MTN003 StudySpecific Training Selected Clinical Considerations Overview of
















































- Slides: 102

MTN-003 Study-Specific Training Selected Clinical Considerations

Overview of Presentation l l l l l Medical/menstrual history Concomitant medications Vital signs and physical exams Pelvic exams Genital bleeding assessment STI/RTI management Urinary tract infections Pregnancy management HIV management

Medical/Menstrual History l Baseline l l l Purpose is to establish eligibility AND document baseline medical condition, for comparison with condition during follow-up “Focused” history since participant became sexually active (lifetime history not required) Probe for most accurate information possible on current health status vis-à-vis the reported history

Medical/Menstrual History l Baseline l l Use listing of body systems on the Baseline Medical and Menstrual History form (recommended source document) to probe for history related to each system Record symptoms, illnesses, allergies, surgeries Record chronic and acute conditions Record resolved and ongoing conditions l Details on gastrointestinal and urinary symptoms will be especially important

Medical/Menstrual History l Baseline l For genital symptoms, probe for and record as much detail as possible l Genital sores l Genital/vaginal itching l Genital/vaginal burning l Genital/vaginal pain l Pain during sex l Abnormal genital/vaginal discharge l Unusual genital/vaginal odor l Other genital symptoms (specify)

Medical/Menstrual History l Baseline l For menstrual history l Age at menarche l First and last day of last period l Usual menstrual cycle § If amenorrheic expected or unexpected l Usual menstrual flow l Usual menstrual symptoms l Usual non-menstrual bleeding l Any other menstrual problems

Medical/Menstrual History l Baseline l For reproductive history l Contraceptive history l Pregnancies and outcomes l Obstetric, gynecologic, and/or other reproductive problems, procedures, surgeries l Sexual assault

Medical/Menstrual History l Baseline l l Complete Concomitant Medications Log form (recommended source document) in tandem with medical/menstrual history: l Prescription medications/preparations l Non-prescription medications/preparations l All routes of administration, including topical and intravaginal l Vitamins, other nutritional supplements, herbal, naturopathic, and traditional preparations Similarly complete Contraceptives Log form

Medical/Menstrual History l Baseline l l l Cross-reference concomitant medication information with medical/menstrual history Probe for any medications taken for all ongoing symptoms/illnesses/conditions Pay special attention to symptoms likely to recur during study participation (e. g. , dysmenorrhea, intermenstrual spotting)

Medical/Menstrual History l Follow-up l l l Performed at all scheduled visits and at interim visits if participant presents with symptoms “Interval” since last visit Follow-up Medical and Menstrual History form is recommended source document Refer to previously completed history forms and update from there Cross-reference Monthly Symptoms form which is administered before history taking

Medical/Menstrual History l Follow-up l l Not necessary to actively review and ask about each body system For most systems l Ask about current status of previously-reported conditions l Ask about symptoms reported on the Monthly Symptoms form (make sure details match) l Then ask open-ended question such as “have you had any other symptoms or problems since your last visit? ” to complete the history

Medical/Menstrual History l Follow-up l l l For genital symptoms l Actively ask about each symptom listed on the Follow -up Medical and Menstrual History form For menstrual information l Record dates of last menstrual period § If no menses since last visit expected or unexpected For other reproductive history information l Record any updates / changes since last visit

Medical/Menstrual History l Follow-up l l l Update Concomitant Medications Log form (recommended source document) in tandem with interval history Specifically ask if each prior medication still being taken Specifically ask if medications taken for any new symptoms or illnesses reported in interval history Generally ask if any new medications taken since last visit Similarly update Contraceptives Log form when applicable

Physical Exam l Required at l l l Screening Part 2 ● Product Use End Visit (PUEV) Month 1 ● When clinically indicated Quarterly Physical Exam form is recommended source document For any observed abnormalities, cross reference with medical history and concomitant medications and probe for additional information as needed

Physical Exam l Vital signs l l l Height Weight Oral temperature Blood pressure Pulse Respirations l Clinical assessments of l l l Head and eyes Ears, nose, and throat Neck Lymph nodes Heart Lungs Abdomen Extremities Neurological Skin Breasts Other assessments at discretion of examining clinician

Weight l Measured as part of all physical exams and whenever blood is drawn for creatinine testing l Needed to calculate Cr. Cl rate l Measure in kilograms and round to nearest whole number l Calibrate scale twice per year (more frequently if required by local standards)

Weight l l Consistent weighing procedures should be followed for all participants at all time points l Each site may choose to consistently weigh participants fully clothed or wearing clinic gowns l Sites with seasonal weather variations should consider using gowns or adopting other procedures to ensure that accurate weights are measured throughout the year Monitor for unintended weight loss and grade severity per DAIDS Toxicity Table

Height l l Measured as part of physical exams at Screening Part 2, Semi-Annually, and at PUEV Measure in centimeters l Do not use device on weight scale l Use wall chart l Use consistent procedures across participants and over time (e. g. , remove shoes)

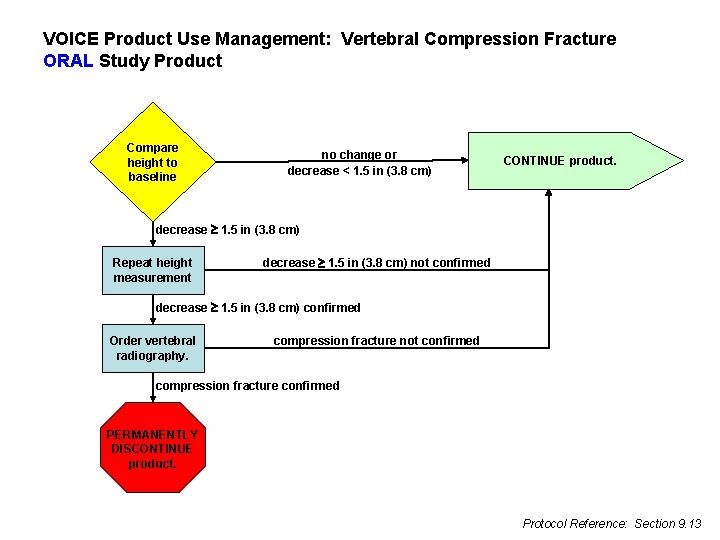
Height l If decrease of 3. 8 cm is detected, repeat measurement for confirmation l If confirmed, order x-ray to assess for vertebral compression fracture

Some Challenges?

