Multi virus specific T cells Spyridonidis Alexandros SCAT












- Slides: 59

(Multi) virus specific T cells Spyridonidis Alexandros SCAT Lab Meeting 03/22/2012

Cellular Immunotherapy for the treatment of Viral infection and reactivation after Bone Marrow Transplantation qmajor cause of morbidity and mortality q. CMV, EBV, Ad. V, BK, HHV-6, HHV-7, RSV q. Depending on protocol, patient characteristics etc. . q. Obligatory for Haplo protocols qdrug treatments q. Efficacy q. Partial effective (CMV, …. . Adv? , RSV? ) q. Not effective (EBV, BK, HHV-6, HHV-7) q. Side effects (cytopenias, renal insufficiency) q. Expensive q. Render viruses resistant q. Why cellular immunotherapy q. Central role of immune system qinverse relationship between viral antigenemia and circulating virus-reactive T cells q 40% CMV reactivation after autologous BMt but <5% disease q. Efficacy q. Clinical studies (Seattle, Baylor, Wurzburg, Leiden)

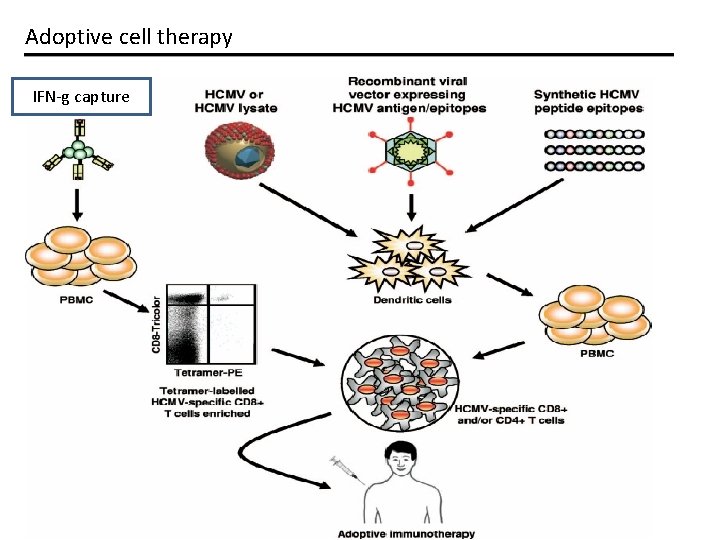
Adoptive cell therapy IFN-g capture

Variables in generation of virus specific T cells q. APCs (DCs) q. Mo DCs vs Plasmacytoid DCs q. TLR q. Virus stimuli qpeptide mix (CD 4, CD 8, polyclonal) q. Other q. Culture q. Media q. Cytokines, others q. IL-7 (10 IU/ml), IL-15 (5 IU/ml), IL-2 (100 IU), IL-12 q. Wnt β- catenin inhibitor q. T cell Donor q. Initial donor q. Patient (? ) q. Third party

Product q. Potency markers q. Viable q. CD 4 and CD 8 q. Polyclonal? (minimize viral escape) q. Tcm, IL-21 R expression (for long term in vivo repopulation) q. Efficacy q. IFNγ –producing q. Other tests (lysing ability etc) q. Number q. Probably few are enough. Efficacy has been shown with q. IFNγ- capture assay: 1. 2 x 10 e 3 cells/ kg (Ad. V) If a cellular product of 10 x 10 e 6 cells/kg contains 1% virus specific cells = 1 x 10 e 5 cells / kg (virus specific) q. Reproducible q. Lack of alloreactivity q. Purity? q. GMP criteria / release q. GMP materials q. Sterility q. Identity

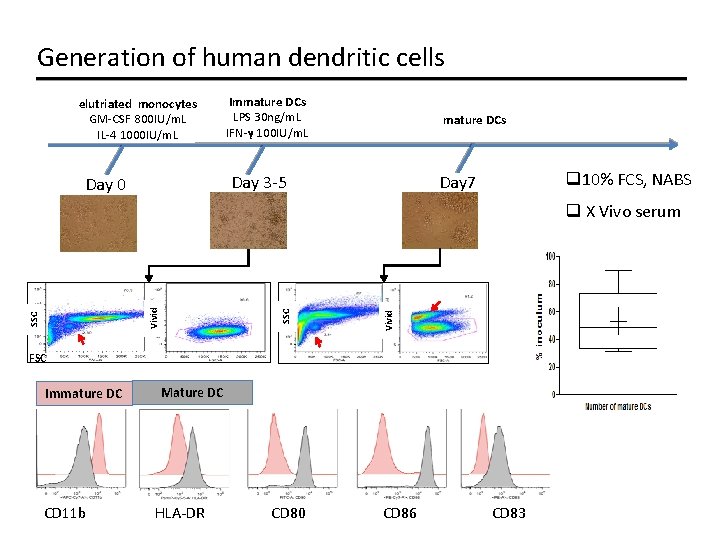
Generation of human dendritic cells elutriated monocytes GM-CSF 800 IU/m. L IL-4 1000 IU/m. L Immature DCs LPS 30 ng/m. L IFN-γ 100 IU/m. L mature DCs Day 3 -5 Day 0 q 10% FCS, NABS Day 7 Vivid SSC Vivid q X Vivo serum FSC Immature DC CD 11 b Mature DC HLA-DR CD 80 CD 86 CD 83

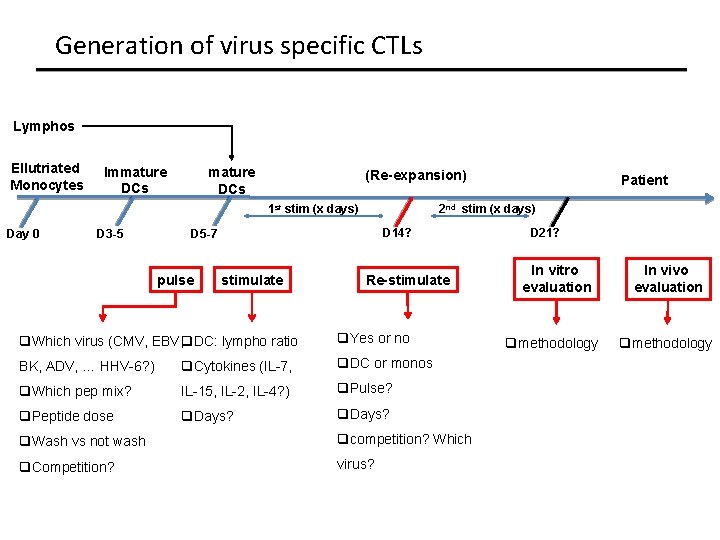
Generation of virus specific CTLs Lymphos Ellutriated Monocytes Immature DCs (Re-expansion) 1 st stim (x days) Day 0 D 3 -5 2 nd stim (x days) D 14? D 5 -7 pulse stimulate q. Which virus (CMV, EBV, q. DC: lympho ratio Patient Re-stimulate q. Yes or no BK, ADV, … HHV-6? ) q. Cytokines (IL-7, q. DC or monos q. Which pep mix? IL-15, IL-2, IL-4? ) q. Pulse? q. Peptide dose q. Days? q. Wash vs not wash qcompetition? Which q. Competition? virus? D 21? In vitro evaluation qmethodology In vivo evaluation qmethodology

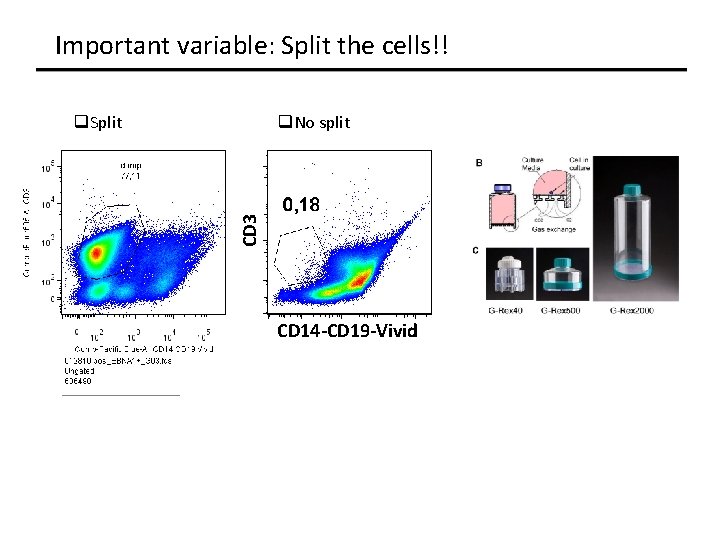
Important variable: Split the cells!! q. No split CD 3 q. Split CD 14 -CD 19 -Vivid

