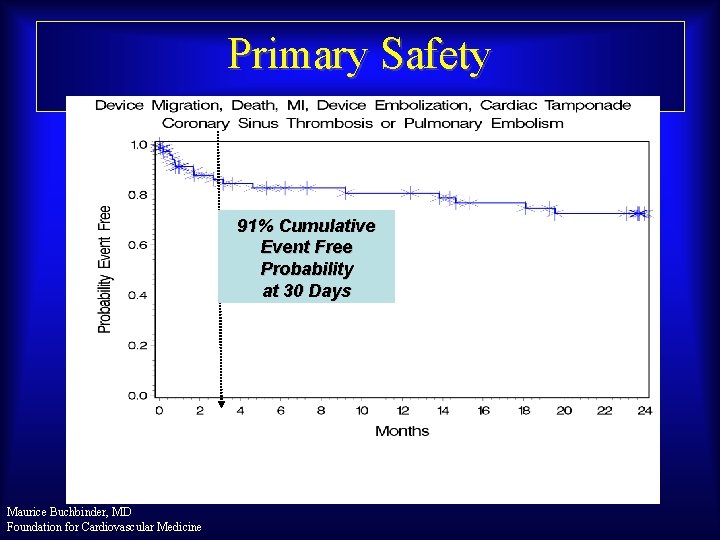
Primary Safety 91% Cumulative Event Free Probability at 30 Days Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

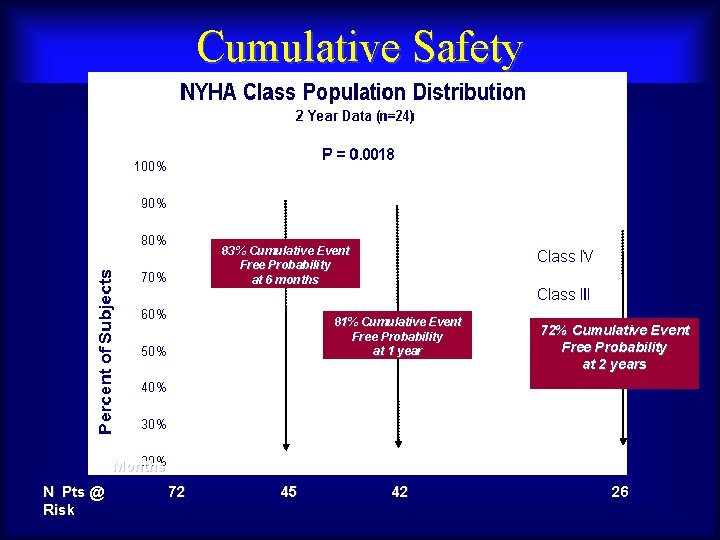
Cumulative Safety 83% Cumulative Event Free Probability at 6 months 81% Cumulative Event Free Probability at 1 year 72% Cumulative Event Free Probability at 2 years Months N Pts @ Maurice. Risk Buchbinder, MD 72 Foundation for Cardiovascular Medicine 45 42 26

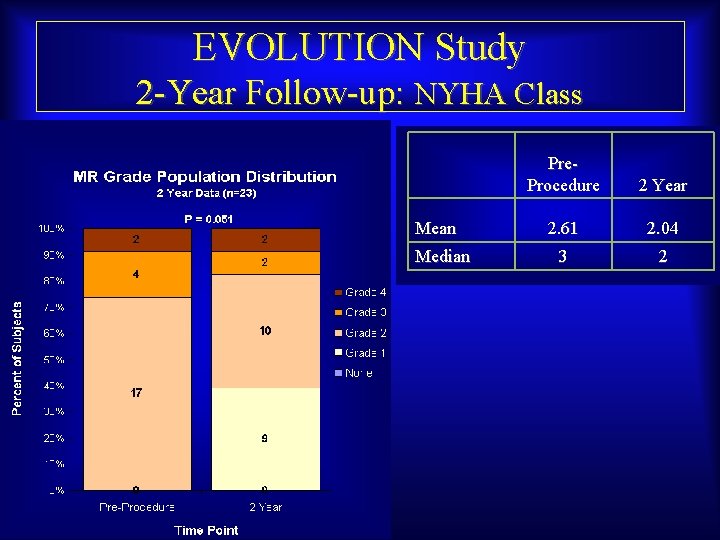
EVOLUTION Study 2 -Year Follow-up: NYHA Class Mean Median Maurice Buchbinder, MD Foundation for Cardiovascular Medicine Pre. Procedure 2 Year 2. 61 2. 04 3 2

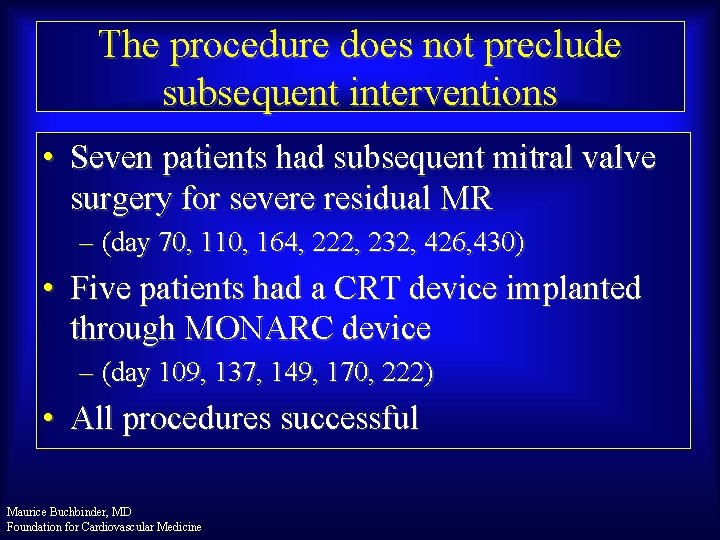
The procedure does not preclude subsequent interventions • Seven patients had subsequent mitral valve surgery for severe residual MR – (day 70, 110, 164, 222, 232, 426, 430) • Five patients had a CRT device implanted through MONARC device – (day 109, 137, 149, 170, 222) • All procedures successful Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

