Neonatal Hypoglycemia Alaa Al Nofal M D Assistant






































- Slides: 54

Neonatal Hypoglycemia Alaa Al Nofal, M. D Assistant Professor of Pediatrics Pediatric Endocrinology University of South Dakota – Sanford School of Medicine


Objectives Ø Transitional Neonatal Hypoglycemia Ø Prolonged and Permanent Hypoglycemia Ø Causes of Permanent Hypoglycemia Ø Genetics Causes of Hyperinsulinemic Hypoglycemia Ø Other Causes of Hypoglycemia Ø Diazoxide

What is The Definition of Hypoglycemia in a Full Term Neonate <48 h Old? 1. Plasma glucose concentration less than 40 mg/dl 2. Capillary glucose concentration less than 35 mg/dl 3. Plasma glucose less than 47 mg/dl 4. Plasma glucose less than 60 mg/dl 5. Symptomatic neonate with glucose level less than 60 mg/dl 6. All/none of the above

Koh et al, Arch Dis Child 1988 Cornblath et al, Pediatrics 1990

WHO, hypoglycemia of the newborn 1997 Duvanel et al, JPeds 1999 Cornblath et al, Pediatrics 2000

Hay, JPeds 2009 Sweet et al, J pediatrics pharmacol ther. 2013 Rozance, Curr Opin Endocrinol Diabes Obes 2015

Al Nofal, SDPA conference 2018

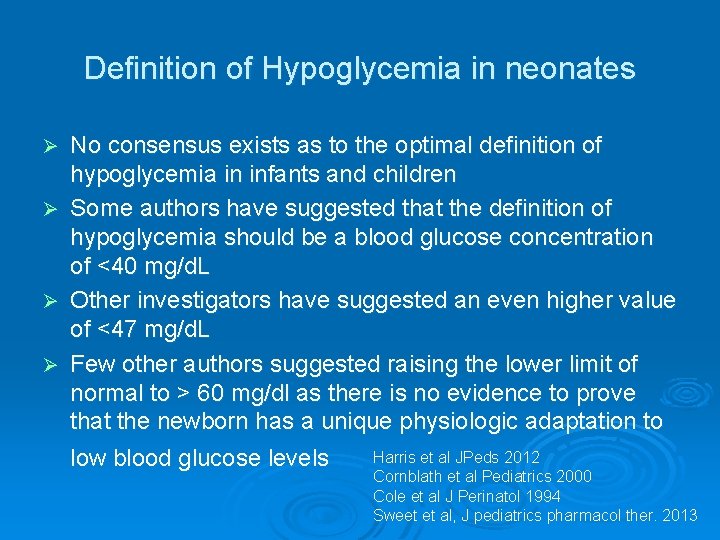
Definition of Hypoglycemia in neonates Ø Ø No consensus exists as to the optimal definition of hypoglycemia in infants and children Some authors have suggested that the definition of hypoglycemia should be a blood glucose concentration of <40 mg/d. L Other investigators have suggested an even higher value of <47 mg/d. L Few other authors suggested raising the lower limit of normal to > 60 mg/dl as there is no evidence to prove that the newborn has a unique physiologic adaptation to Harris et al JPeds 2012 low blood glucose levels Cornblath et al Pediatrics 2000 Cole et al J Perinatol 1994 Sweet et al, J pediatrics pharmacol ther. 2013


It is known that plasma glucose concentration is lower in the first 1 -3 days of life in normal newborn infants than at later age

Ø Plasma glucose was checked fasting at 3 - 6 hour of age Ø Mean glucose level in term AGA infants was found to be 54 mg/dl – 126 newborn Pediatrics 1971

But why?

It All Starts in The Womb

Basics of Fetal Glucose Metabolism Fetal fuel metabolism is based primarily on oxidation of glucose Ø The fetus depends entirely on maternal supply and placental transfer of glucose Ø l l Ø There is no fetal glucose production under normal conditions In most cases, gluconeogenesis appears only after birth The fetal brain is exposed to circulating glucose concentrations only slightly below those of maternal plasma l ~9 mg/dl lower than mother’s glucose Stanley, Jpeds 2015 Hay Jpeds 2009

Basics of Fetal Glucose Metabolism Fetal insulin secretion is responsive to fetal plasma glucose concentrations Ø Fetal glucose concentrations are determined primarily by maternal glucose concentration Ø Fetal insulin primarily functions to regulate growth Ø Continuous fetal secretion of insulin would be important to maintain fetal growth Ø Stanley, Jpeds 2015 Hay Jpeds 2009