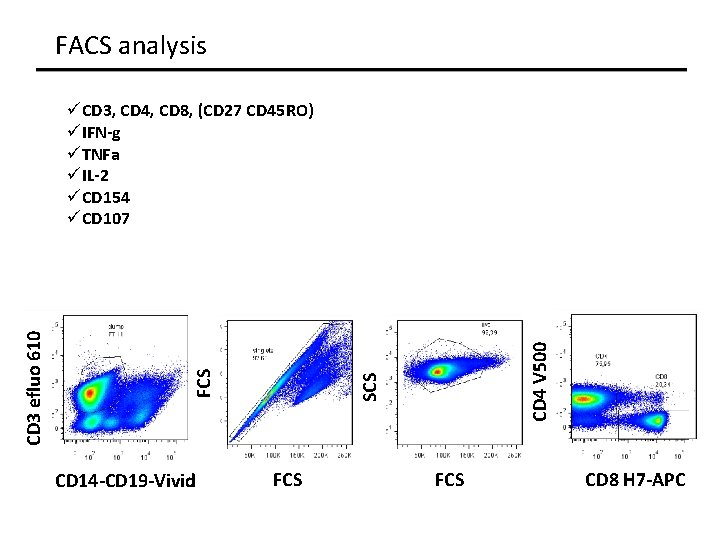
FACS analysis CD 14 -CD 19 -Vivid CD 4 V 500 SCS FCS CD 3 efluo 610 üCD 3, CD 4, CD 8, (CD 27 CD 45 RO) üIFN-g üTNFa üIL-2 üCD 154 üCD 107 FCS CD 8 H 7 -APC

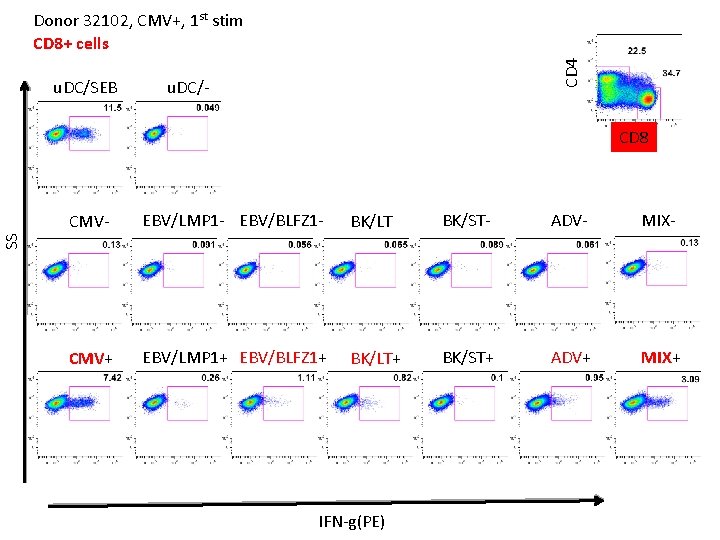
u. DC/SEB CD 4 Donor 32102, CMV+, 1 st stim CD 8+ cells u. DC/- CD 8 EBV/LMP 1 - EBV/BLFZ 1 - BK/LT - BK/ST- ADV- MIX- CMV+ EBV/LMP 1+ EBV/BLFZ 1+ BK/LT+ BK/ST+ ADV+ MIX+ SS CMV- IFN-g(PE)

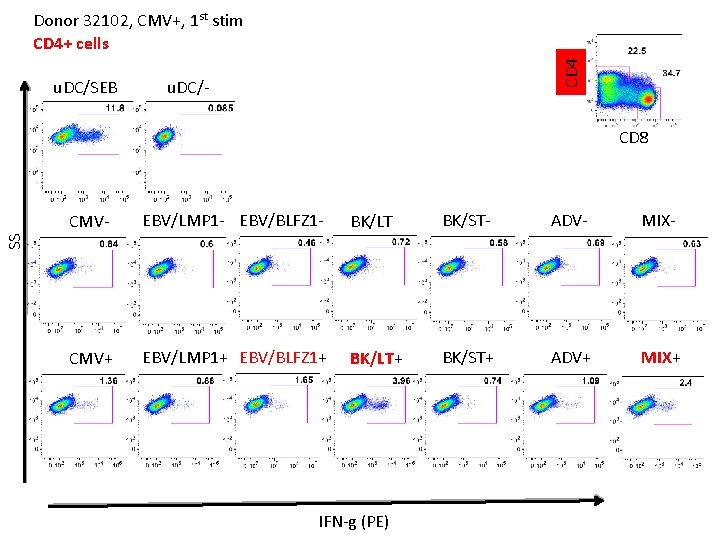
u. DC/SEB CD 4 Donor 32102, CMV+, 1 st stim CD 4+ cells u. DC/- CD 8 EBV/LMP 1 - EBV/BLFZ 1 - BK/LT - BK/ST- ADV- MIX- CMV+ EBV/LMP 1+ EBV/BLFZ 1+ BK/LT+ BK/ST+ ADV+ MIX+ SS CMV- IFN-g (PE)

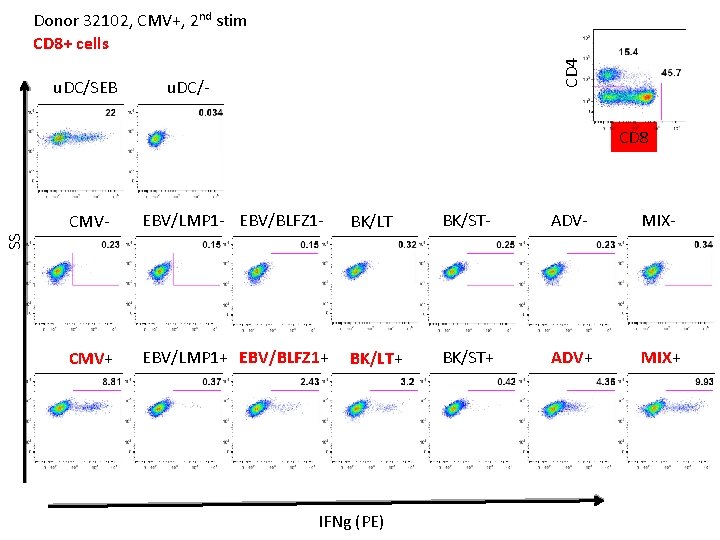
u. DC/SEB CD 4 Donor 32102, CMV+, 2 nd stim CD 8+ cells u. DC/- CD 8 EBV/LMP 1 - EBV/BLFZ 1 - BK/LT - BK/ST- ADV- MIX- CMV+ EBV/LMP 1+ EBV/BLFZ 1+ BK/LT+ BK/ST+ ADV+ MIX+ SS CMV- IFNg (PE)


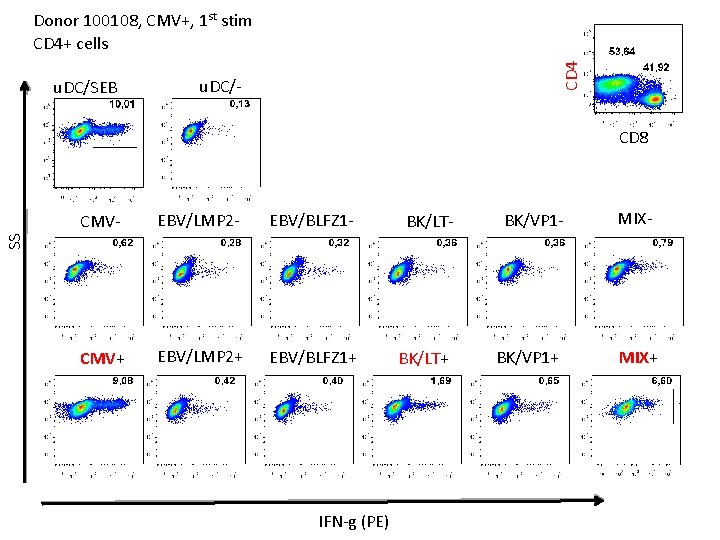
u. DC/SEB CD 4 Donor 100108, CMV+, 1 st stim CD 4+ cells u. DC/- CD 8 EBV/LMP 2 - EBV/BLFZ 1 - BK/LT- BK/VP 1 - CMV+ EBV/LMP 2+ EBV/BLFZ 1+ BK/LT+ BK/VP 1+ MIX- SS CMV- IFN-g (PE) MIX+


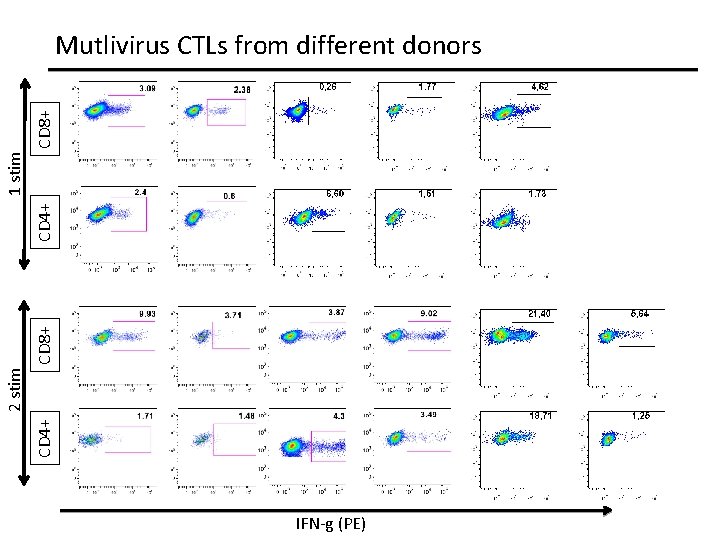
CD 4+ 2 stim CD 8+ CD 4+ 1 stim CD 8+ Mutlivirus CTLs from different donors IFN-g (PE)