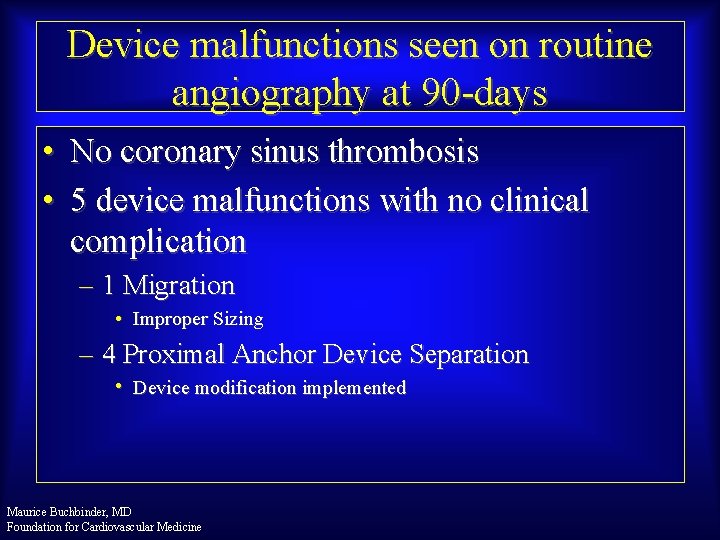
Device malfunctions seen on routine angiography at 90 -days • No coronary sinus thrombosis • 5 device malfunctions with no clinical complication – 1 Migration • Improper Sizing – 4 Proximal Anchor Device Separation • Device modification implemented Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

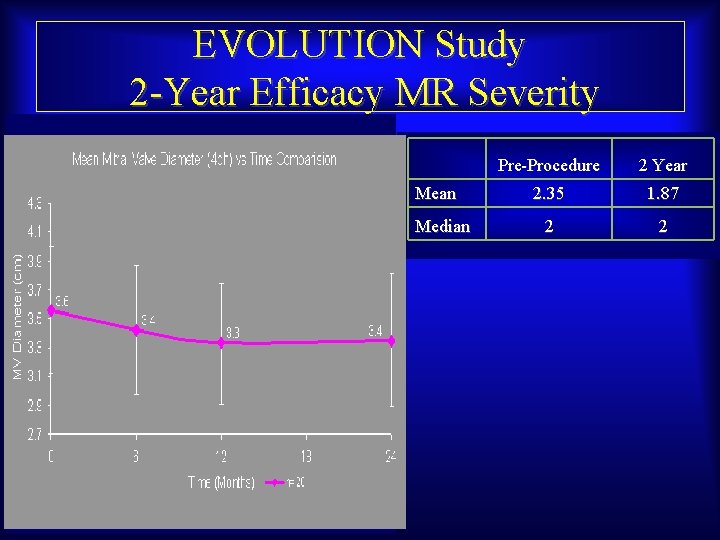
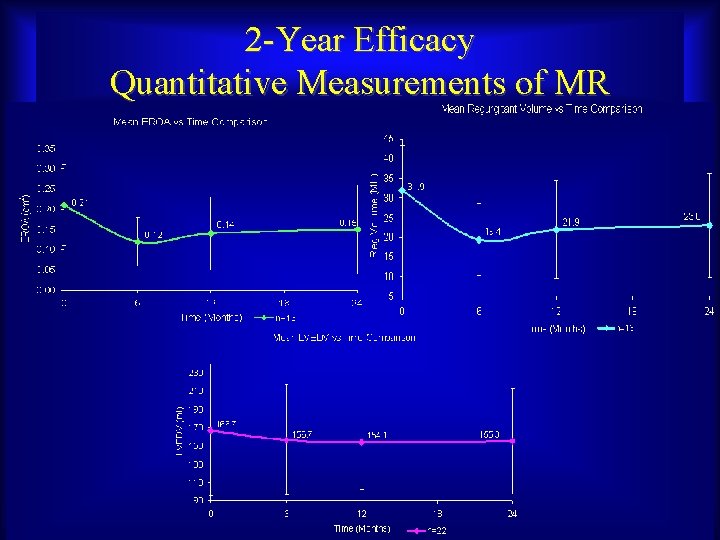
EVOLUTION Study 2 -Year Efficacy MR Severity Mean Median Maurice Buchbinder, MD Foundation for Cardiovascular Medicine Pre-Procedure 2 Year 2. 35 1. 87 2 2

2 -Year Efficacy Quantitative Measurements of MR Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

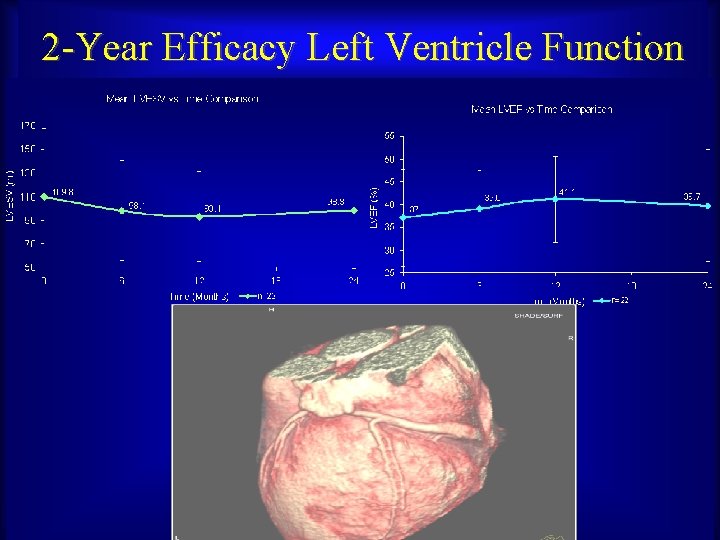
2 -Year Efficacy Left Ventricle Function Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

Conclusions • EVOLUTION interim data with the MONARC system suggests: • Implantation in the coronary sinus is feasible • At 24 -months, 72% of patients are event-free as defined by the protocol • Encouraging 24 -months results in terms of MR reduction and physiological parameters. Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

EVOLUTION II Study Overview • Prospective, non-randomized, multi-centre study. • The primary objectives of this study are to confirm the safety, efficacy, and performance of the MONARC™ system in treating patients with functional mitral regurgitation. Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