Basics of Fetal Glucose Metabolism Ø Pregnant women produce higher amount of ketones. l The generation of high plasma ketone levels would provide the fetus with an alternative fuel to protect the brain when plasma glucose is low Ø Making it unnecessary for the fetus to switch off insulin secretion and thereby avoid limiting growth Stanley, Jpeds 2015

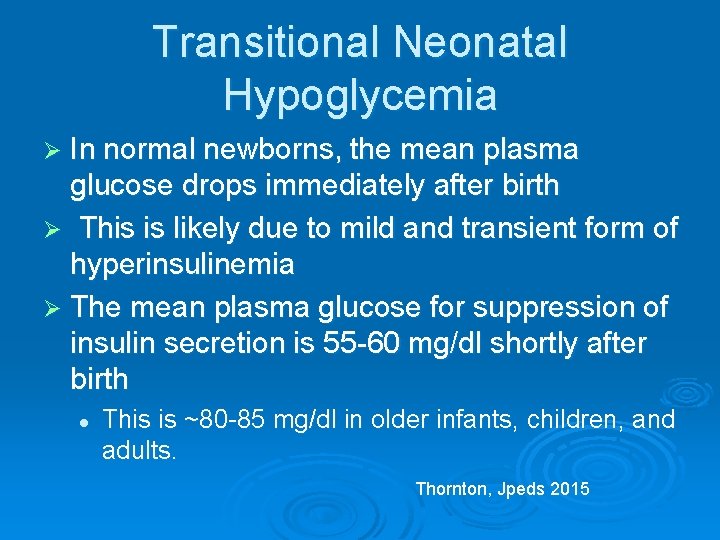
Transitional Neonatal Hypoglycemia Ø In normal newborns, the mean plasma glucose drops immediately after birth Ø This is likely due to mild and transient form of hyperinsulinemia Ø The mean plasma glucose for suppression of insulin secretion is 55 -60 mg/dl shortly after birth l This is ~80 -85 mg/dl in older infants, children, and adults. Thornton, Jpeds 2015

Transitional Neonatal Hypoglycemia Ø As the glucose-stimulated insulin secretion mechanism matures, mean plasma glucose concentration in normal newborns increase. Ø It is similar to those of infants and children by 72 h of life l l The standard for normal neonates must never be extrapolated beyond 2 -3 days of life If possible, delay diagnostic evaluation for persistent hypoglycemia until 2 -3 days of life Thornton, Jpeds 2015

Basics of Fetal Glucose Metabolism Fetus needs glucose from the mother Fetus’ glucose level is ~9 mg/dl lower than mother’s Fetus needs insulin to grow Continuous fetal secretion of insulin would be important to maintain fetal growth Fetus has lower glucose threshold to turn off insulin secretion Stanley, Jpeds 2015 Hay Jpeds 2009

The mean plasma glucose at 8 hours of fasting after birth was 57+/-12 mg/dl Stanely et al Pediatrics 1979

The mean plasma glucose in normal newborn infants who fasted 24 h after birth was 57 -69 mg/dl. Murdina et al Jpeds 1954

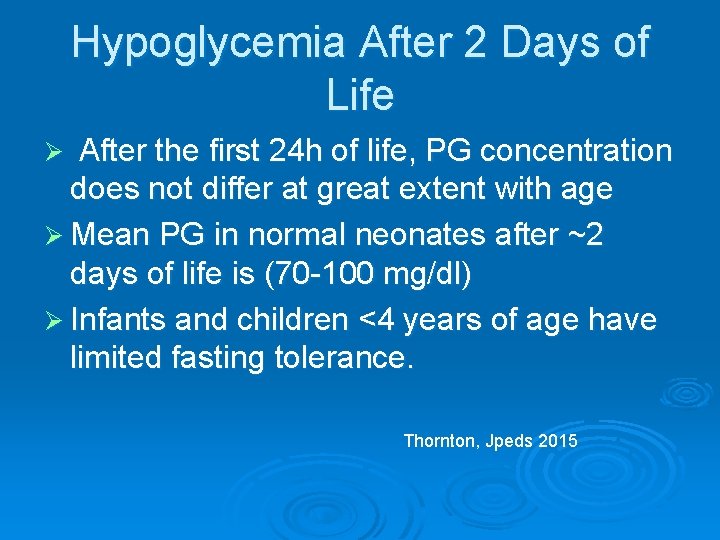
Hypoglycemia After 2 Days of Life After the first 24 h of life, PG concentration does not differ at great extent with age Ø Mean PG in normal neonates after ~2 days of life is (70 -100 mg/dl) Ø Infants and children <4 years of age have limited fasting tolerance. Ø Thornton, Jpeds 2015