The role of CMV in CMV seropos patients

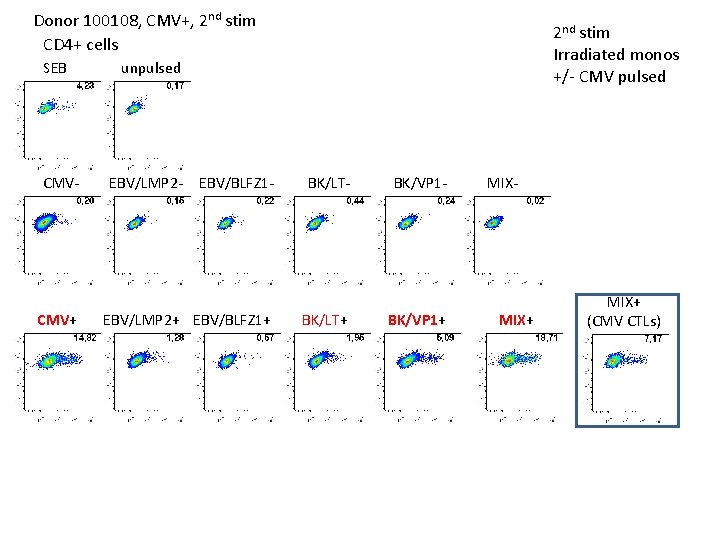
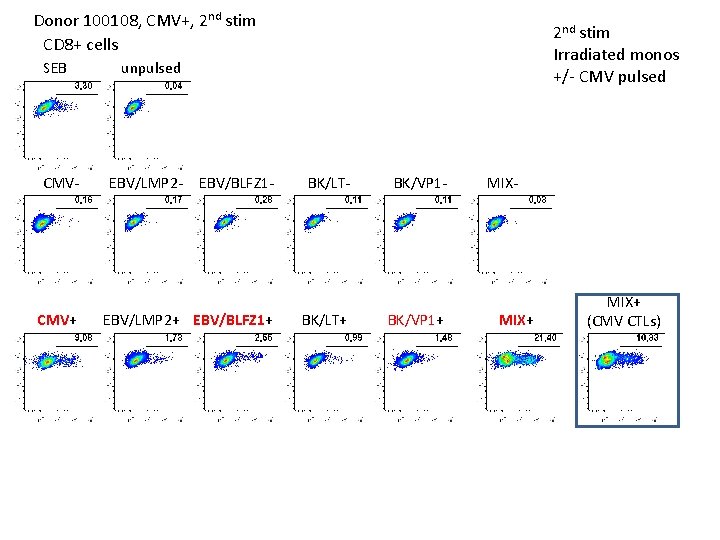
Donor 100108, CMV+, 2 nd stim CD 4+ cells SEB CMV- CMV+ 2 nd stim Irradiated monos +/- CMV pulsed unpulsed EBV/LMP 2 - EBV/BLFZ 1 - EBV/LMP 2+ EBV/BLFZ 1+ BK/LT- BK/LT+ BK/VP 1 - BK/VP 1+ MIX- MIX+ (CMV CTLs)

Donor 100108, CMV+, 2 nd stim CD 8+ cells SEB CMV- CMV+ 2 nd stim Irradiated monos +/- CMV pulsed unpulsed EBV/LMP 2 - EBV/BLFZ 1 - EBV/LMP 2+ EBV/BLFZ 1+ BK/LT- BK/LT+ BK/VP 1 - BK/VP 1+ MIX- MIX+ (CMV CTLs)

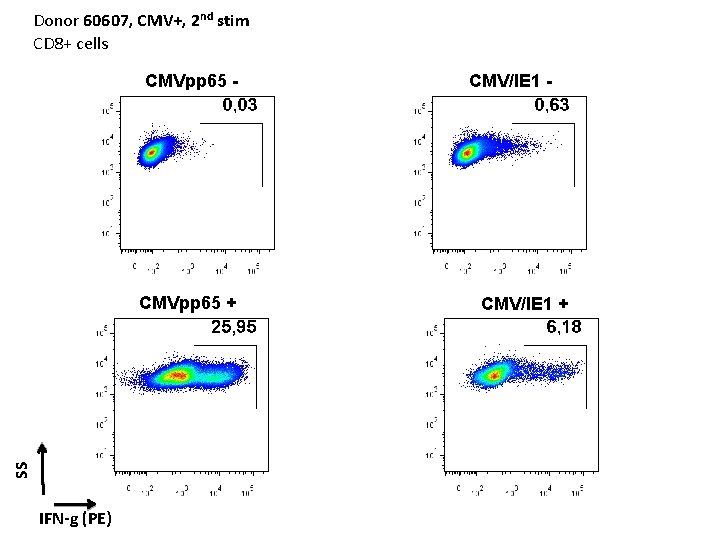
Donor 60607, CMV+, 2 nd stim CD 8+ cells CMVpp 65 - SS CMVpp 65 + IFN-g (PE) CMV/IE 1 - CMV/IE 1 +

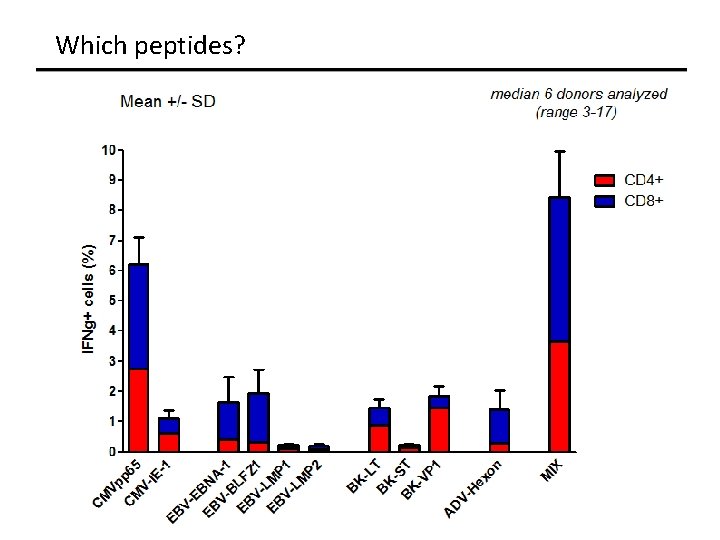
Which peptides?

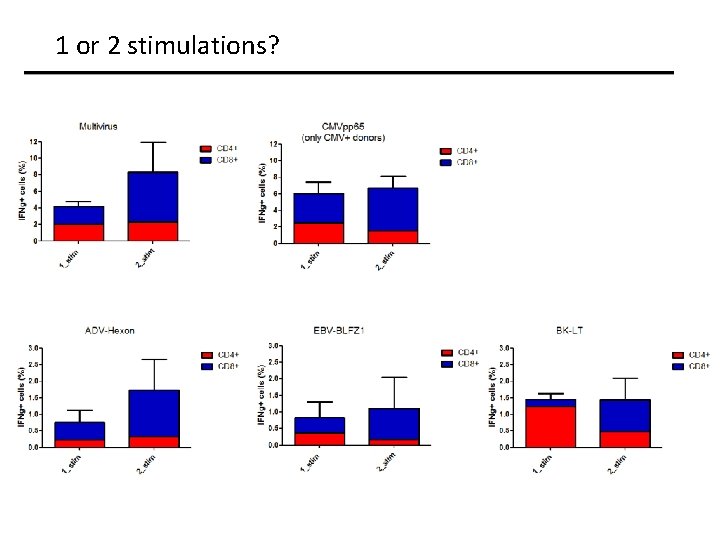
1 or 2 stimulations?