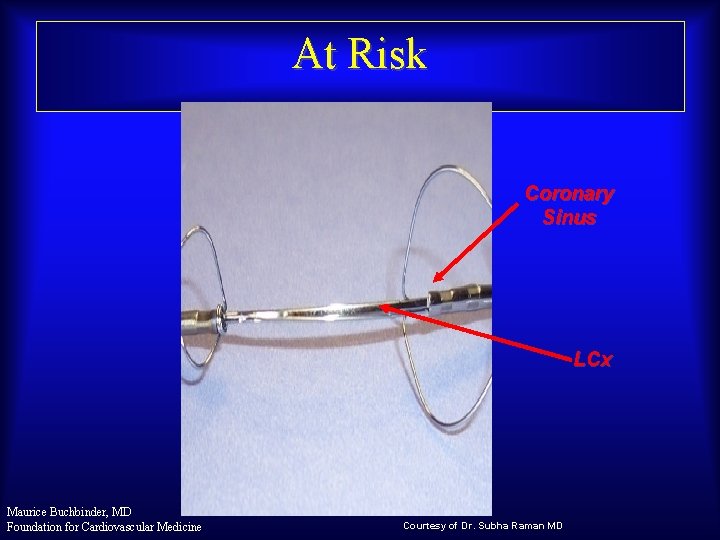
At Risk Coronary Sinus LCx Maurice Buchbinder, MD Foundation for Cardiovascular Medicine Courtesy of Dr. Subha Raman MD

Coronary Sinus Approach Cardiac Dimensions • The Carillon™ device – – Reduces annulus by traction Tension is controlled/adjusted by physician intra-procedurally Results immediately observable by TEE Recapture feature provides “safety net” and ability to optimize result Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

Study Designs • AMADEUS – Prospective, single-arm, 30 patient multi-center trial – Evaluate patients with HF and FMR @ 1 & 6 months (CARILLON® XE implant) • TITAN – Prospective, single-arm, 36 patient multi-center trial – Evaluate patients with HF and FMR @ 1, 6, 12, 18 & 24 months (CARILLON® XE 2 implant) Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

Study Designs • Inclusion Criteria – – Dilated ischemic or non-ischemic cardiomyopathy (LVEDd > 55 mm) NYHA Class II–IV / FMR 2+ to 4+ / EF < 40% 6 MWT distance between 150 m & 450 m Stable on heart failure meds • Primary End Point – 30 -day Major Adverse Events • Secondary End Points – NYHA class / MR reduction / QOL (KCCQ) – 6 -minute walk test / Maximum exercise time Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

Baseline Demographics Intent-to-Treat Population Patients were at high cardiovascular risk Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

AMADEUS™ & TITAN™ Patient Enrollment Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

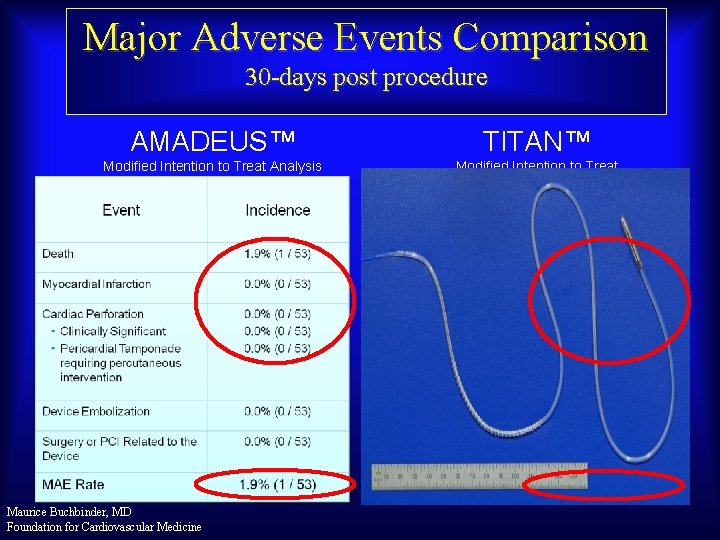
Major Adverse Events Comparison 30 -days post procedure AMADEUS™ TITAN™ Modified Intention to Treat Analysis Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

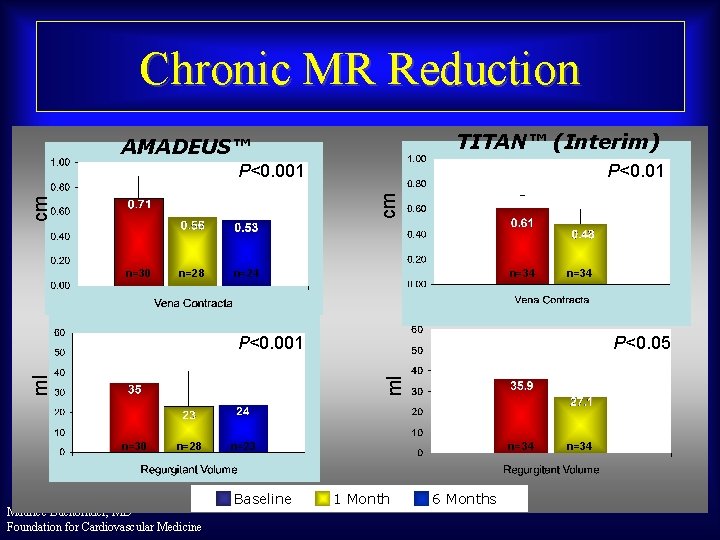
Chronic MR Reduction TITAN™ (Interim) AMADEUS™ P<0. 01 cm cm P<0. 001 n=30 n=28 n=24 n=34 P<0. 05 ml ml P<0. 001 n=30 n=28 Maurice Buchbinder, MD Foundation for Cardiovascular Medicine n=23 Baseline n=34 1 Month 6 Months n=34