Etiology of neonatal hypoglycemia 1. Transient neonatal hypoglycemia Ø Day 1 of life: developmental immaturity of fasting adaptation First 2 days of life: transient hypoglycemia due to maternal factors - Maternal diabetes - Intravenous glucose administration during labor and delivery - Medications: oral hypoglycemics, terbutaline, propranolol Ø

Etiology of neonatal hypoglycemia 2. Prolonged neonatal hypoglycemia Perinatal stress-induced hyperinsulinism (low birth weight, birth asphyxia, maternal toxemia or preeclampsia, prematurity) Ø Beckwith–Wiedemann syndrome/Overgrowth syndromes Ø Hypopituitarism Ø

Etiology of neonatal hypoglycemia 3. Permanent neonatal hypoglycemia Ø Congenital hyperinsulinism - ATP-sensitive potassium channel hyperinsulinism - Glutamate dehydrogenase hyperinsulinism - Glucokinase hyperinsulinism - Short-chain 3 -hydroxyacyl-Co. A dehydrogenase hyperinsulinism - Congenital disorders of glycosylation Ø Counter-regulatory hormone deficiency - Hypopituitarism - Adrenal insufficiency Gluconeogenesis or glycogenolysis enzyme defects Ø Fatty acid oxidation disorders Ø

Workup for Neonatal Hypoglycemia Ø Thorough History and Chart Review l l l Gestational age Birth Weight Maternal History Family History Feeding History

Workup for Neonatal Hypoglycemia Ø Physical Exam l l l Macrosomia Signs of hypopituitarism (cleft palate, micropenis) Hepatomegaly Signs of Adrenal Insufficiency (hyperpigmentation) Signs of Beckwith-Wiedemann syndrome (omphalocele, hemihypertrophy, macroglossia)

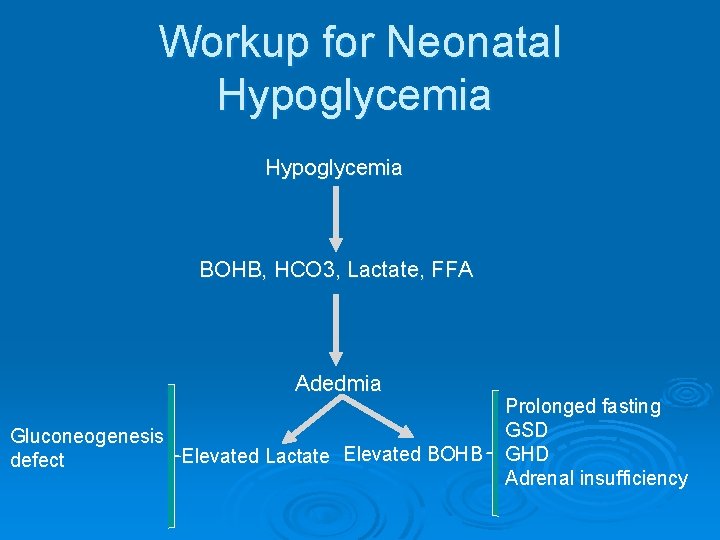
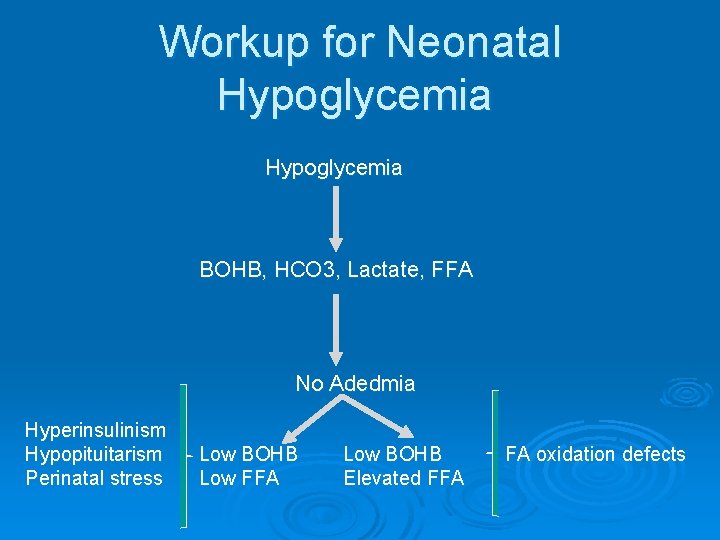
Workup for Neonatal Hypoglycemia Ø Laboratory Evaluation (critical sample) l l l Newborn screen Confirmatory plasma glucose HCo 3 BOHB Lactate FFA