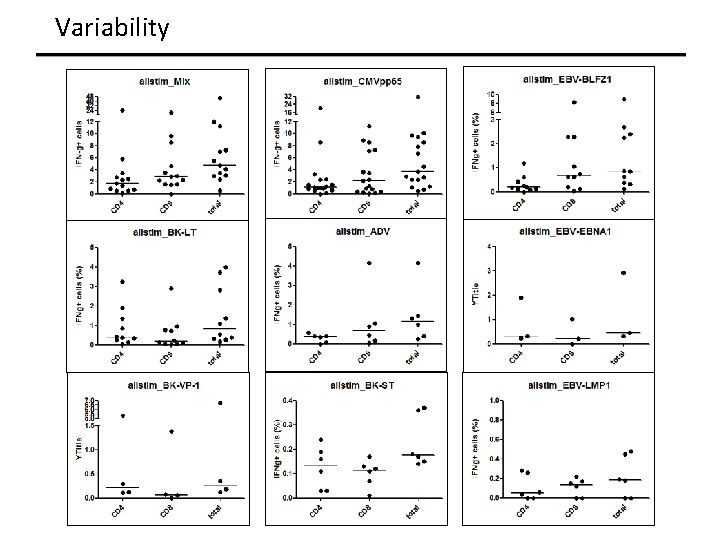
Variability

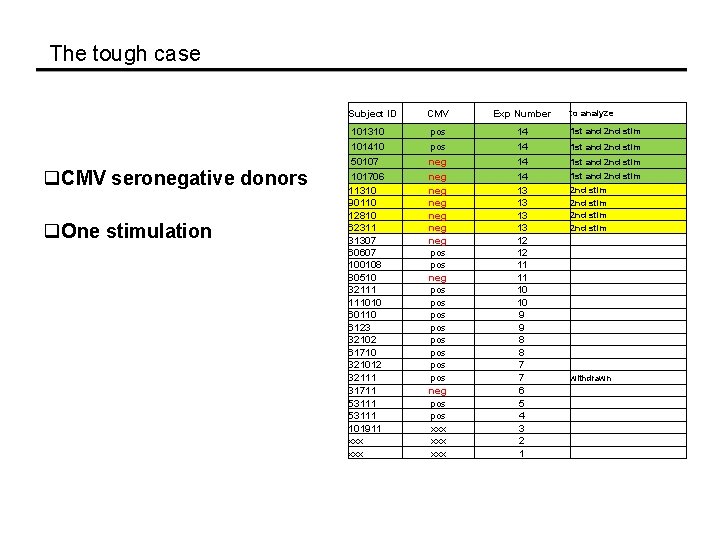
The tough case Subject ID q. CMV seronegative donors q. One stimulation CMV Exp Number 101310 pos 14 1 st and 2 nd stim 101410 50107 101706 11310 90110 12810 62311 31307 60607 100108 80510 32111 111010 60110 6123 32102 61710 321012 32111 31711 53111 101911 xxx pos neg neg pos pos pos neg pos xxx xxx 14 14 14 13 13 12 12 11 11 10 10 9 9 8 8 7 7 6 5 4 3 2 1 1 st and 2 nd stim to analyze 1 st and 2 nd stim 2 nd stim withdrawn

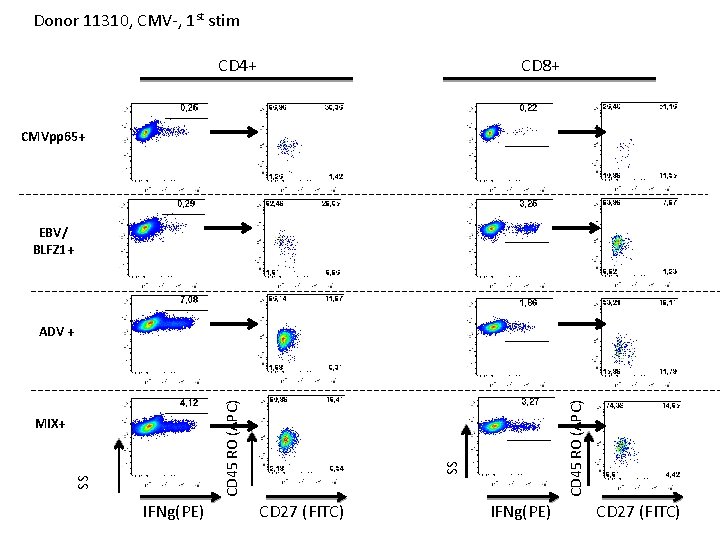
Exp #13 Donor 11310, CMV 1 st stim

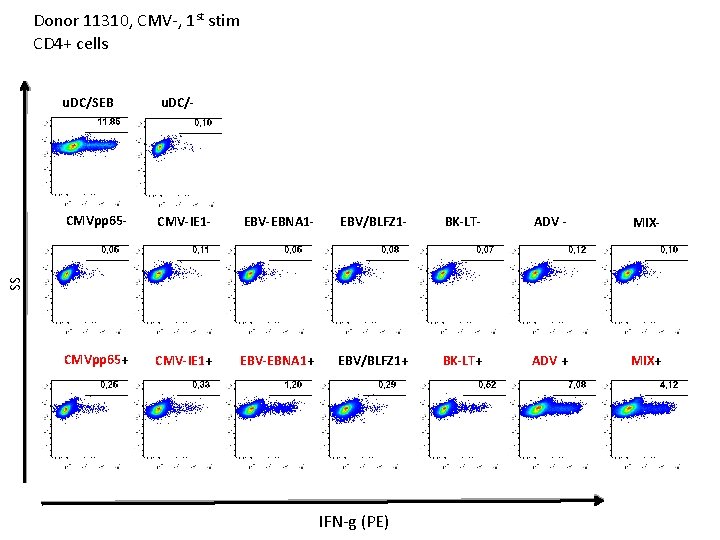
Donor 11310, CMV-, 1 st stim CD 4+ cells u. DC/- CMVpp 65 - CMV-IE 1 - EBV-EBNA 1 - EBV/BLFZ 1 - BK-LT- ADV - MIX- CMVpp 65+ CMV-IE 1+ EBV-EBNA 1+ EBV/BLFZ 1+ BK-LT+ ADV + MIX+ SS u. DC/SEB IFN-g (PE)

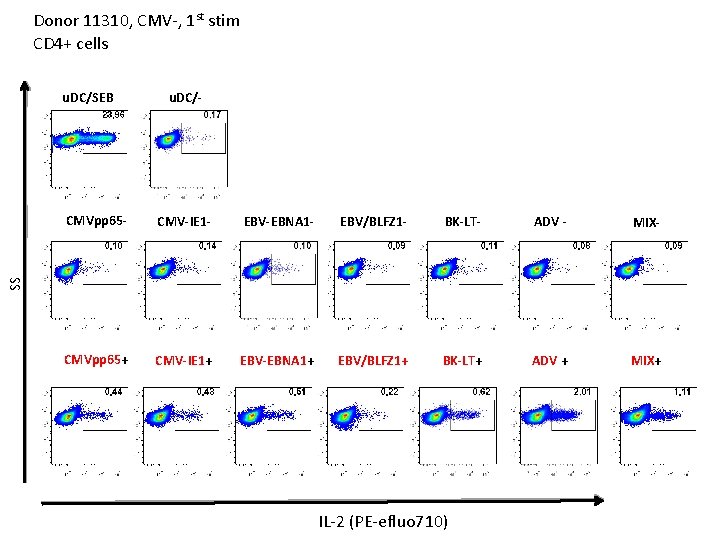
Donor 11310, CMV-, 1 st stim CD 4+ cells u. DC/SEB u. DC/- CMV-IE 1 - EBV-EBNA 1 - EBV/BLFZ 1 - BK-LT- ADV - MIX- CMVpp 65+ CMV-IE 1+ EBV-EBNA 1+ EBV/BLFZ 1+ BK-LT+ ADV + MIX+ SS CMVpp 65 - IL-2 (PE-efluo 710)

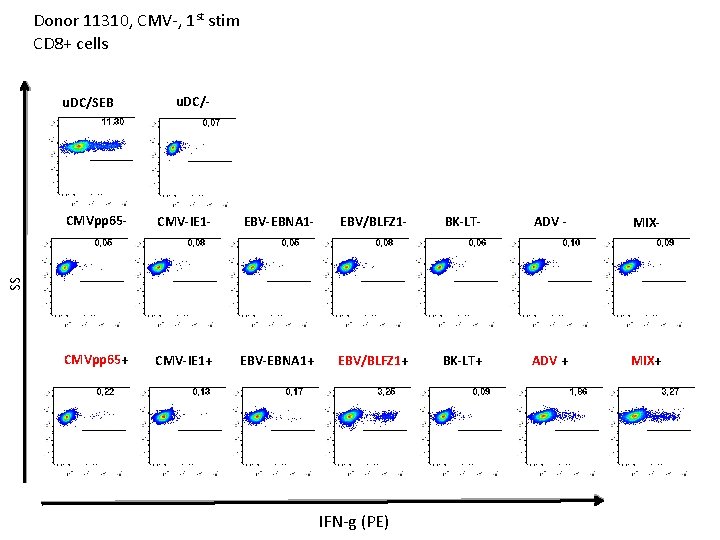
Donor 11310, CMV-, 1 st stim CD 8+ cells u. DC/SEB u. DC/- CMV-IE 1 - EBV-EBNA 1 - EBV/BLFZ 1 - BK-LT- ADV - MIX- CMVpp 65+ CMV-IE 1+ EBV-EBNA 1+ EBV/BLFZ 1+ BK-LT+ ADV + MIX+ SS CMVpp 65 - IFN-g (PE)

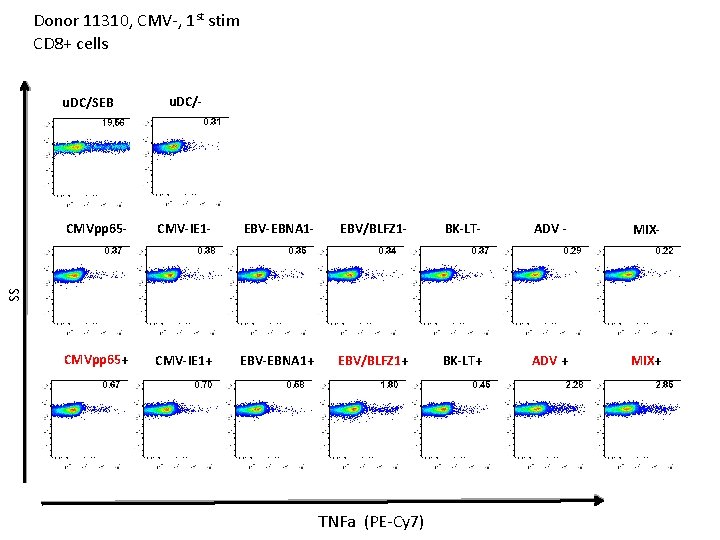
Donor 11310, CMV-, 1 st stim CD 8+ cells u. DC/SEB u. DC/- CMV-IE 1 - EBV-EBNA 1 - EBV/BLFZ 1 - BK-LT- ADV - MIX- CMVpp 65+ CMV-IE 1+ EBV-EBNA 1+ EBV/BLFZ 1+ BK-LT+ ADV + MIX+ SS CMVpp 65 - TNFa (PE-Cy 7)