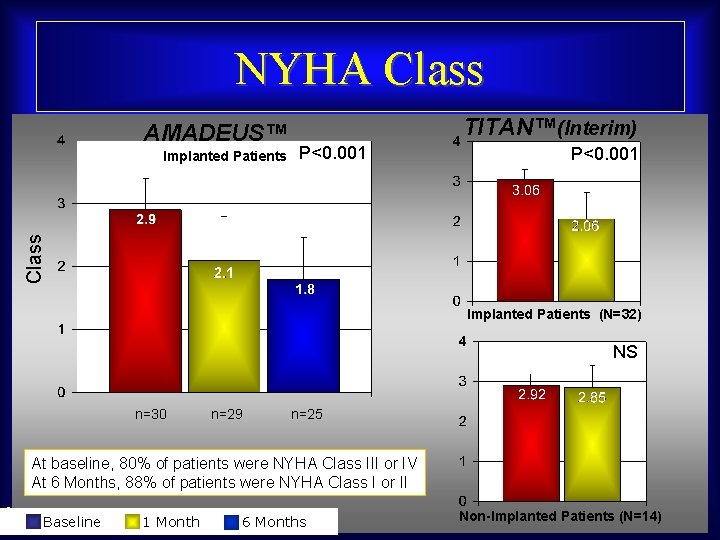
NYHA Class AMADEUS™ P<0. 001 Class Implanted Patients TITAN™(Interim) Implanted Patients (N=32) NS n=30 n=29 n=25 At baseline, 80% of patients were NYHA Class III or IV At 6 Months, 88% of patients were NYHA Class I or II Maurice Buchbinder, MD Baseline 1 Month Foundation for Cardiovascular Medicine 6 Months Non-Implanted Patients (N=14)

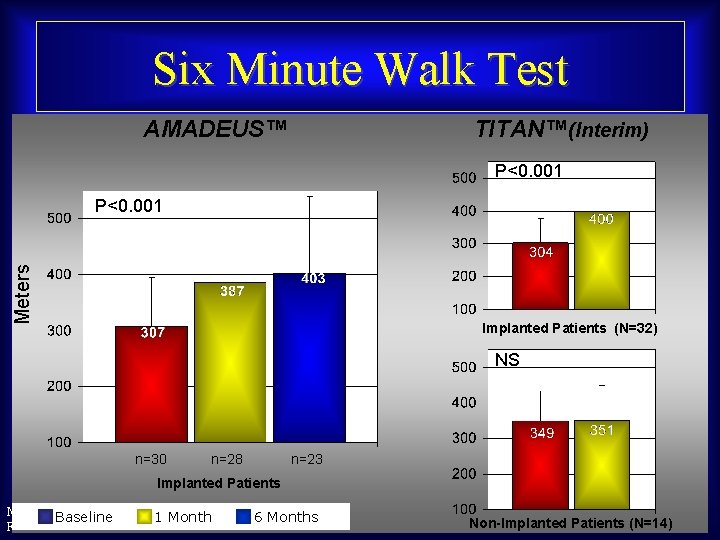
Six Minute Walk Test AMADEUS™ TITAN™(Interim) P<0. 001 Meters P<0. 001 Implanted Patients (N=32) NS n=30 n=28 n=23 Implanted Patients Maurice Buchbinder, MD Baseline 1 Month Foundation for Cardiovascular Medicine 6 Months Non-Implanted Patients (N=14)

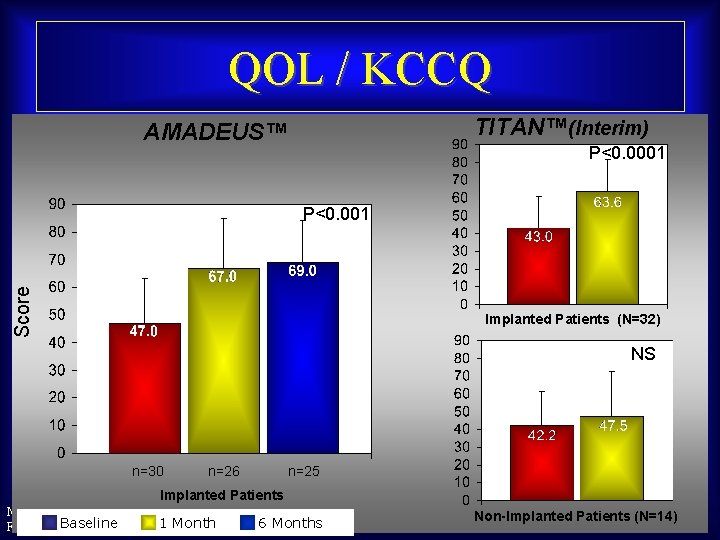
QOL / KCCQ TITAN™(Interim) AMADEUS™ P<0. 0001 Score P<0. 001 Implanted Patients (N=32) NS n=30 n=26 n=25 Implanted Patients Maurice Buchbinder, MD 1 Month Foundation. Baseline for Cardiovascular Medicine 6 Months Non-Implanted Patients (N=14)

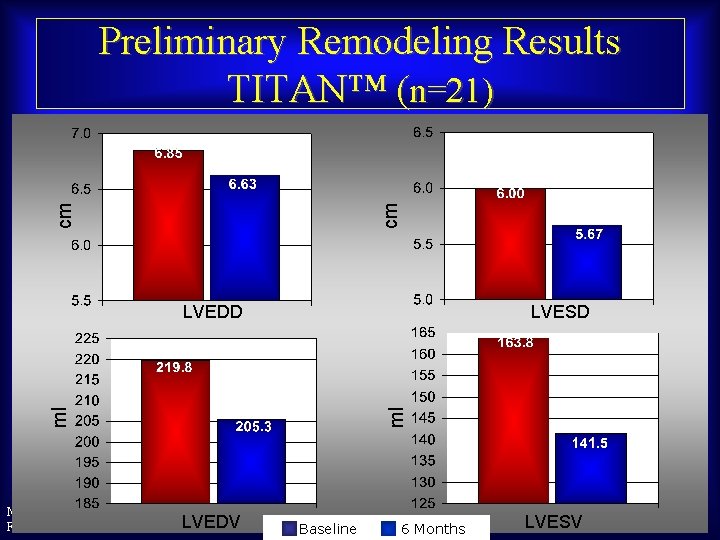
cm cm Preliminary Remodeling Results TITAN™ (n=21) LVESD ml ml LVEDD Maurice Buchbinder, MD LVEDV Foundation for Cardiovascular Medicine Baseline 6 Months LVESV

Conclusions • Low MAE rates @ 30 days, learning curve! • Permanent implantation and significant MR reduction were achieved in the majority of eligible patients. • Significant improvements in functional parameters – 6 MWT, NYHA, QOL • Maybe a mortality and LV remodeling benefit Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