Solution

Blood Pressure l l Measured as part of all physical exams Should also be measured at other visits to monitor participants with abnormal pressures Hypertension (>140 systolic, >90 diastolic) should be confirmed with repeat measurement at the same visit Grade severity per DAIDS Toxicity Table

Blood Pressure l VOICE sites should ideally provide antihypertensive treatment for study participants when clinically indicated l l l Participants with grade 3 and higher hypertension should be counseled and treated Participants with grade 1 and 2 hypertension should be counseled and may be treated at Io. R discretion Also consider hypertension in the context of contraceptive choice

Blood Pressure l Potential participants with significant uncontrolled hypertension during screening may not be immediately enrolled but may be enrolled after control is established and documented l l May require a second screening attempt Antihypertensives, including thiazide diuretics and ACE inhibitors, are not contraindicated in VOICE l l l PSRT available for consultation Consider re-checking creatinine for confirmation of eligibility if antihypertensives started during screening Otherwise follow local standard of care for monitoring and treatment

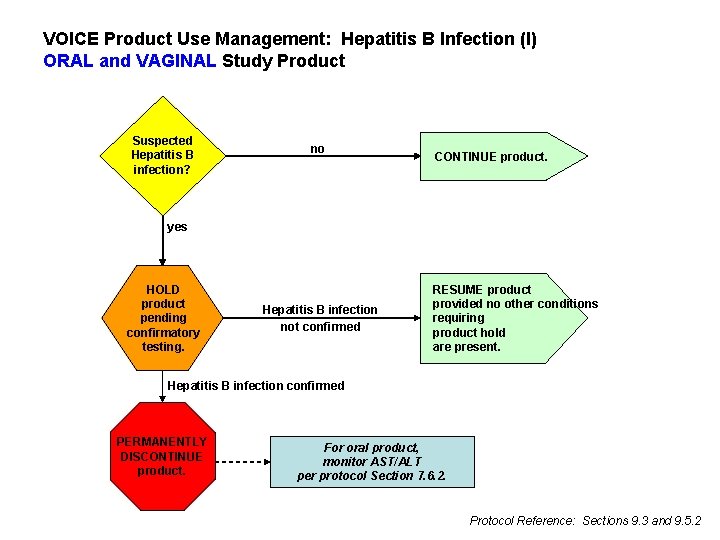
Hepatitis B l All participants undergo testing for Hepatitis B surface antigen (HBs. Ag) and surface antibody (HBs. Ab) at Screening Part 1 l HBs. Ag+ not eligible counsel and refer l HBs. Ag- and HBs. Ab- Hep B susceptible l HBs. Ag- and HBs. Ab+ not Hep B susceptible l If susceptible and enroll, offer vaccine If not susceptible, vaccine not indicated l

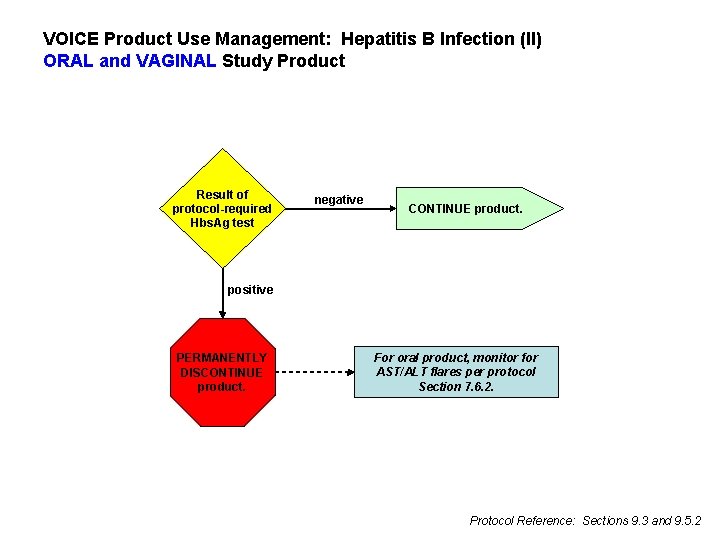
Hepatitis B l l If susceptible and enroll and accept vaccine, ideally administer first vaccination at enrollment and second and third vaccinations at study Months 1 and 6 If susceptible and enroll and decline vaccine l l Continue to offer vaccination throughout follow-up Perform HBs. Ag testing annually (All participants have HBs. Ag testing at PUEV) If assigned to oral study product, additionally perform HBs. Ag testing six months after PUEV

Pelvic Exam Performed at Screening Part 2, Semi-Annually, and at PUEV, per l l l WHO/CONRAD Manual MTN-003 SSP Manual checklists Pelvic exam training video Pay careful attention to which evaluations are required at all exams, which are required at some but not all exams, and which are required only when clinically indicated

Pelvic Exam l l Ideally use two-person team: examining clinician and assistant Ensure all possibly-required supplies and paperwork are easily accessible in exam room Review specimen collection requirements for each visit in preparation for each exam Pay careful attention to the required sequence of swab collection and required handling of each swab l Especially important to use the correct swab for the BV rapid test (cotton swab from kit)

Pelvic Exam l Vaginal fluid specimen collection requirements l l l For rapid tests and wet mount – lateral vaginal wall and posterior fornix are acceptable For archive – posterior fornix only For p. H assessment – lateral vaginal wall only l Swab fluids then apply to p. H strip

Pelvic Exam Terminology l To document findings, use terms from the FGGT and the pelvic exam case report forms l l When the term from the case report form is more specific than the term from the FGGT, use the term from the case report form Use routine QC/QA opportunities to help ensure consistency of terminology across staff and exams

Pap Smear Management l Perform at Screening Part 2, at PUEV, and when clinically indicated and/or per local standard of care l l Note: Women with a documented normal result within the 12 months prior to enrollment need not have Pap smear during the screening period. Manage results per 2006 Consensus Guidelines for the Management of Women with Abnormal Cervical Cancer Screening Tests and local guidelines

Pap Smear Management l l l During screening, Grade 2 (HSIL) and higher grade results are exclusionary For all abnormal results during screening, note exclusion for gynecologic procedures within 42 days of enrollment Note: Women with abnormal Pap smears can be enrolled upon completion of the initial phase of evaluation if no treatment is indicated … need for a repeat Pap within 6 months does not preclude enrollment prior to result becoming available.