Workup for Neonatal Hypoglycemia BOHB, HCO 3, Lactate, FFA Adedmia Gluconeogenesis Elevated Lactate Elevated BOHB defect Prolonged fasting GSD GHD Adrenal insufficiency

Workup for Neonatal Hypoglycemia BOHB, HCO 3, Lactate, FFA No Adedmia Hyperinsulinism Hypopituitarism Perinatal stress Low BOHB Low FFA Low BOHB Elevated FFA FA oxidation defects

Hormonal Causes of Neonatal Hypoglycemia Hyperinsulinism Ø Adrenal Insufficiency Ø Growth Hormone Deficiency Ø

Congenital Hyperinsulinemia

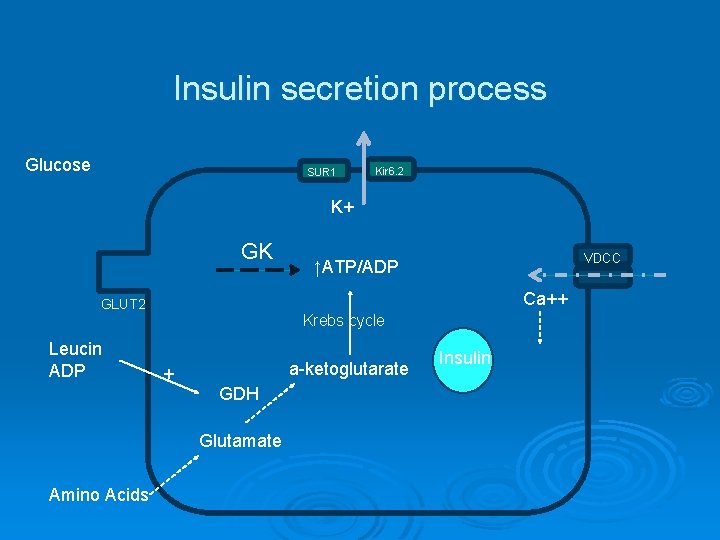
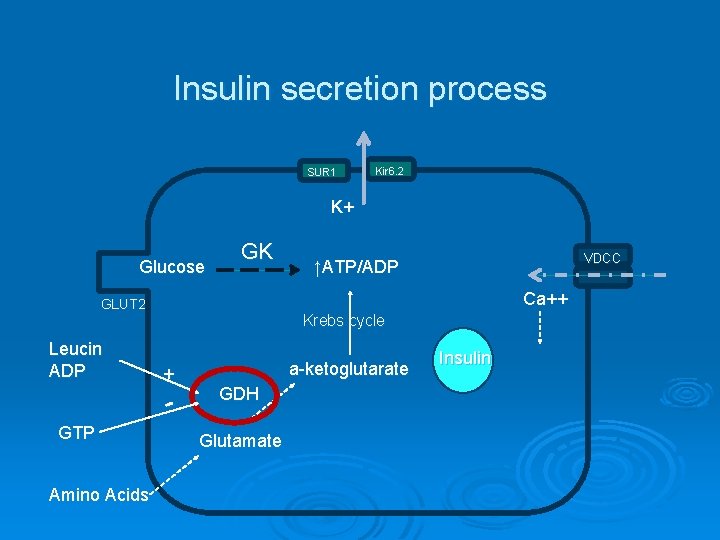
Insulin secretion process Glucose SUR 1 Kir 6. 2 K+ GK VDCC ↑ATP/ADP Ca++ GLUT 2 Krebs cycle Leucin ADP + a-ketoglutarate GDH Glutamate Amino Acids Insulin

What is the most common form of congenital hyperinsulinemic hypoglycemia? ATP-sensitive potassium channel congenital hyperinsulinism