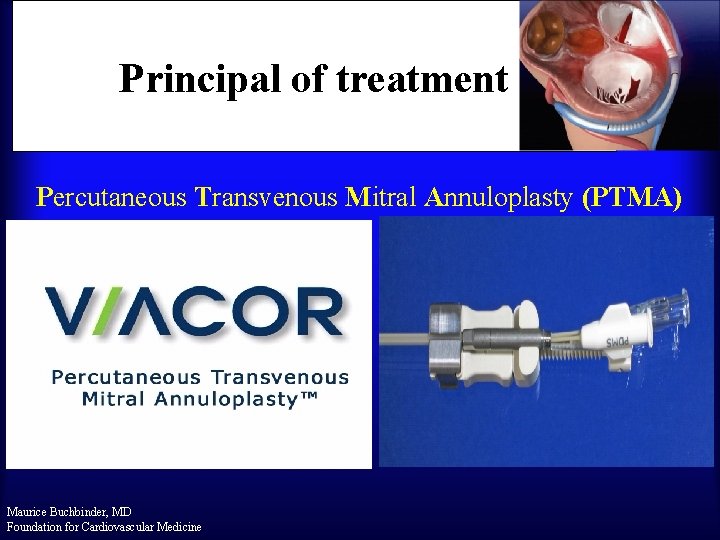
Principal of treatment Percutaneous Transvenous Mitral Annuloplasty (PTMA) Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

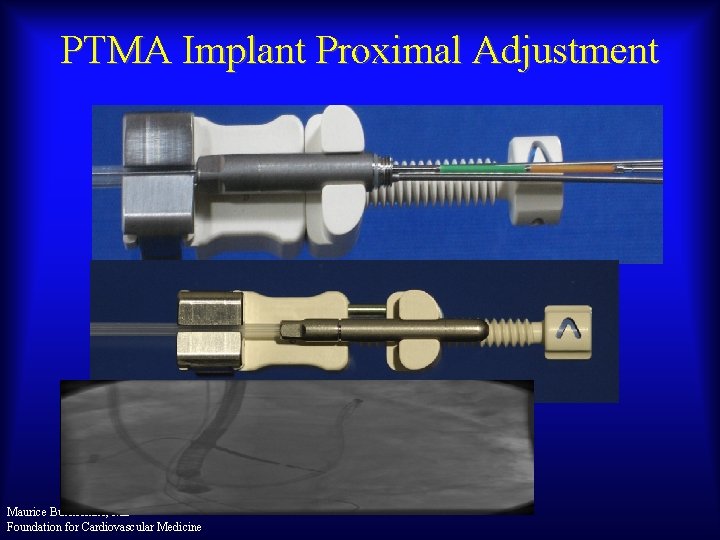
PTMA Implant Proximal Adjustment Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

METHODS 1. Left subclavian puncture, 6 F sheath. 2. Coronary sinus cannulation AL 1, EBU or MP using 0. 035 inch Magic Torque or coronary wire. 3. Venogram using any occlusion balloon, LAO 30 O / caudal 30 O and 90 O lateral view. 4. If anatomy deemed suitable, change 6 F sheath for a coronary sinus 8 F sheath. 5. Advance PTMA 7 F sheath over Magic torque until distal marker reaches AIV (obturator in two lumens, wire in third lumen). 6. Exchange obturation for nitinol rods with incremental stiffnesses under TEE guidance. 7. Optimized rods selection. 8. Fine tune rods position within stabilized sheath. 9. Observe for 30 minutes stability and persistant effect. 10. Check left coronary patency. Maurice Buchbinder, MD Foundation for Cardiovascular Medicine 11. Finalize subclavian pocket.

PTOLEMY 1 Trial: Europe & Canada • • Indications: Improvement of heart failure with moderate to severe valve regurgitation • • • Number of Subjects: Up to 15 per country implanted Up to 30 per country enrolled • • • Follow-up periods: Discharge, 1, 3, 6, & 12 mo 2 – 5 years implanted patients • • Design: Single-arm feasibility study Number of Centers: 2 Centers in Germany 2 Centers in Belgium 1 Center in Canada Maurice Buchbinder, MD Foundation for Cardiovascular Medicine • Endpoints: • • Safety, Primary endpoint Freedom from Major Adverse Events for 30 days • • Efficacy – Technical success – AP dimension reduction – MR reduction at 6 months • • • – QOL and - 6 minute walk improvement Observations

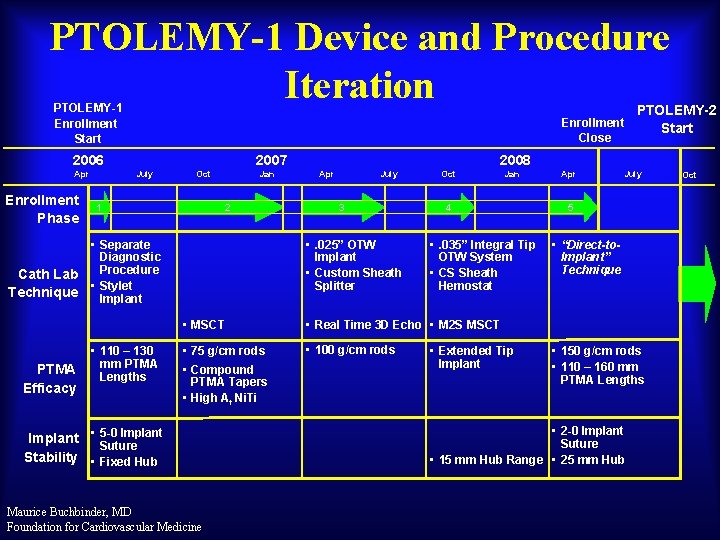
PTOLEMY-1 Device and Procedure Iteration PTOLEMY-1 Enrollment Start Enrollment Close 2006 Apr Enrollment Phase Cath Lab Technique PTMA Efficacy 2007 July Oct 1 Jan 2 • Separate Diagnostic Procedure • Stylet Implant • 110 – 130 mm PTMA Lengths 2008 Apr July 3 • . 025” OTW Implant • Custom Sheath Splitter Oct • . 035” Integral Tip OTW System • CS Sheath Hemostat • Real Time 3 D Echo • M 2 S MSCT • 75 g/cm rods • 100 g/cm rods Implant • 5 -0 Suture Stability • Fixed Hub Maurice Buchbinder, MD Foundation for Cardiovascular Medicine Jan 4 • MSCT • Compound PTMA Tapers • High Af Ni. Ti PTOLEMY-2 Start • Extended Tip Implant Apr July 5 • “Direct-to. Implant” Technique • 150 g/cm rods • 110 – 160 mm PTMA Lengths • 2 -0 Implant Suture • 15 mm Hub Range • 25 mm Hub Oct