Pap Smear Management l During follow-up, HSIL and more severe abnormalities require l l l Temporary hold of vaginal study product No change for oral study product See protocol Section 9. 8

Genital Bleeding Assessment l l l Non-menstrual genital bleeding is common, especially in the context of recent uptake of hormonal contraception Refer to SSP Section 10 for procedures, algorithms, and terminology to standardize assessment of genital bleeding across sites All genital bleeding must be assessed and documented as: l Menstrual or non-menstrual l Expected or unexpected

Genital Bleeding Assessment l Expected bleeding is considered normal l l Menses Early menses Intermenstrual bleeding explained by contraceptive method Expected / explained absence of menses is also considered normal

Genital Bleeding Assessment l Unexpected bleeding is considered abnormal l l Metrorrhagia = intermenstrual bleeding Menorrhagia = regularly timed but prolonged and/or heavy menstrual bleeding Menometrorrhagia = prolonged menstrual bleeding occurring at irregular intervals Bleeding during pregnancy Unexpected / unexplained infrequent bleeding (missed menses, oligomenorrhea, amenorrhea) is also considered abnormal

Genital Bleeding Assessment l l l Instruct participants to report all episodes of genital bleeding Document all reported and observed bleeding Complete Genital Bleeding Assessment form and/or pelvic exam as needed to assess all unexpected bleeding

STI/RTI Management l Sexually transmitted infections (STI) and other reproductive tract infections (RTI) will be assessed during screening and throughout follow-up l l l l Bacterial vaginosis Vaginal candidiasis Chlamydia infection Gonorrhea infection Syphilis infection Vaginal trichomoniasis [Genital herpes]

STI/RTI Management l l STIs/RTIs should be treated per WHO guidelines, except that asymptomatic candidiasis should not routinely be treated WHO guidelines are minimum standard, if local guidelines set higher standard, follow local guidelines Day-to-day, consult WHO and local guidelines as needed Provide directly observed single dose regimens whenever possible

Clarification for BV l l l WHO guidelines include both single and multi-dose regimens of metronidazole for treatment of symptomatic BV l Multi-dose = recommended l Single-dose = alternate Multi-dose regimen is more effective and should be used in most cases Single-dose regimen may be used on a case-by-case basis if considered more appropriate for a given participant (e. g. , for participant known to have difficulty adhering to longer course regimens)

STI/RTI Management l STI/RTI are considered resolved when treatment has been completed and symptoms, if any, have resolved l l No test of cure is required NB: For infections diagnosed during screening, treatment must be completed, and symptoms must resolve, before enrollment

Clarification for Syphilis l Clinical management of syphilis should include repeat serology (RPR) at six-month intervals to confirm treatment effectiveness l l If RPR titre does not decrease four-fold or revert to seronegative within six months after treatment, treatment should be repeated NB: For syphilis infections diagnosed during screening, four-fold decrease not required before enrollment

Urinary Tract Infections l l Diagnosed in VOICE based on the presence of symptoms and dipstick urinalysis positive for both nitrites and leukocyte esterase (LE) Symptoms include l l l Frequent urge to urinate Passage of only a small volume of urine Pain and/or burning during urination Lower abdominal pain and/or uncomfortable pressure above the pubic bone Milky/cloudy, reddish or bloody urine

Urinary Tract Infections l l l At Screening Part 1, dipstick for nitrites and LE is done regardless of symptoms After Screening Part 1, dipstick for nitrites and LE is done only among symptomatic participants Asymptomatic participants should not be treated, regardless of dipstick results

Urinary Tract Infections l Symptomatic participants with dipstick positive for both nitrites and LE should be treated l l During screening, treatment must be completed and symptoms must resolve before enrollment Symptomatic participants with dipstick negative for either nitrites or LE may be treated according to clinical judgment; however this would not be diagnosed as a UTI for VOICE

Urinary Tract Infections l Operational note l l Dipstick strips for nitrites and LE also test for protein and glucose Even if testing for protein and glucose is not required or indicated when testing for nitrites and LE, results for protein and glucose must be documented, assessed for clinical significance, and acted upon per protocol

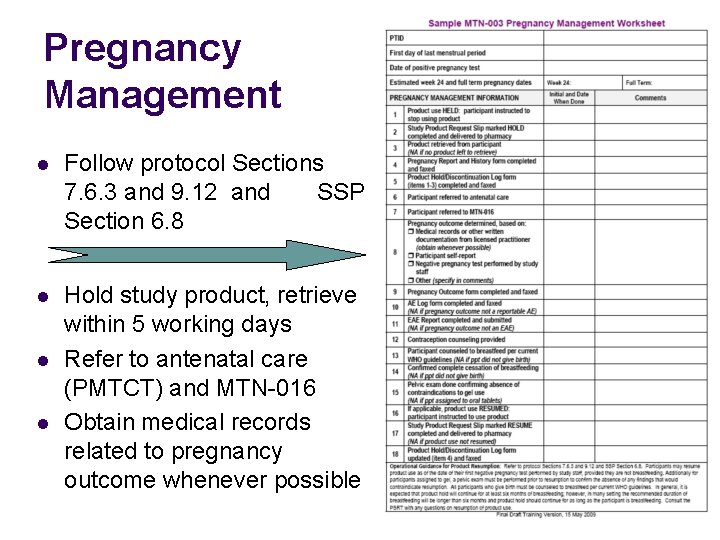
Pregnancy Management l Follow protocol Sections 7. 6. 3 and 9. 12 and SSP Section 6. 8 l Hold study product, retrieve within 5 working days Refer to antenatal care (PMTCT) and MTN-016 Obtain medical records related to pregnancy outcome whenever possible l l

Pregnancy Management l l l Provide counseling on infant feeding per WHO and local guidelines Provide contraceptive counseling Resume study product after pregnancy outcome provided participant is not breast feeding l l l At least six months breast feeding expected Negative pregnancy test performed by study staff For gel participants pelvic exam confirming no findings to contraindicate gel use