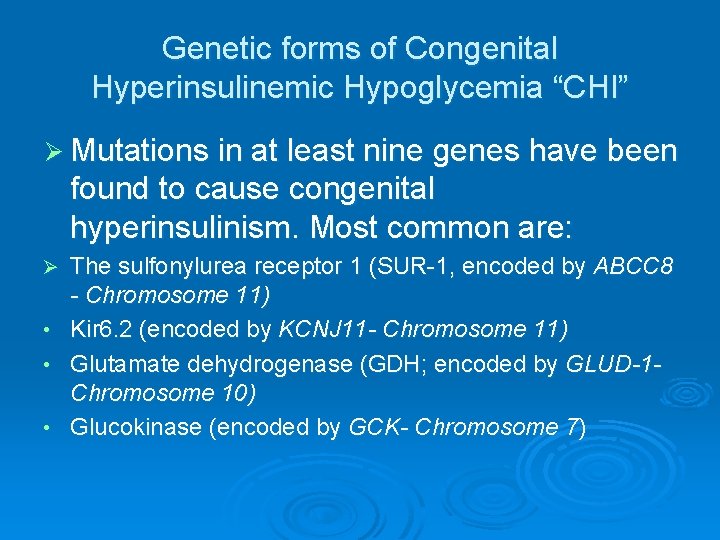
Genetic forms of Congenital Hyperinsulinemic Hypoglycemia “CHI” Ø Mutations in at least nine genes have been found to cause congenital hyperinsulinism. Most common are: Ø • • • The sulfonylurea receptor 1 (SUR-1, encoded by ABCC 8 - Chromosome 11) Kir 6. 2 (encoded by KCNJ 11 - Chromosome 11) Glutamate dehydrogenase (GDH; encoded by GLUD-1 Chromosome 10) Glucokinase (encoded by GCK- Chromosome 7)

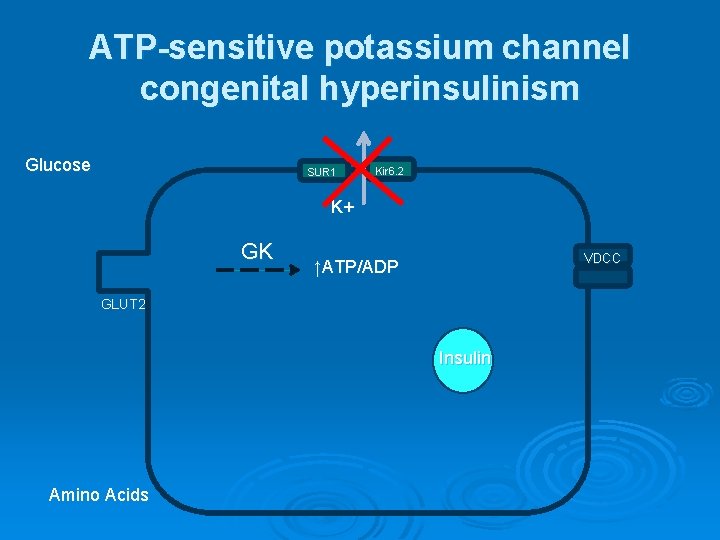
ATP-sensitive potassium channel congenital hyperinsulinism Glucose SUR 1 Kir 6. 2 K+ GK VDCC ↑ATP/ADP GLUT 2 Insulin Amino Acids

ATP-sensitive potassium channel congenital hyperinsulinism The most common and severe form of CHI Ø More than 100 mutations of ABCC 8 and 20 mutations of KCNJ 11 have been found Ø Most reported mutations of ABCC 8 and KCNJ 11 are recessive; however, a few dominantly expressed mutations of ABCC 8 and one of KCNJ 11 have been reported Ø The dominant defects retain responsiveness to diazoxide and tend to be milder Ø

ATP-sensitive potassium channel congenital hyperinsulinism Ø Two histologic forms present: - Focal - Diffuse Ø Large for gestational age Ø Neonatal onset hypoglycemia Ø Unresponsive to diazoxide (usually) Ø Usually require pancreatectomy to control hypoglycemia.

Insulin secretion process SUR 1 Kir 6. 2 K+ Glucose GK Ca++ GLUT 2 Leucin ADP Krebs cycle + GTP Amino Acids VDCC ↑ATP/ADP a-ketoglutarate GDH Glutamate Insulin