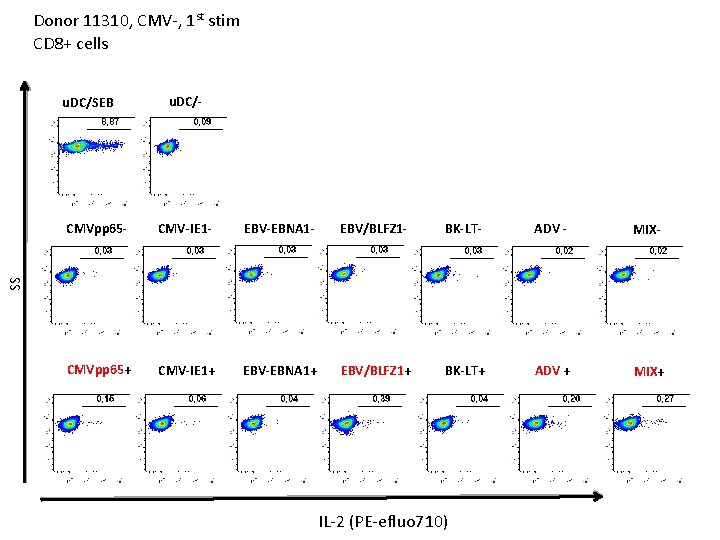
Donor 11310, CMV-, 1 st stim CD 8+ cells u. DC/SEB u. DC/- CMV-IE 1 - EBV-EBNA 1 - EBV/BLFZ 1 - BK-LT- ADV - MIX- CMVpp 65+ CMV-IE 1+ EBV-EBNA 1+ EBV/BLFZ 1+ BK-LT+ ADV + MIX+ SS CMVpp 65 - IL-2 (PE-efluo 710)

Donor 11310, CMV-, 1 st stim CD 4+ CD 8+ CMVpp 65+ EBV/ BLFZ 1+ IFNg(PE) CD 45 RO (APC) SS MIX+ SS CD 45 RO (APC) ADV + CD 27 (FITC) IFNg(PE) CD 27 (FITC)

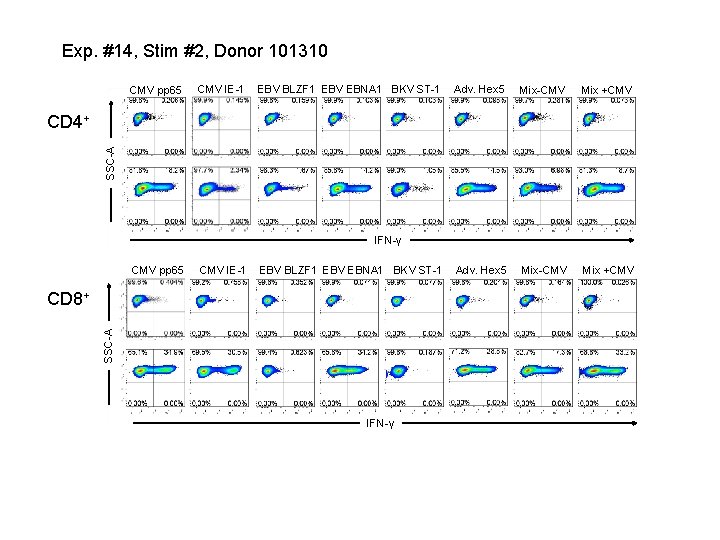
Exp. #14, Stim #2, Donor 101310 CMV pp 65 CMV IE-1 EBV BLZF 1 EBV EBNA 1 BKV ST-1 Adv. Hex 5 Mix-CMV Mix +CMV SSC-A CD 4+ IFN-γ CMV pp 65 CMV IE-1 EBV BLZF 1 EBV EBNA 1 BKV ST-1 SSC-A CD 8+ IFN-γ

Exp. #14, Stim #2, Donor 101310 CMV pp 65 CMV IE-1 EBV BLZF 1 EBV EBNA 1 BKV ST-1 Adv. Hex 5 Mix-CMV Mix +CMV SSC-A CD 4+ TNF-α CMV pp 65 CMV IE-1 EBV BLZF 1 EBV EBNA 1 BKV ST-1 SSC-A CD 8+ TNF-α Adv. Hex 5 Mix-CMV Mix +CMV


Exp #13 Donor 012180, CMV 1 st stim

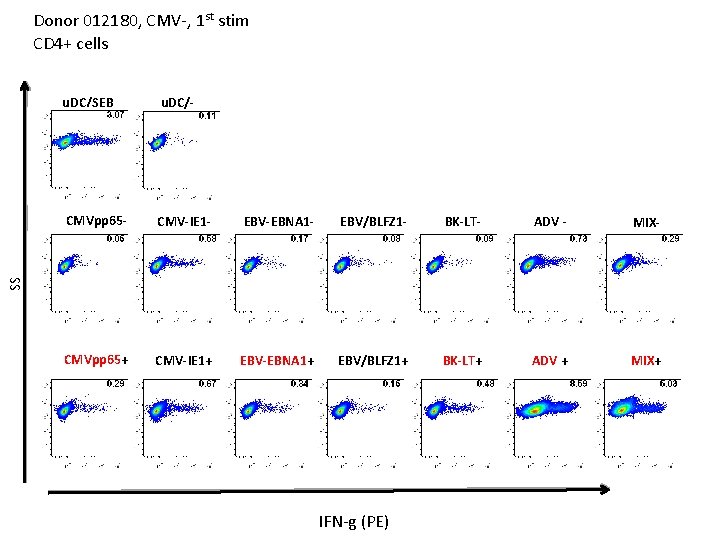
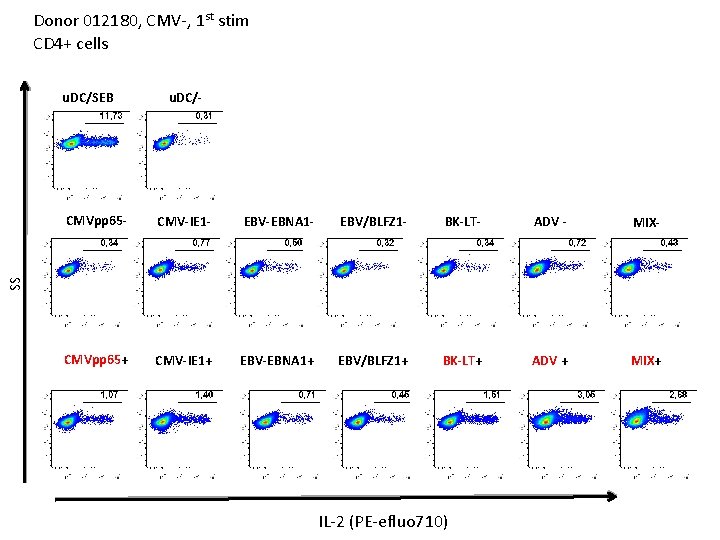
Donor 012180, CMV-, 1 st stim CD 4+ cells u. DC/- CMVpp 65 - CMV-IE 1 - EBV-EBNA 1 - EBV/BLFZ 1 - BK-LT- ADV - MIX- CMVpp 65+ CMV-IE 1+ EBV-EBNA 1+ EBV/BLFZ 1+ BK-LT+ ADV + MIX+ SS u. DC/SEB IFN-g (PE)

Donor 012180, CMV-, 1 st stim CD 4+ cells u. DC/SEB u. DC/- CMV-IE 1 - EBV-EBNA 1 - EBV/BLFZ 1 - BK-LT- ADV - MIX- CMVpp 65+ CMV-IE 1+ EBV-EBNA 1+ EBV/BLFZ 1+ BK-LT+ ADV + MIX+ SS CMVpp 65 - IL-2 (PE-efluo 710)