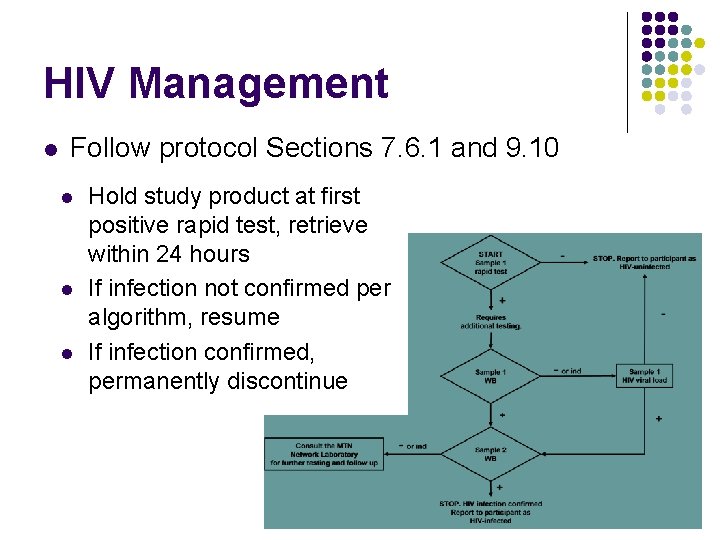
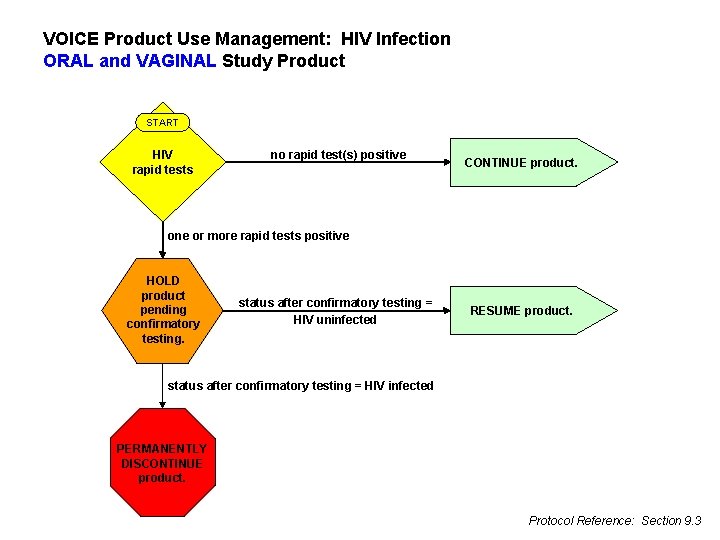
HIV Management l Follow protocol Sections 7. 6. 1 and 9. 10 l l l Hold study product at first positive rapid test, retrieve within 24 hours If infection not confirmed per algorithm, resume If infection confirmed, permanently discontinue

HIV Management l l Provide post-test counseling and ongoing supportive counseling Offer continued participation in VOICE Refer to MTN-015 Refer to available sources of medical and psychosocial care and support

HIV Management l Actively follow-up on all referrals at subsequent visits to determine l l If participant sought the care to which she was referred Whether care/services were received If additional referrals are needed Document all counseling, referrals, outcomes, and follow-up plans and actions

HIV Management l If participant chooses to remain in VOICE l l l If participant remains in VOICE but declines or defers joining MTN-015, perform as part of VOICE l l Tailor procedures accordingly Perform Hepatitis B antibody testing if applicable CD 4 cell count HIV RNA PCR (viral load) Plasma archive } At 1, 3, 6, and every 6 months after seroconversion May request resistance test results when clinically indicated

Product Use Management Flowcharts SSP section 10, Appendix 10 -3 l l l l l HIV infection Hepatitis B Infection Prohibited Medications PEP Pregnancy Breastfeeding RTIs/STIs Adverse Events Nausea, Vomiting, Diarrhea AST/ALT Elevation l l l l l Lactic Acidosis Elevated Creatinine Decreased Creatinine Clearance Hypophosphatemia Proteinuria Glycosuria Vertebral Compression Fracture Pap Smear Gynecologic Surgery

VOICE Product Use Management: HIV Infection ORAL and VAGINAL Study Product START HIV rapid tests no rapid test(s) positive CONTINUE product. one or more rapid tests positive HOLD product pending confirmatory testing. status after confirmatory testing = HIV uninfected RESUME product. status after confirmatory testing = HIV infected PERMANENTLY DISCONTINUE product. Protocol Reference: Section 9. 3

VOICE Product Use Management: Hepatitis B Infection (I) ORAL and VAGINAL Study Product Suspected Hepatitis B infection? no CONTINUE product. yes HOLD product pending confirmatory testing. Hepatitis B infection not confirmed RESUME product provided no other conditions requiring product hold are present. Hepatitis B infection confirmed PERMANENTLY DISCONTINUE product. For oral product, monitor AST/ALT per protocol Section 7. 6. 2. Protocol Reference: Sections 9. 3 and 9. 5. 2

VOICE Product Use Management: Hepatitis B Infection (II) ORAL and VAGINAL Study Product Result of protocol-required Hbs. Ag test negative CONTINUE product. positive PERMANENTLY DISCONTINUE product. For oral product, monitor for AST/ALT flares per protocol Section 7. 6. 2. Protocol Reference: Sections 9. 3 and 9. 5. 2

VOICE Product Use Management: Prohibited Medications ORAL and VAGINAL Study Product HOLD product until participant reports she is no longer taking the prohibited medication. Protocol Reference: Section 9. 3

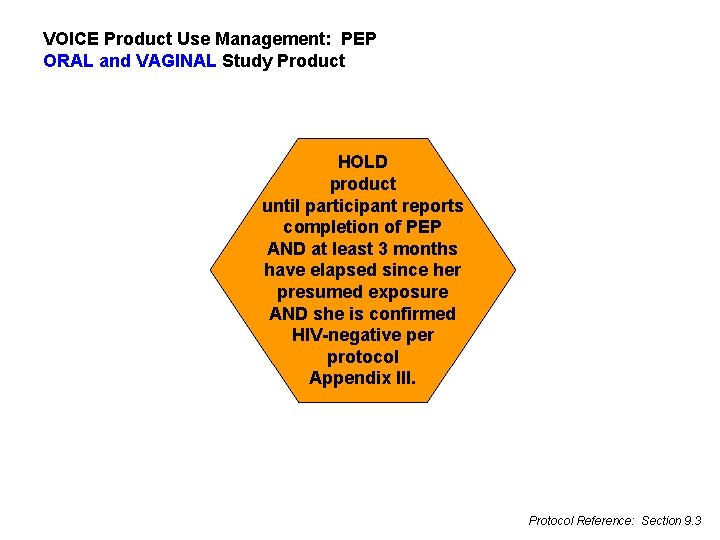
VOICE Product Use Management: PEP ORAL and VAGINAL Study Product HOLD product until participant reports completion of PEP AND at least 3 months have elapsed since her presumed exposure AND she is confirmed HIV-negative per protocol Appendix III. Protocol Reference: Section 9. 3