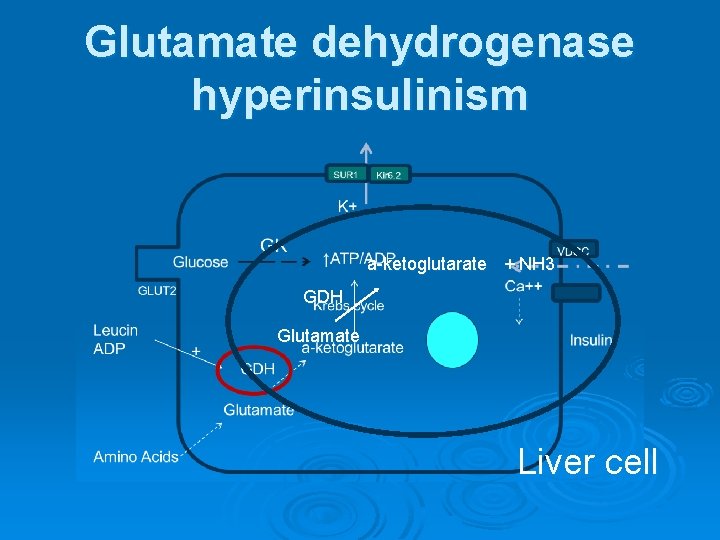
Glutamate dehydrogenase hyperinsulinism a-ketoglutarate + NH 3 GDH Glutamate Liver cell

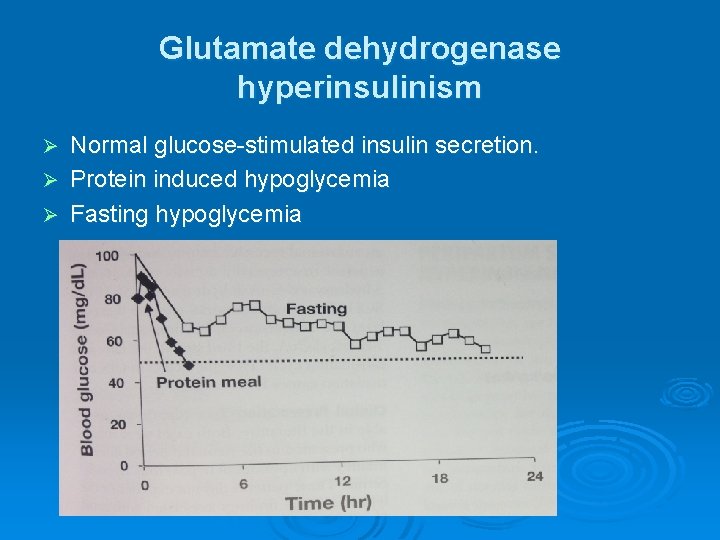
Glutamate dehydrogenase hyperinsulinism Normal glucose-stimulated insulin secretion. Ø Protein induced hypoglycemia Ø Fasting hypoglycemia Ø

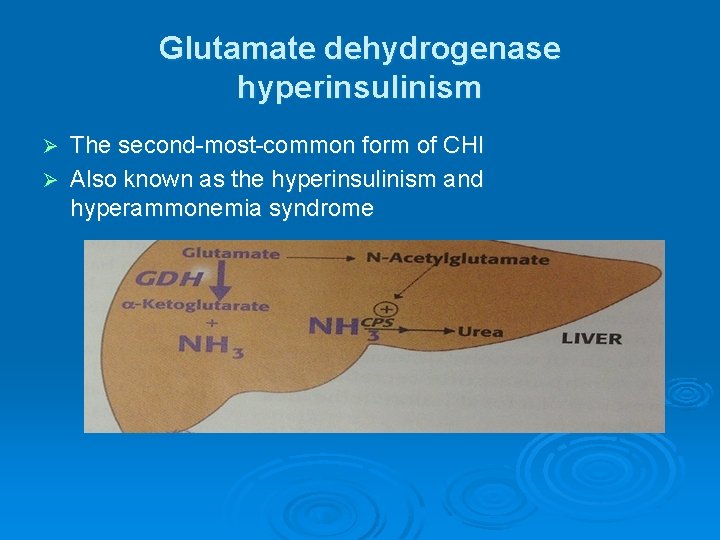
Glutamate dehydrogenase hyperinsulinism The second-most-common form of CHI Ø Also known as the hyperinsulinism and hyperammonemia syndrome Ø

Glutamate dehydrogenase hyperinsulinism Ø Ø Ø Normal birth weight Hypoglycemia does not often manifest until later in infancy Recurrent episodes of fasting and postprandial hypoglycemia associated with persistent asymptomatic elevation of plasma ammonia levels Patients respond well to diazoxide Patients are instructed to avoid pure protein meals and to eat carbohydrates before protein