PTOLEMY-1 Patient Study Flow • Start: May 2006 • Close April 2008 Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

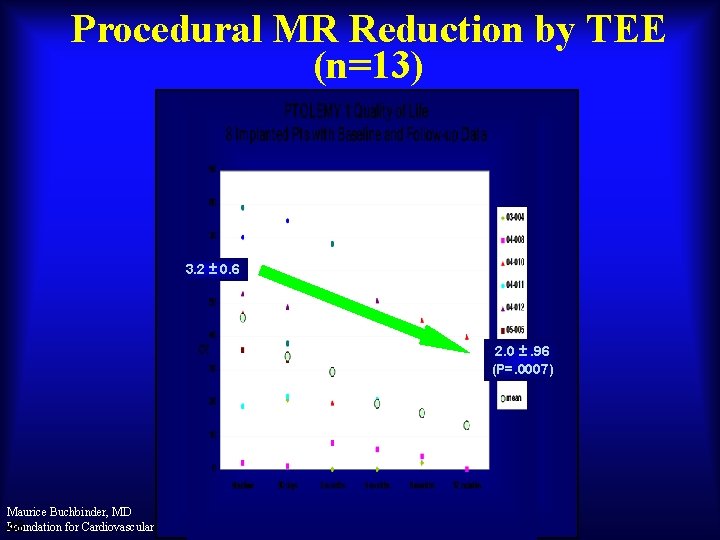
Procedural MR Reduction by TEE (n=13) 3. 2 ± 0. 6 2. 0 ±. 96 (P=. 0007) Maurice Buchbinder, MD Foundation for Cardiovascular Medicine 48

Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

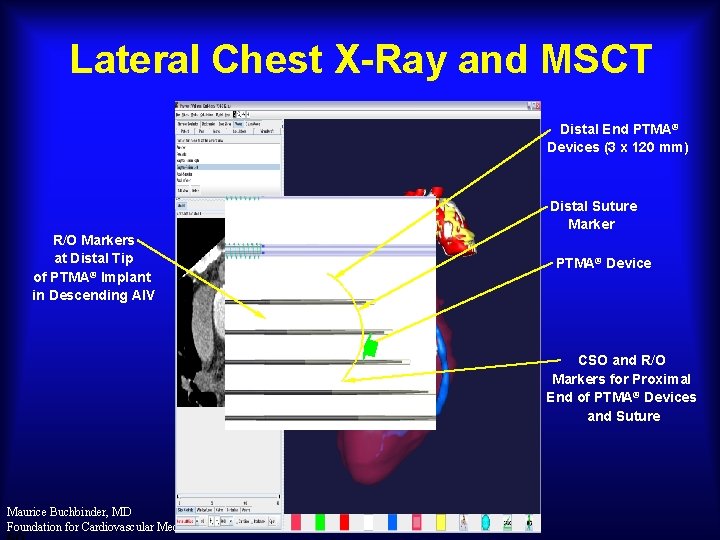
Lateral Chest X-Ray and MSCT Distal End PTMA® Devices (3 x 120 mm) Distal Suture Marker R/O Markers at Distal Tip of PTMA® Implant in Descending AIV PTMA® Device CSO and R/O Markers for Proximal End of PTMA® Devices and Suture Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

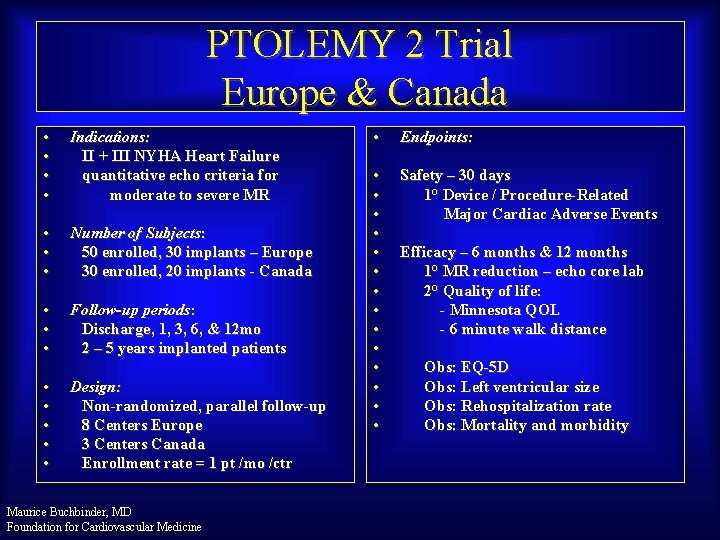
PTOLEMY 2 Trial Europe & Canada • • Indications: II + III NYHA Heart Failure quantitative echo criteria for moderate to severe MR • • • Number of Subjects: 50 enrolled, 30 implants – Europe 30 enrolled, 20 implants - Canada • • • Follow-up periods: Discharge, 1, 3, 6, & 12 mo 2 – 5 years implanted patients • • • Design: Non-randomized, parallel follow-up 8 Centers Europe 3 Centers Canada Enrollment rate = 1 pt /mo /ctr Maurice Buchbinder, MD Foundation for Cardiovascular Medicine • Endpoints: • • • • Safety – 30 days 1° Device / Procedure-Related Major Cardiac Adverse Events Efficacy – 6 months & 12 months 1° MR reduction – echo core lab 2° Quality of life: - Minnesota QOL - 6 minute walk distance Obs: EQ-5 D Obs: Left ventricular size Obs: Rehospitalization rate Obs: Mortality and morbidity