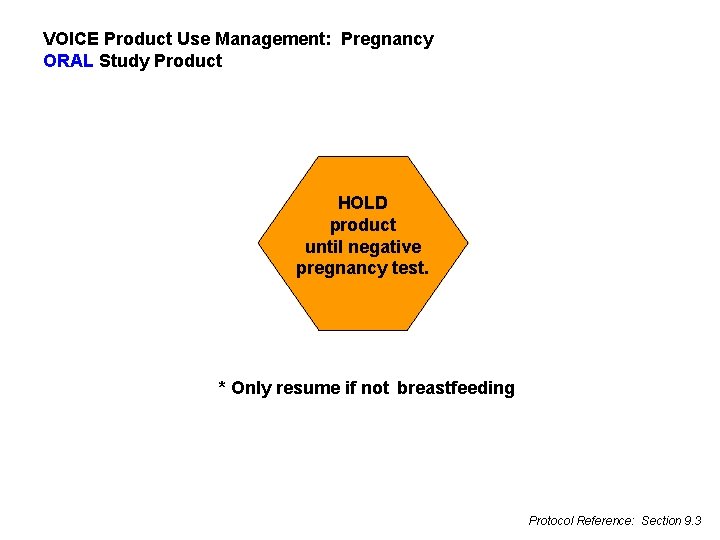
VOICE Product Use Management: Pregnancy ORAL Study Product HOLD product until negative pregnancy test. * Only resume if not breastfeeding Protocol Reference: Section 9. 3

VOICE Product Use Management: Pregnancy VAGINAL Study Product HOLD product until negative pregnancy test AND pelvic exam confirms absence of findings that contraindicate resumption. * Only resume if not breastfeeding Protocol Reference: Sections 9. 3 and 7. 6. 3

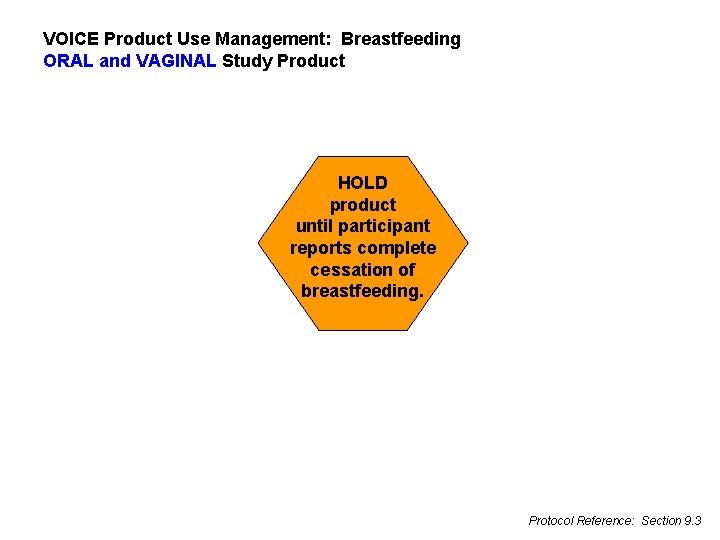
VOICE Product Use Management: Breastfeeding ORAL and VAGINAL Study Product HOLD product until participant reports complete cessation of breastfeeding. Protocol Reference: Section 9. 3

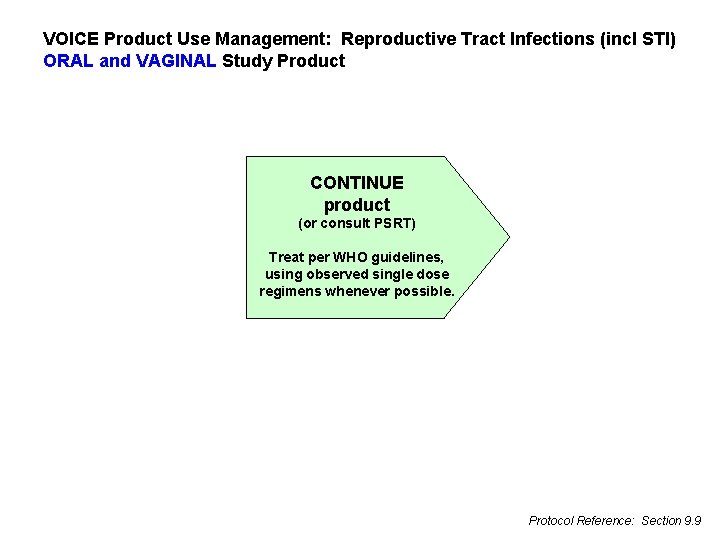
VOICE Product Use Management: Reproductive Tract Infections (incl STI) ORAL and VAGINAL Study Product CONTINUE product (or consult PSRT) Treat per WHO guidelines, using observed single dose regimens whenever possible. Protocol Reference: Section 9. 9

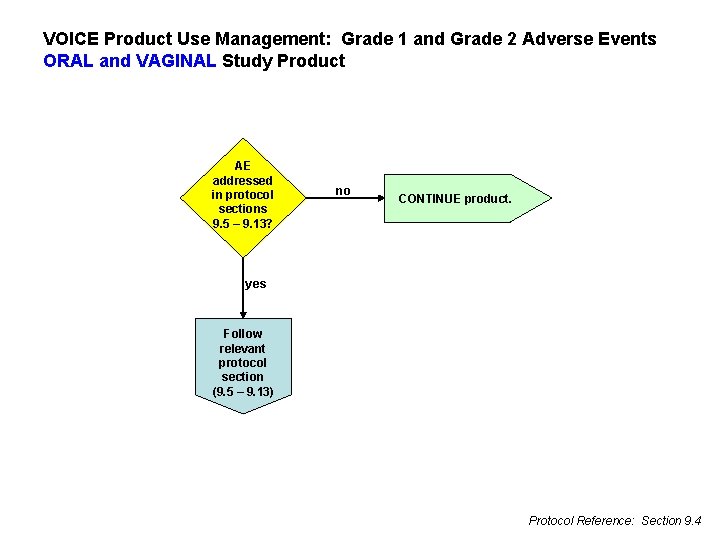
VOICE Product Use Management: Grade 1 and Grade 2 Adverse Events ORAL and VAGINAL Study Product AE addressed in protocol sections 9. 5 – 9. 13? no CONTINUE product. yes Follow relevant protocol section (9. 5 – 9. 13) Protocol Reference: Section 9. 4