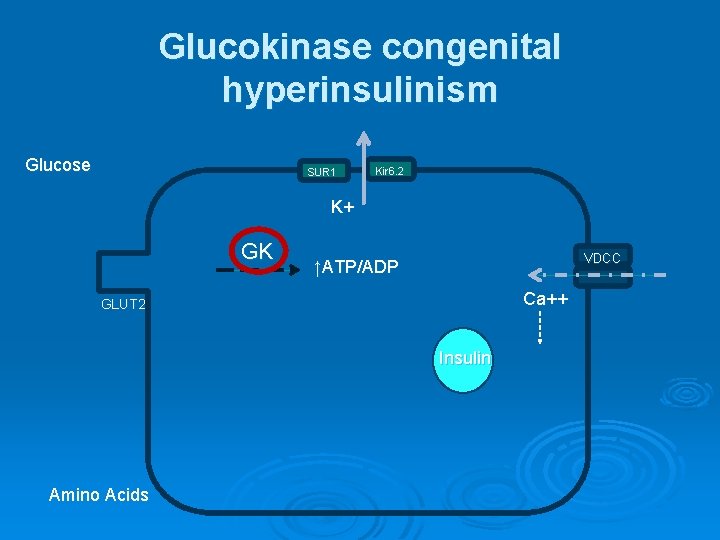
Glucokinase congenital hyperinsulinism Glucose SUR 1 Kir 6. 2 K+ GK VDCC ↑ATP/ADP Ca++ GLUT 2 Insulin Amino Acids

Glucokinase congenital hyperinsulinism Ø GK is expressed exclusively in the liver and pancreas Consider the B-Cell “glucosensor” Ø Activating mutations result in increased affinity of glucokinase for glucose and inappropriate secretion of insulin (Lower threshold for insulin secretion) Ø Diazoxide is usually effective Ø


OTHER FORMS OF HYPERINSULINISM IN NEONATES Perinatal stress-induced hyperinsulinism Birth asphyxia, maternal toxemia, prematurity, intrauterine growth retardation Ø Continuous feeding might be needed to maintain blood glucose Ø Usually resolve in 3 -4 months Ø

OTHER FORMS OF HYPERINSULINISM IN NEONATES Infant of the diabetic mother Ø Ø Ø Infant is exposed to hyperglycemia in utero Fetus up regulates insulin secretion macrosomia Glucagon deficiency may contribute to hypoglycemia in infant of a diabetic mother Hypoglycemia generally resolves within 2 days Responds to diazoxide, but generally not needed Usually treated with intravenous dextrose

OTHER FORMS OF HYPERINSULINISM IN NEONATES Beckwith–Wiedemann syndrome Characterized by somatic overgrowth, macroglossia, hemihypertrophy and transverse creases of the ear lobes, hypoglycemia, and predisposition to childhood tumors Ø Hypoglycemia occurs in up to 50% of patients with BWS Ø Diazoxide should be attempted Ø

Diazoxide Suppresses insulin secretion by maintaining the KATP channel in the open position Ø Dose 5 -15 mg/kg/day orally divided into 2 -3 doses Ø Response is evident in 48 hours Ø Side effects are 1. Primarily cosmetic - Hypertrichosis - Coarsening of the face 2. DKA 3. Fluid retention- particularly in preterm infants 4. Rarely leukopenia and thrombocytopenia Ø

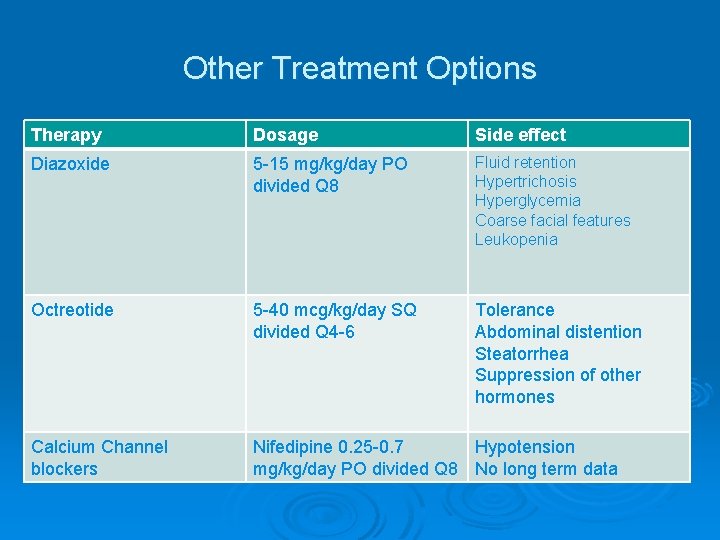
Other Treatment Options Therapy Dosage Side effect Diazoxide 5 -15 mg/kg/day PO divided Q 8 Fluid retention Hypertrichosis Hyperglycemia Coarse facial features Leukopenia Octreotide 5 -40 mcg/kg/day SQ divided Q 4 -6 Tolerance Abdominal distention Steatorrhea Suppression of other hormones Calcium Channel blockers Nifedipine 0. 25 -0. 7 Hypotension mg/kg/day PO divided Q 8 No long term data