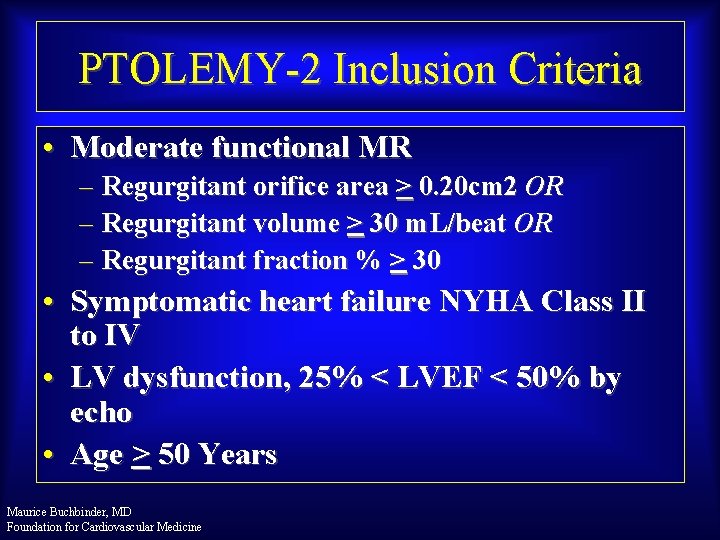
PTOLEMY-2 Inclusion Criteria • Moderate functional MR – Regurgitant orifice area > 0. 20 cm 2 OR – Regurgitant volume > 30 m. L/beat OR – Regurgitant fraction % > 30 • Symptomatic heart failure NYHA Class II to IV • LV dysfunction, 25% < LVEF < 50% by echo • Age > 50 Years Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

PTOLEMY-2 Device & Implant Technique • Frozen device design • PTMA device evolved from PTOLEMY-1 – Added rod lengths up to 160 mm – Maximum available treatment stiffness 150% of PTOLEMY-1 – Implant capable of internal 25 mm rod position adjustment • Implant technique standardized to use position metrics derived from CT Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

PTMA Implant System Elements • Two implant system lengths • 5 implant rod lengths: 110, 120, 130, 145, 160 mm; stiffness increased 50% over PTOLEMY-1 • 25 mm internal final position adjustment Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

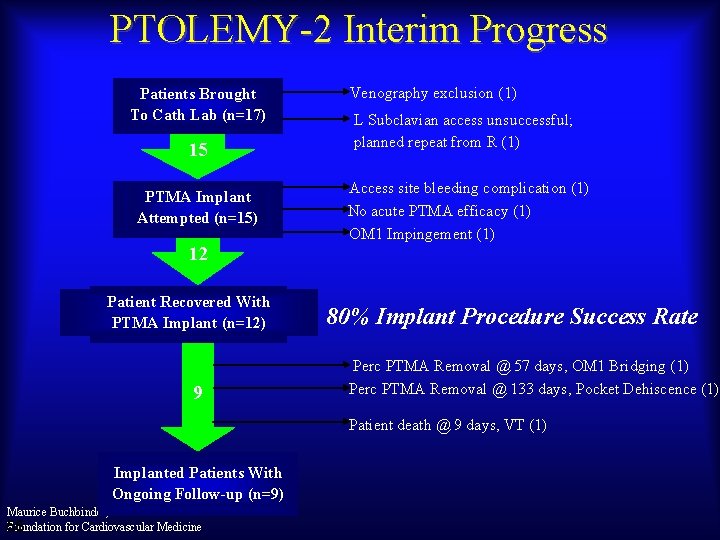
PTOLEMY-2 Interim Progress Patients Brought To Cath Lab (n=17) 15 PTMA Implant Attempted (n=15) Venography exclusion (1) L Subclavian access unsuccessful; planned repeat from R (1) Access site bleeding complication (1) No acute PTMA efficacy (1) OM 1 Impingement (1) 12 Patient Recovered With PTMA Implant (n=12) 9 80% Implant Procedure Success Rate Perc PTMA Removal @ 57 days, OM 1 Bridging (1) Perc PTMA Removal @ 133 days, Pocket Dehiscence (1) Patient death @ 9 days, VT (1) Implanted Patients With Ongoing Follow-up (n=9) Maurice Buchbinder, MD Foundation for Cardiovascular Medicine 55

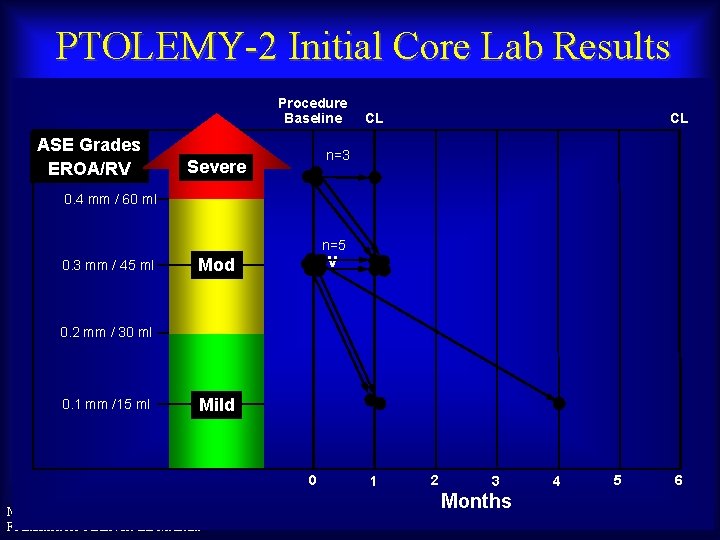
PTOLEMY-2 Initial Core Lab Results Procedure Baseline ASE Grades EROA/RV CL CL n=3 Severe 0. 4 mm / 60 ml n=5 0. 3 mm / 45 ml Mod – v 0. 2 mm / 30 ml 0. 1 mm /15 ml Mild 0 Maurice Buchbinder, MD Foundation for Cardiovascular Medicine 1 2 3 Months 4 5 6