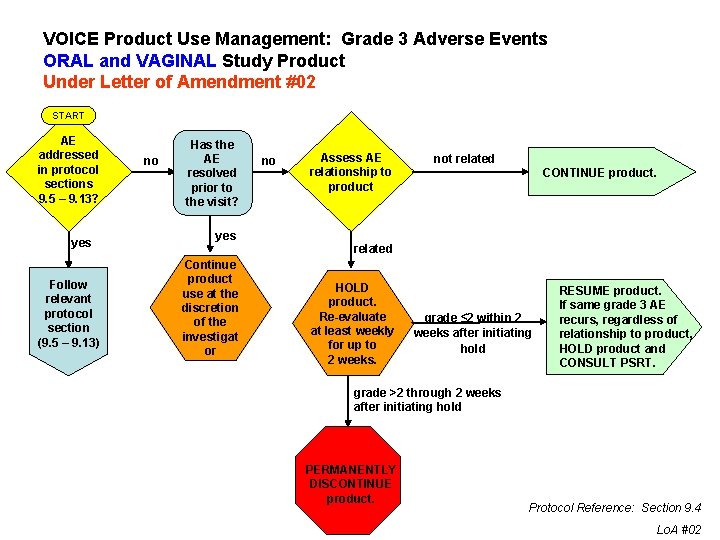
VOICE Product Use Management: Grade 3 Adverse Events ORAL and VAGINAL Study Product Under Letter of Amendment #02 START AE addressed in protocol sections 9. 5 – 9. 13? yes Follow relevant protocol section (9. 5 – 9. 13) no Has the AE resolved prior to the visit? yes Continue product use at the discretion of the investigat or no Assess AE relationship to product not related CONTINUE product. related HOLD product. Re-evaluate at least weekly for up to 2 weeks. grade ≤ 2 within 2 weeks after initiating hold RESUME product. If same grade 3 AE recurs, regardless of relationship to product, HOLD product and CONSULT PSRT. grade >2 through 2 weeks after initiating hold PERMANENTLY DISCONTINUE product. Protocol Reference: Section 9. 4 Lo. A #02

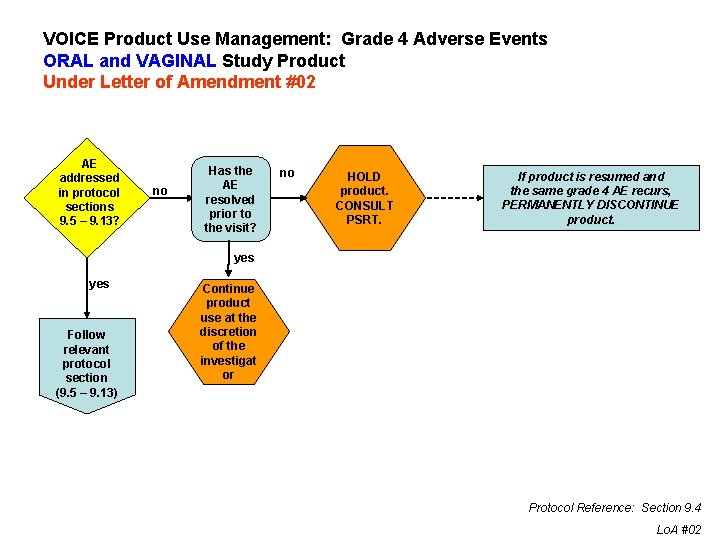
VOICE Product Use Management: Grade 4 Adverse Events ORAL and VAGINAL Study Product Under Letter of Amendment #02 AE addressed in protocol sections 9. 5 – 9. 13? no Has the AE resolved prior to the visit? no HOLD product. CONSULT PSRT. If product is resumed and the same grade 4 AE recurs, PERMANENTLY DISCONTINUE product. yes Follow relevant protocol section (9. 5 – 9. 13) Continue product use at the discretion of the investigat or Protocol Reference: Section 9. 4 Lo. A #02

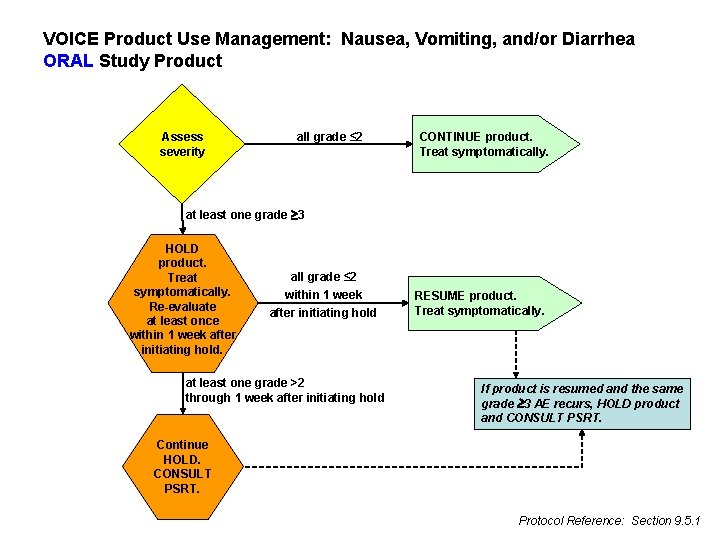
VOICE Product Use Management: Nausea, Vomiting, and/or Diarrhea ORAL Study Product Assess severity all grade ≤ 2 CONTINUE product. Treat symptomatically. at least one grade 3 HOLD product. Treat symptomatically. Re-evaluate at least once within 1 week after initiating hold. all grade ≤ 2 within 1 week after initiating hold at least one grade >2 through 1 week after initiating hold RESUME product. Treat symptomatically. If product is resumed and the same grade 3 AE recurs, HOLD product and CONSULT PSRT. Continue HOLD. CONSULT PSRT. Protocol Reference: Section 9. 5. 1

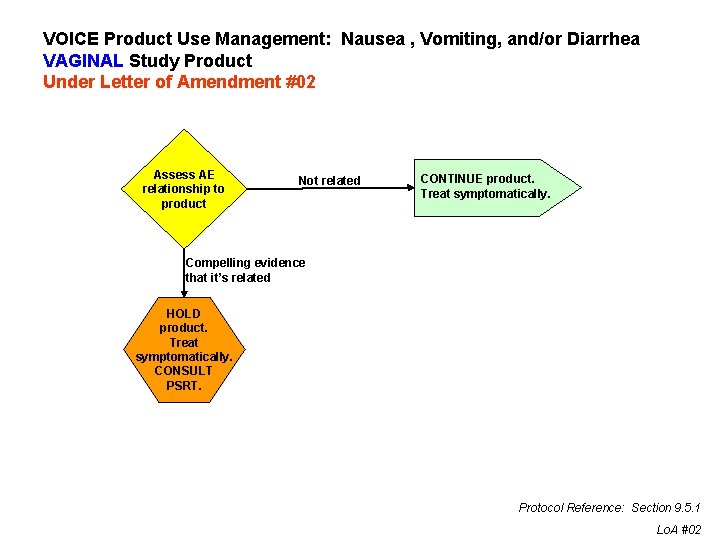
VOICE Product Use Management: Nausea , Vomiting, and/or Diarrhea VAGINAL Study Product Under Letter of Amendment #02 Assess AE relationship to product Not related CONTINUE product. Treat symptomatically. Compelling evidence that it’s related HOLD product. Treat symptomatically. CONSULT PSRT. Protocol Reference: Section 9. 5. 1 Lo. A #02