Summary Ø Ø Ø No consensus exists as to the optimal definition of hypoglycemia in newborns Transitional Hypoglycemia most closely resembles congenital hyperinsulinism There are many Genetic forms of Congenital Hyperinsulinemic Hypoglycemia - Diazoxide is the main treatment for most of them Other causes of hyperinsulinemic hypoglycemia include, infants of diabetic mothers, Beckwith-Wiedemann syndrome, congenital disorders of glycosylation Surgical intervention may be needed in case of failure of medical treatment

THANK YOU QUESTIONS
 Slidetodoc.com
Slidetodoc.com Alaa alameldeen
Alaa alameldeen Fouad belkacem
Fouad belkacem Alaa mohammad fouad
Alaa mohammad fouad Hypoglycemia
Hypoglycemia Whipple's triad
Whipple's triad Nursing diagnosis for diabetes mellitus slideshare
Nursing diagnosis for diabetes mellitus slideshare Hypoglycemia cpg
Hypoglycemia cpg Hypoglycemia unawareness
Hypoglycemia unawareness Hypoglycemia factitia
Hypoglycemia factitia Hypoglycemia without diabetes
Hypoglycemia without diabetes Repaglinide mechanism of action
Repaglinide mechanism of action Starvation hypoglycemia
Starvation hypoglycemia Hypoglycemia mnemonic
Hypoglycemia mnemonic Clasificacion de kramer ictericia
Clasificacion de kramer ictericia Neonatal hlr
Neonatal hlr Circulação fetal
Circulação fetal Neonatal sepsis symptoms
Neonatal sepsis symptoms Cuidados de enfermería en oxigenoterapia neonatal
Cuidados de enfermería en oxigenoterapia neonatal Site:slidetodoc.com
Site:slidetodoc.com Differential diagnosis of jaundice in pediatrics
Differential diagnosis of jaundice in pediatrics Iap ug teaching slides 2015-16
Iap ug teaching slides 2015-16 Tetanus
Tetanus Phototherapy working principle
Phototherapy working principle Oxigeno por halo
Oxigeno por halo Neonatal cholestasis naspghan
Neonatal cholestasis naspghan Hastalık düzeyini belirleyen ölçütler
Hastalık düzeyini belirleyen ölçütler Estercobilina nas fezes
Estercobilina nas fezes Nipedipina
Nipedipina Glasgow coma scale chart
Glasgow coma scale chart Hemoglucotest en neonatos
Hemoglucotest en neonatos Neonatal resuscitation
Neonatal resuscitation L
L Segurneo guia farmacologica
Segurneo guia farmacologica Mortality rate formula
Mortality rate formula Vasculopatia lenticuloestriada neonatal
Vasculopatia lenticuloestriada neonatal Triade neonatal
Triade neonatal Neonatal sepsis nelson pediatrics
Neonatal sepsis nelson pediatrics Circulação neonatal
Circulação neonatal What does apgar scale measure
What does apgar scale measure Neonatal mortality rate formula
Neonatal mortality rate formula Psychiatric nursing diagnosis
Psychiatric nursing diagnosis Neonatal energy triangle
Neonatal energy triangle Parâmetros iniciais ventilação mecânica neonatal
Parâmetros iniciais ventilação mecânica neonatal Ictericia neonatal clasificacion
Ictericia neonatal clasificacion Neonatal sepsis pathophysiology diagram
Neonatal sepsis pathophysiology diagram Hoja de balance hidrico en enfermeria
Hoja de balance hidrico en enfermeria Gig pig aig
Gig pig aig Ascites veins
Ascites veins Dope reanimacion neonatal
Dope reanimacion neonatal Curva bilirrubina neonatal
Curva bilirrubina neonatal Organization of neonatal unit
Organization of neonatal unit Site:slidetodoc.com
Site:slidetodoc.com Baby reflexes chart
Baby reflexes chart Poliglobulia neonatal
Poliglobulia neonatal