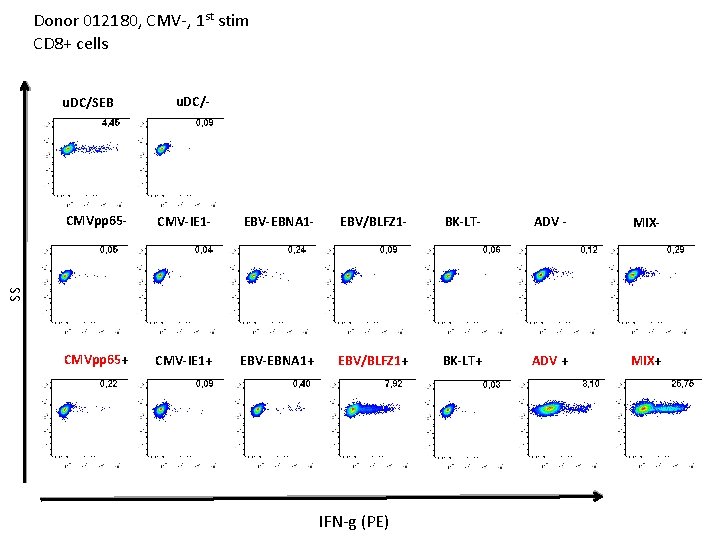
Donor 012180, CMV-, 1 st stim CD 8+ cells u. DC/SEB u. DC/- CMV-IE 1 - EBV-EBNA 1 - EBV/BLFZ 1 - BK-LT- ADV - MIX- CMVpp 65+ CMV-IE 1+ EBV-EBNA 1+ EBV/BLFZ 1+ BK-LT+ ADV + MIX+ SS CMVpp 65 - IFN-g (PE)

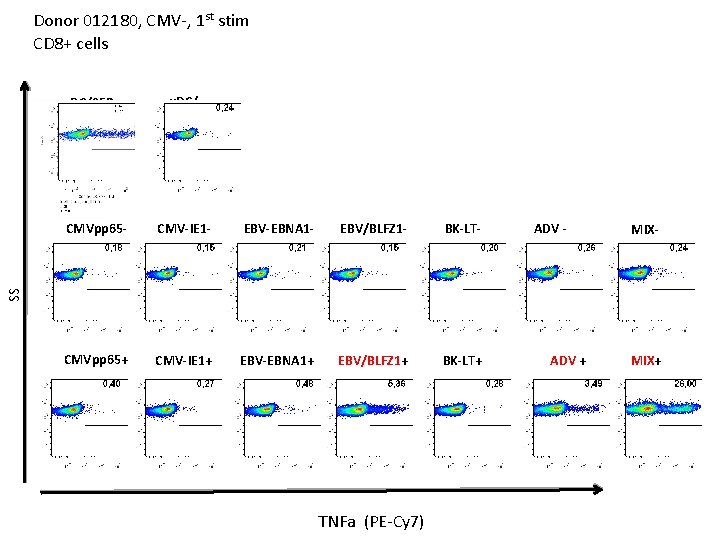
Donor 012180, CMV-, 1 st stim CD 8+ cells u. DC/SEB u. DC/- CMV-IE 1 - EBV-EBNA 1 - EBV/BLFZ 1 - BK-LT- CMVpp 65+ CMV-IE 1+ EBV-EBNA 1+ EBV/BLFZ 1+ BK-LT+ ADV - MIX- SS CMVpp 65 - TNFa (PE-Cy 7) ADV + MIX+

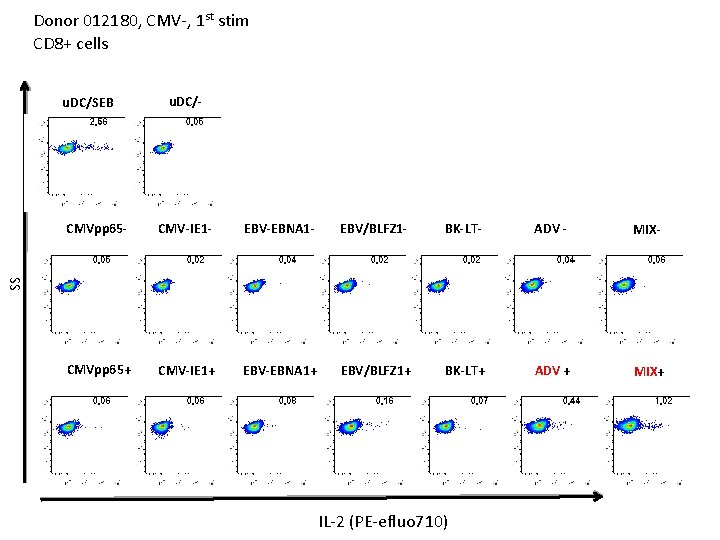
Donor 012180, CMV-, 1 st stim CD 8+ cells u. DC/SEB u. DC/- CMV-IE 1 - EBV-EBNA 1 - EBV/BLFZ 1 - BK-LT- ADV - MIX- CMVpp 65+ CMV-IE 1+ EBV-EBNA 1+ EBV/BLFZ 1+ BK-LT+ ADV + MIX+ SS CMVpp 65 - IL-2 (PE-efluo 710)


Exp #13 Donor 062311, CMV 1 st stim

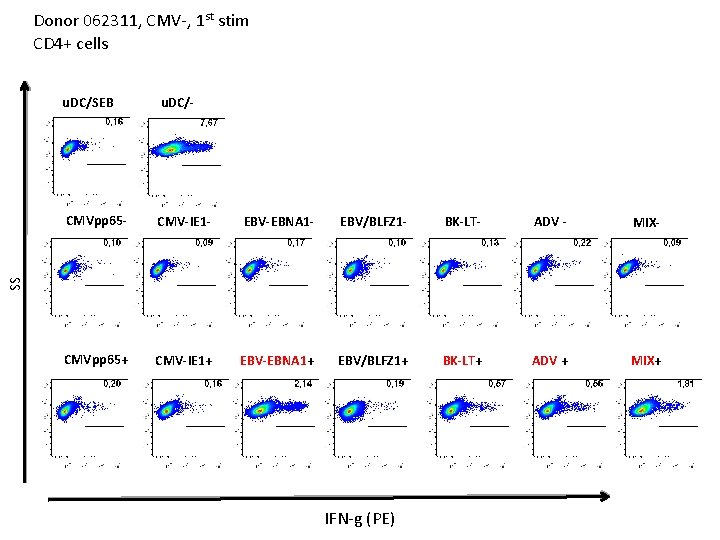
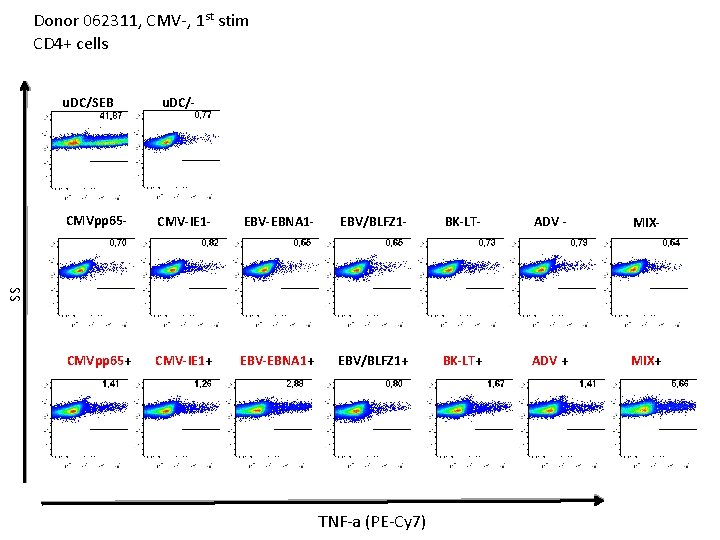
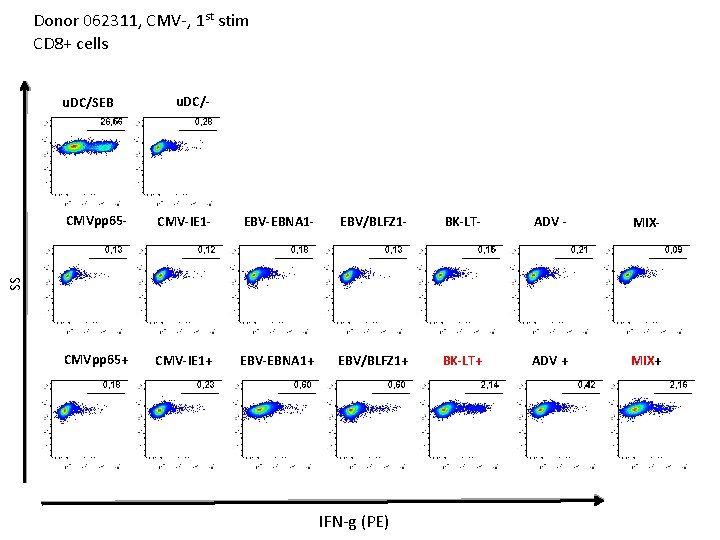
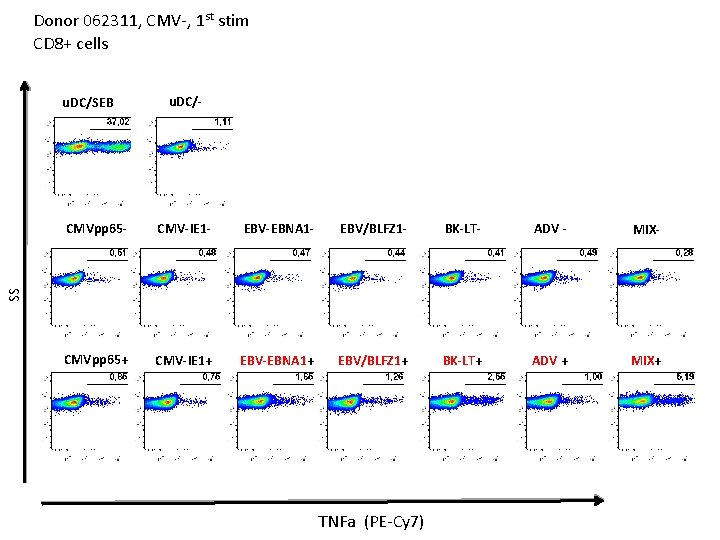
Donor 062311, CMV-, 1 st stim CD 4+ cells u. DC/- CMVpp 65 - CMV-IE 1 - EBV-EBNA 1 - EBV/BLFZ 1 - BK-LT- ADV - MIX- CMVpp 65+ CMV-IE 1+ EBV-EBNA 1+ EBV/BLFZ 1+ BK-LT+ ADV + MIX+ SS u. DC/SEB IFN-g (PE)