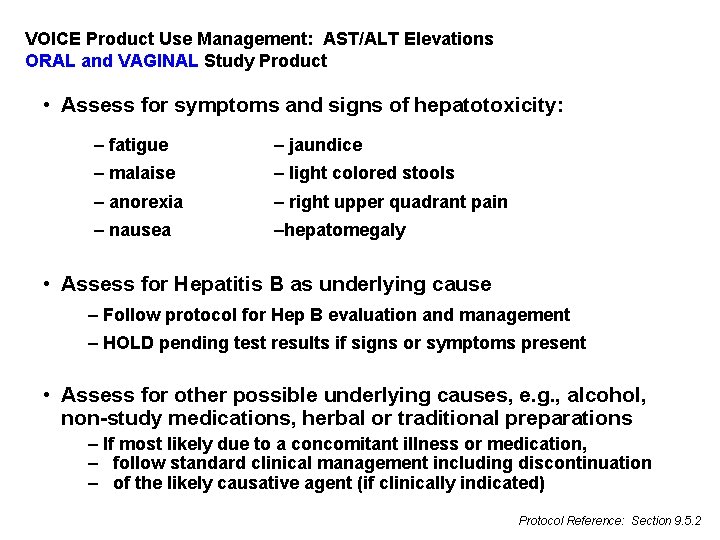
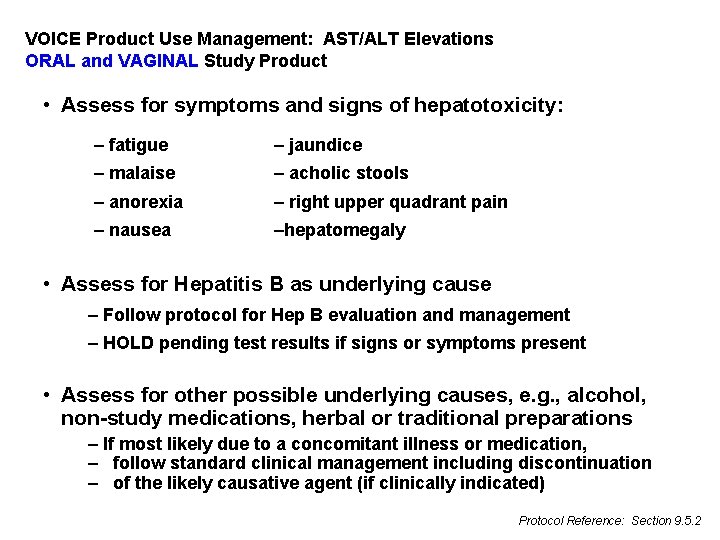
VOICE Product Use Management: AST/ALT Elevations ORAL and VAGINAL Study Product • Assess for symptoms and signs of hepatotoxicity: – fatigue – jaundice – malaise – light colored stools – anorexia – right upper quadrant pain – nausea –hepatomegaly • Assess for Hepatitis B as underlying cause – Follow protocol for Hep B evaluation and management – HOLD pending test results if signs or symptoms present • Assess for other possible underlying causes, e. g. , alcohol, non-study medications, herbal or traditional preparations – If most likely due to a concomitant illness or medication, – follow standard clinical management including discontinuation – of the likely causative agent (if clinically indicated) Protocol Reference: Section 9. 5. 2

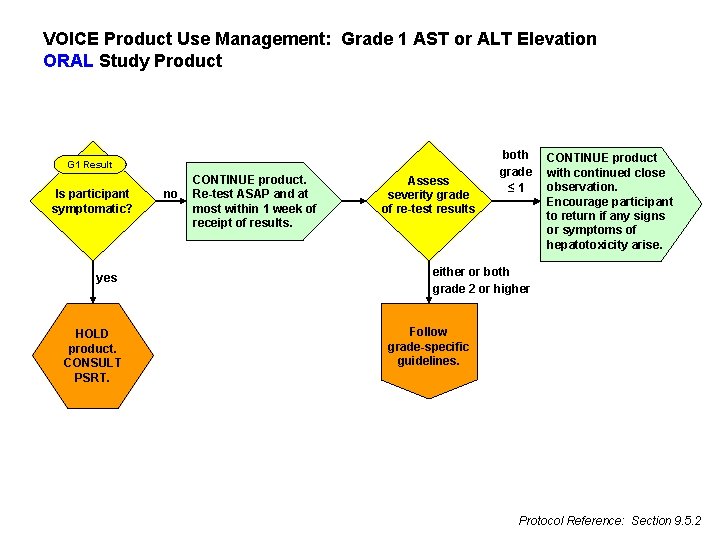
VOICE Product Use Management: Grade 1 AST or ALT Elevation ORAL Study Product G 1 Result Is participant symptomatic? yes HOLD product. CONSULT PSRT. no CONTINUE product. Re-test ASAP and at most within 1 week of receipt of results. Assess severity grade of re-test results both grade ≤ 1 CONTINUE product with continued close observation. Encourage participant to return if any signs or symptoms of hepatotoxicity arise. either or both grade 2 or higher Follow grade-specific guidelines. Protocol Reference: Section 9. 5. 2

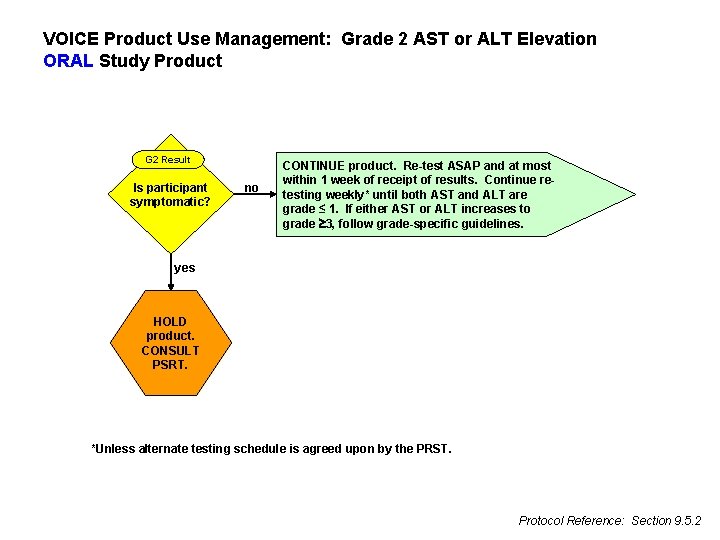
VOICE Product Use Management: Grade 2 AST or ALT Elevation ORAL Study Product G 2 Result Is participant symptomatic? no CONTINUE product. Re-test ASAP and at most within 1 week of receipt of results. Continue retesting weekly* until both AST and ALT are grade ≤ 1. If either AST or ALT increases to grade 3, follow grade-specific guidelines. yes HOLD product. CONSULT PSRT. *Unless alternate testing schedule is agreed upon by the PRST. Protocol Reference: Section 9. 5. 2