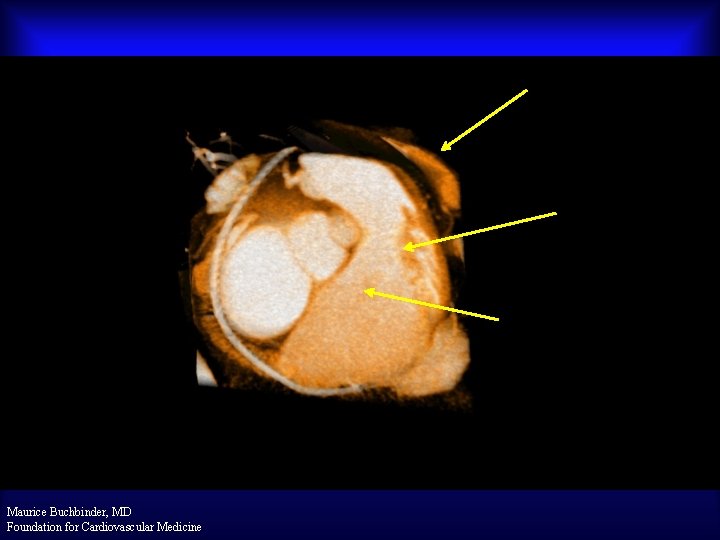
Tether Section in Right Atrium Proximal R/O Marker Treatment Section Engaging CSO Posterior View Maurice Buchbinder, MD Foundation for Cardiovascular Medicine

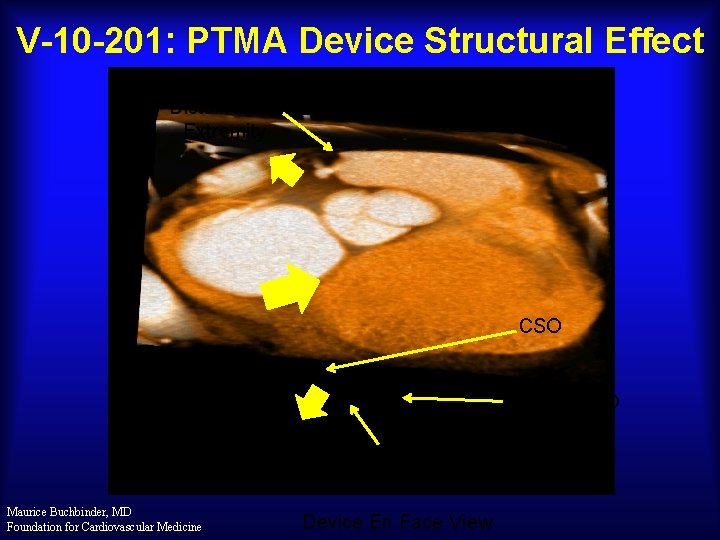
V-10 -201: PTMA Device Structural Effect Distal Nitinol Extremity CSO Proximal R/O Marker Treatment Section Proximal End Maurice Buchbinder, MD Foundation for Cardiovascular Medicine Device En Face View

V-10 -201: Echo Core Lab Mitral Annular AP Dimensions m m . 5 8 3 Baseline Maurice Buchbinder, MD Foundation for Cardiovascular Medicine m 8 . 31 60 Days m

Edge-to-edge • e. Valve • Edwards Mobius Coronary sinus annuloplasty • Edwards Monarc • Cardiac Dimensions Carillon • Viacor PTMA Indirect annuloplasty • Ample PS 3 • Myocor i-Coapsys • St. Jude AAR Direct annuloplasty • Mitralign • Guided Delivery Systems • Quantum. Cor, Cordis DPA • Mi. Cardia, Mitral Solutions Mitral valve replacement Maurice Buchbinder, MD • Endovalve Foundation for Cardiovascular Medicine Device Landscape: Percutaneous MV Repair
 Apical pulse and mitral valve
Apical pulse and mitral valve Ef slope in echo
Ef slope in echo Pressure half time formula
Pressure half time formula Tendyne mitral valve
Tendyne mitral valve Chordae tendineae
Chordae tendineae Pulmonary trunk
Pulmonary trunk Dna repair mechanism notes
Dna repair mechanism notes Mismatch repair
Mismatch repair Percutaneous umbilical blood sampling
Percutaneous umbilical blood sampling Percutaneous image-guided lumbar decompression
Percutaneous image-guided lumbar decompression Common bile duct diameter
Common bile duct diameter Terminal testicular cancer
Terminal testicular cancer Ellis curve
Ellis curve Transhepatic cholangiography
Transhepatic cholangiography Soal perkalian sinus dan cosinus
Soal perkalian sinus dan cosinus Tachy brady syndrome
Tachy brady syndrome Servo valve types
Servo valve types Severe ms heart
Severe ms heart Valva mitral
Valva mitral Mitral klapan prolapsi
Mitral klapan prolapsi Rvh cxr
Rvh cxr Estenose mitral
Estenose mitral Valvuloplastia mitral percutánea
Valvuloplastia mitral percutánea Mitral klapan
Mitral klapan Pathophysiology of valvular heart disease
Pathophysiology of valvular heart disease Aortic regurgitation murmur
Aortic regurgitation murmur Mitral facies pathophysiology
Mitral facies pathophysiology Rhuematic
Rhuematic L
L Silhouette mitrale triangulaire
Silhouette mitrale triangulaire Valvula mitral en paracaidas
Valvula mitral en paracaidas Carvallo belirtisi
Carvallo belirtisi Lvvo heart
Lvvo heart Systolic mumur
Systolic mumur Atheromatous thoracic aorta
Atheromatous thoracic aorta Mitral facies
Mitral facies Valva mitral
Valva mitral Postiam
Postiam Mitral stenosis pulmonary hypertension
Mitral stenosis pulmonary hypertension Tendinous cords
Tendinous cords Adlov
Adlov Mitral stenosis measurements
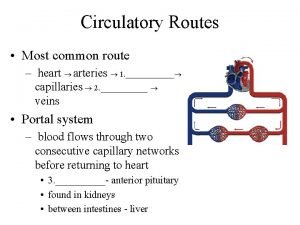
Mitral stenosis measurements Coronary circulatory routes
Coronary circulatory routes Acute coronary syndrome
Acute coronary syndrome Coronary steal syndrome
Coronary steal syndrome Coronary calcium score guidelines
Coronary calcium score guidelines Crash course circulatory system
Crash course circulatory system Ischemic heart disease classification
Ischemic heart disease classification Coronary personality
Coronary personality Coronary artery disease
Coronary artery disease Mesa coronary calcium score
Mesa coronary calcium score Coronary ligament
Coronary ligament Coronary artery disease pathophysiology
Coronary artery disease pathophysiology Course of right coronary artery
Course of right coronary artery Qfr coronary
Qfr coronary