Donor 062311, CMV-, 1 st stim CD 4+ cells u. DC/SEB u. DC/- CMV-IE 1 - EBV-EBNA 1 - EBV/BLFZ 1 - BK-LT- ADV - MIX- CMVpp 65+ CMV-IE 1+ EBV-EBNA 1+ EBV/BLFZ 1+ BK-LT+ ADV + MIX+ SS CMVpp 65 - TNF-a (PE-Cy 7)

Donor 062311, CMV-, 1 st stim CD 8+ cells u. DC/SEB u. DC/- CMV-IE 1 - EBV-EBNA 1 - EBV/BLFZ 1 - BK-LT- ADV - MIX- CMVpp 65+ CMV-IE 1+ EBV-EBNA 1+ EBV/BLFZ 1+ BK-LT+ ADV + MIX+ SS CMVpp 65 - IFN-g (PE)

Donor 062311, CMV-, 1 st stim CD 8+ cells u. DC/SEB u. DC/- CMV-IE 1 - EBV-EBNA 1 - EBV/BLFZ 1 - BK-LT- ADV - MIX- CMVpp 65+ CMV-IE 1+ EBV-EBNA 1+ EBV/BLFZ 1+ BK-LT+ ADV + MIX+ SS CMVpp 65 - TNFa (PE-Cy 7)


Exp #13 Donor 090110, CMV 1 st stim

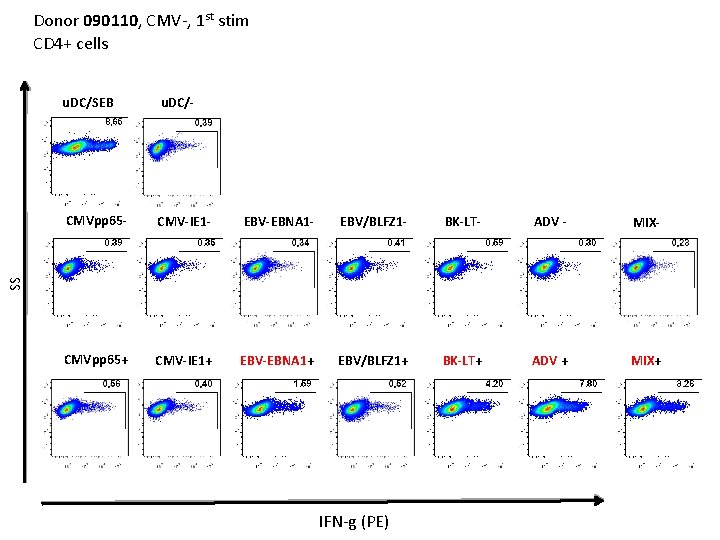
Donor 090110, CMV-, 1 st stim CD 4+ cells u. DC/- CMVpp 65 - CMV-IE 1 - EBV-EBNA 1 - EBV/BLFZ 1 - BK-LT- ADV - MIX- CMVpp 65+ CMV-IE 1+ EBV-EBNA 1+ EBV/BLFZ 1+ BK-LT+ ADV + MIX+ SS u. DC/SEB IFN-g (PE)

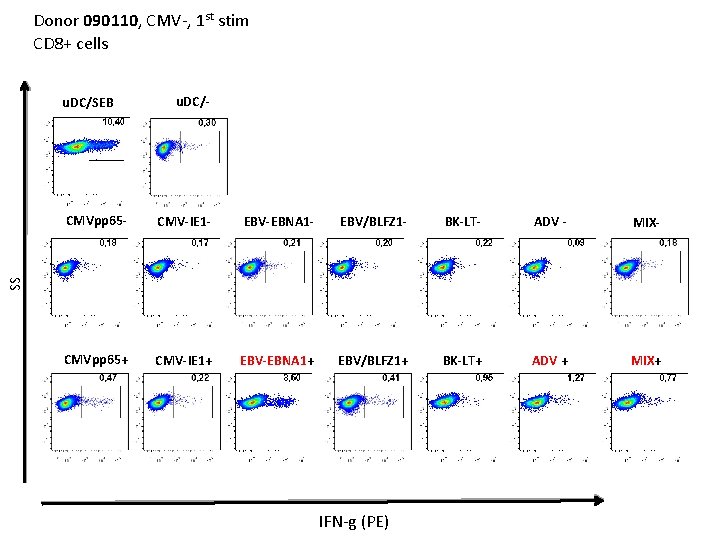
Donor 090110, CMV-, 1 st stim CD 8+ cells u. DC/SEB u. DC/- CMV-IE 1 - EBV-EBNA 1 - EBV/BLFZ 1 - BK-LT- ADV - MIX- CMVpp 65+ CMV-IE 1+ EBV-EBNA 1+ EBV/BLFZ 1+ BK-LT+ ADV + MIX+ SS CMVpp 65 - IFN-g (PE)

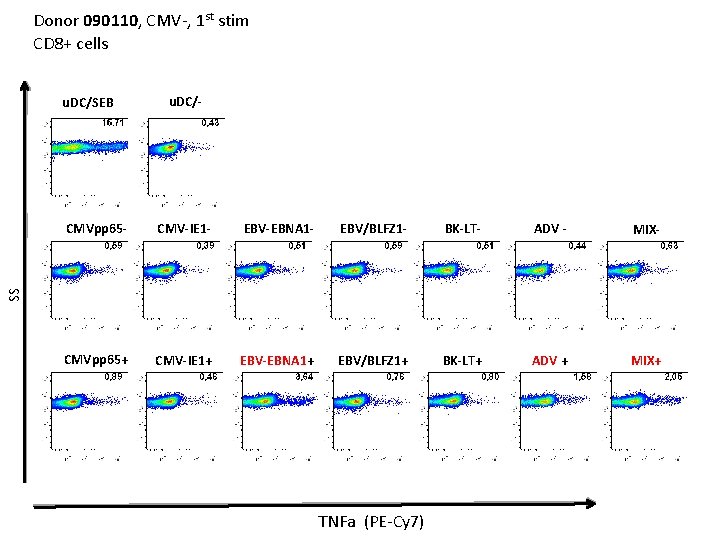
Donor 090110, CMV-, 1 st stim CD 8+ cells u. DC/SEB u. DC/- CMV-IE 1 - EBV-EBNA 1 - EBV/BLFZ 1 - BK-LT- ADV - MIX- CMVpp 65+ CMV-IE 1+ EBV-EBNA 1+ EBV/BLFZ 1+ BK-LT+ ADV + MIX+ SS CMVpp 65 - TNFa (PE-Cy 7)


Reproducibility

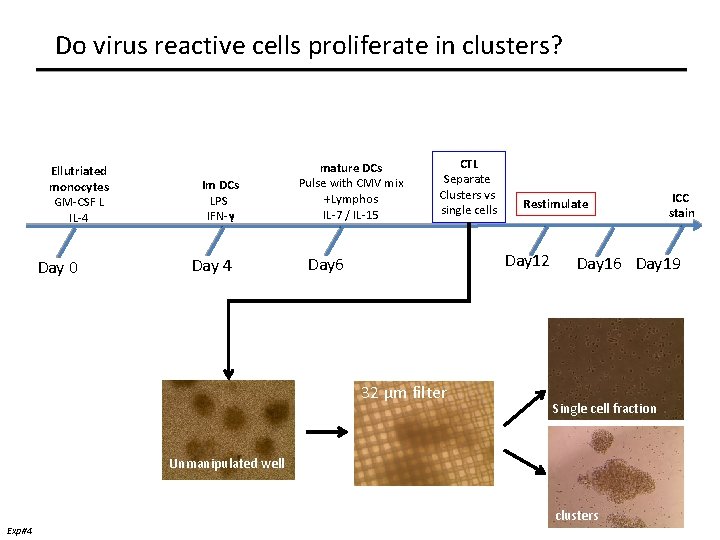
Do virus reactive cells proliferate in clusters? Ellutriated monocytes GM-CSF L IL-4 Day 0 Im DCs LPS IFN-γ Day 4 mature DCs Pulse with CMV mix +Lymphos IL-7 / IL-15 CTL Separate Clusters vs single cells Restimulate Day 12 Day 6 32 μm filter Day 16 Day 19 Single cell fraction Unmanipulated well Exp#4 ICC stain clusters

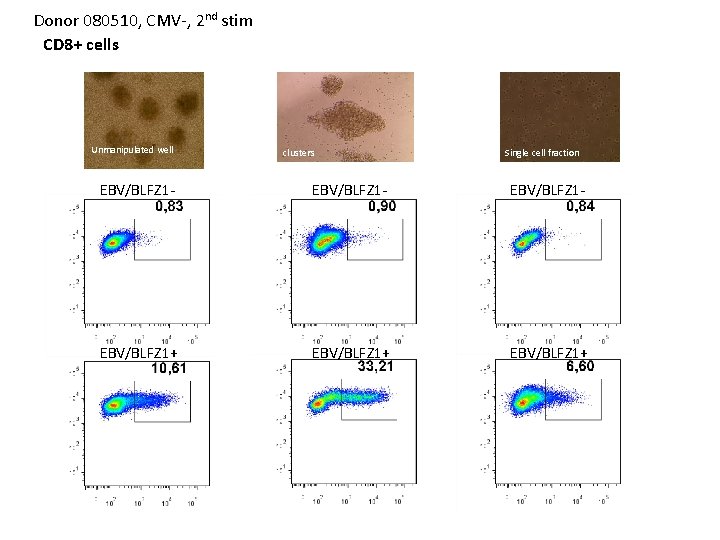
Donor 080510, CMV-, 2 nd stim CD 8+ cells Unmanipulated well clusters Single cell fraction EBV/BLFZ 1 - EBV/BLFZ 1+

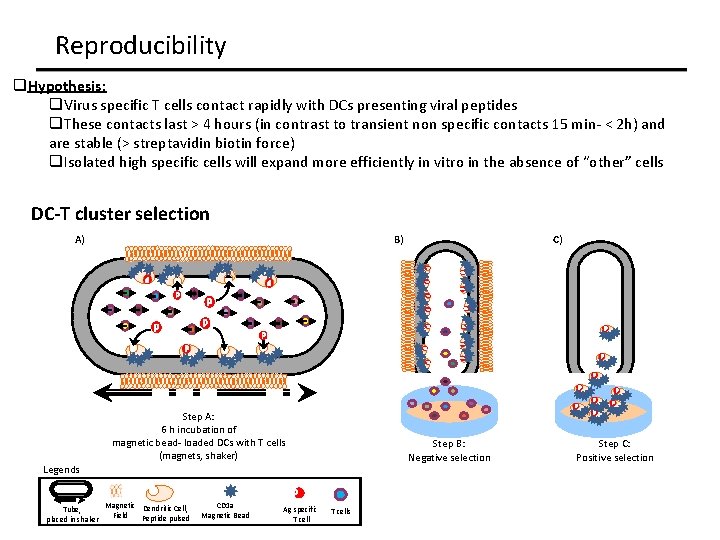
Reproducibility q. Hypothesis: q. Virus specific T cells contact rapidly with DCs presenting viral peptides q. These contacts last > 4 hours (in contrast to transient non specific contacts 15 min- < 2 h) and are stable (> streptavidin biotin force) q. Isolated high specific cells will expand more efficiently in vitro in the absence of “other” cells DC-T cluster selection A) B) Step A: 6 h incubation of magnetic bead- loaded DCs with T cells (magnets, shaker) Step B: Negative selection Legends Tube, placed in shaker Magnetic Dendritic Cell, Field Peptide pulsed CD 1 a Magnetic Bead Ag specific T cell C) T cells Step C: Positive selection

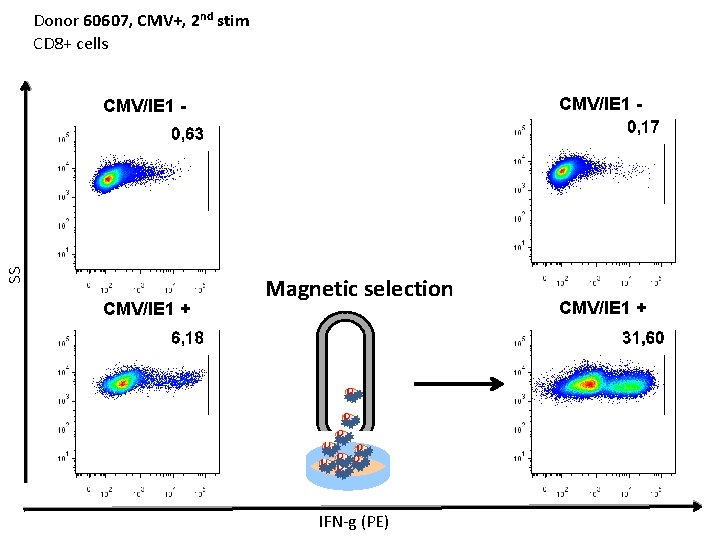
Donor 60607, CMV+, 2 nd stim CD 8+ cells CMV/IE 1 - SS CMV/IE 1 - CMV/IE 1 + Magnetic selection IFN-g (PE) CMV/IE 1 +

Next steps q. Scale up, GMP production q. Test alloreactivity q. Evaluate methods of reproducibility, eg through in vitro selection q. Evaluate whether donor markers can predict results of in vitro expansion (preevaluation of virus cells in donors? ) q. Phase I/II clinical trial

 Alexandros spyridonidis
Alexandros spyridonidis Alexandros potamianos
Alexandros potamianos Potamianos ntua
Potamianos ntua Exp orig
Exp orig Aminophyllini
Aminophyllini Flucinari
Flucinari Scat
Scat David luca scat
David luca scat Brianmac scat test
Brianmac scat test Klavocin bid 1g
Klavocin bid 1g Fishing cat scat
Fishing cat scat Waiting line management system
Waiting line management system Multi loop pid controller regolatore pid multi loop
Multi loop pid controller regolatore pid multi loop Masses of cells form and steal nutrients from healthy cells
Masses of cells form and steal nutrients from healthy cells Haploid and diploid venn diagram
Haploid and diploid venn diagram Nondisjunction in meiosis
Nondisjunction in meiosis Venn diagram for animal and plant cells
Venn diagram for animal and plant cells Younger cells cuboidal older cells flattened
Younger cells cuboidal older cells flattened Somatic vs germ cells
Somatic vs germ cells Cells and life lesson 1 answer key
Cells and life lesson 1 answer key Prokaryotic vs eukaryotic cells venn diagram
Prokaryotic vs eukaryotic cells venn diagram Paranasal sinuses development
Paranasal sinuses development 4 types of eukaryotic cells
4 types of eukaryotic cells Red blood cells and white blood cells difference
Red blood cells and white blood cells difference The organelle trail
The organelle trail Thyroid gland
Thyroid gland Regulation of tubular reabsorption
Regulation of tubular reabsorption Which organisms are prokaryotes
Which organisms are prokaryotes Prokaryotic vs eukaryotic
Prokaryotic vs eukaryotic Weight = specific gravity x volume
Weight = specific gravity x volume Specific volume
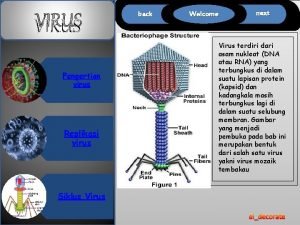
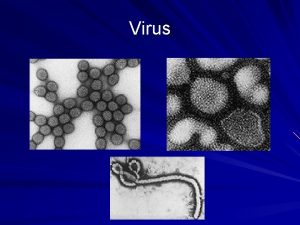
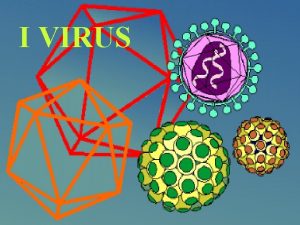
Specific volume Virus
Virus Mumps virus
Mumps virus Vbscript virus
Vbscript virus Xxx virus
Xxx virus Ciclo litico de un virus
Ciclo litico de un virus U n i x
U n i x Origen de los virus
Origen de los virus Prophylaxis of rabies ppt
Prophylaxis of rabies ppt Siklus virus
Siklus virus Mumps virus
Mumps virus Tuberculose é um virus
Tuberculose é um virus John trifiletti
John trifiletti Virus machupo
Virus machupo Rimantidina
Rimantidina Flash belleğe virüs bulaşmasını engelleme
Flash belleğe virüs bulaşmasını engelleme Jitender mehla
Jitender mehla Dermotropos
Dermotropos Virus del policia
Virus del policia Neuralgische schulteramyotrophie rückfall
Neuralgische schulteramyotrophie rückfall Sofiacruzv
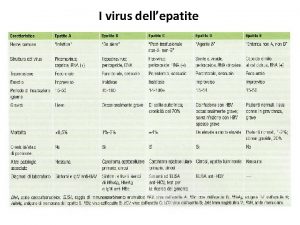
Sofiacruzv Virus vhe
Virus vhe Complex virus
Complex virus Metabolismo heterótrofo
Metabolismo heterótrofo West nyle virus
West nyle virus Icosahedral adalah
Icosahedral adalah Ctrl c ctrl v
Ctrl c ctrl v Virus virion
Virus virion Pico naked
Pico naked Virus complejo supramolecular
Virus complejo supramolecular