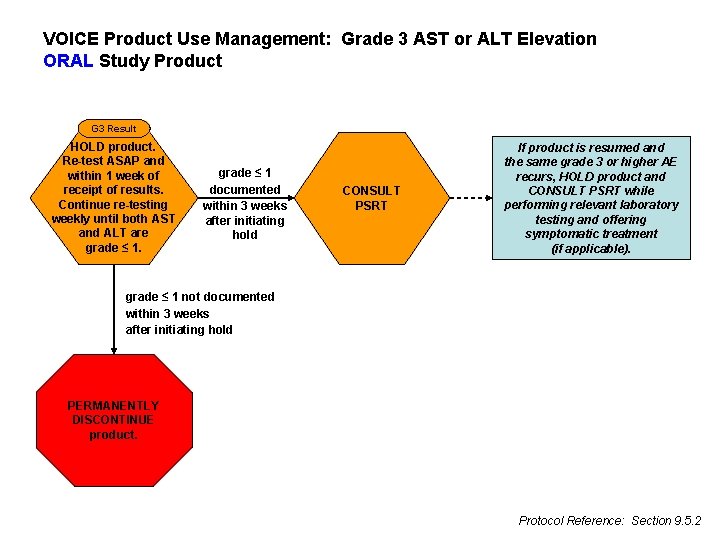
VOICE Product Use Management: Grade 3 AST or ALT Elevation ORAL Study Product G 3 Result HOLD product. Re-test ASAP and within 1 week of receipt of results. Continue re-testing weekly until both AST and ALT are grade ≤ 1 documented within 3 weeks after initiating hold CONSULT PSRT If product is resumed and the same grade 3 or higher AE recurs, HOLD product and CONSULT PSRT while performing relevant laboratory testing and offering symptomatic treatment (if applicable). grade ≤ 1 not documented within 3 weeks after initiating hold PERMANENTLY DISCONTINUE product. Protocol Reference: Section 9. 5. 2

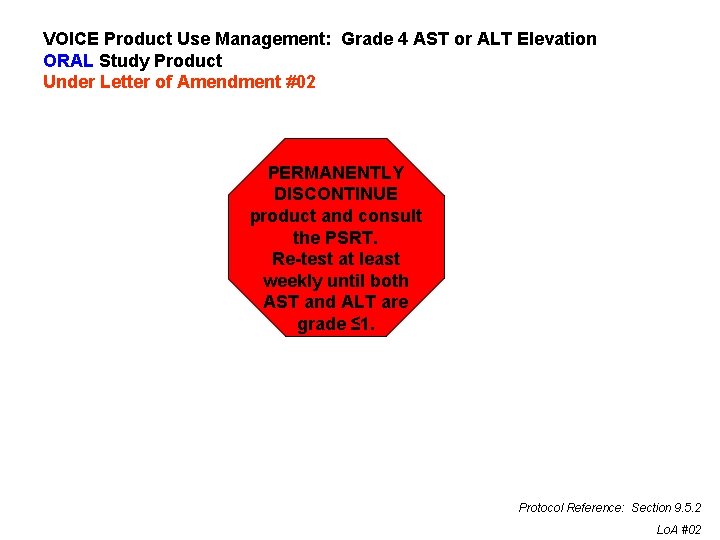
VOICE Product Use Management: Grade 4 AST or ALT Elevation ORAL Study Product Under Letter of Amendment #02 PERMANENTLY DISCONTINUE product and consult the PSRT. Re-test at least weekly until both AST and ALT are grade ≤ 1. Protocol Reference: Section 9. 5. 2 Lo. A #02

VOICE Product Use Management: AST/ALT Elevations ORAL and VAGINAL Study Product • Assess for symptoms and signs of hepatotoxicity: – fatigue – jaundice – malaise – acholic stools – anorexia – right upper quadrant pain – nausea –hepatomegaly • Assess for Hepatitis B as underlying cause – Follow protocol for Hep B evaluation and management – HOLD pending test results if signs or symptoms present • Assess for other possible underlying causes, e. g. , alcohol, non-study medications, herbal or traditional preparations – If most likely due to a concomitant illness or medication, – follow standard clinical management including discontinuation – of the likely causative agent (if clinically indicated) Protocol Reference: Section 9. 5. 2

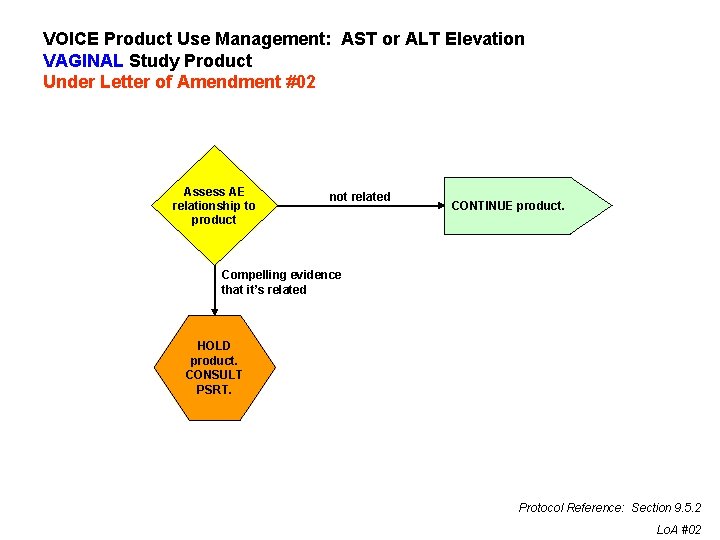
VOICE Product Use Management: AST or ALT Elevation VAGINAL Study Product Under Letter of Amendment #02 Assess AE relationship to product not related CONTINUE product. Compelling evidence that it’s related HOLD product. CONSULT PSRT. Protocol Reference: Section 9. 5. 2 Lo. A #02

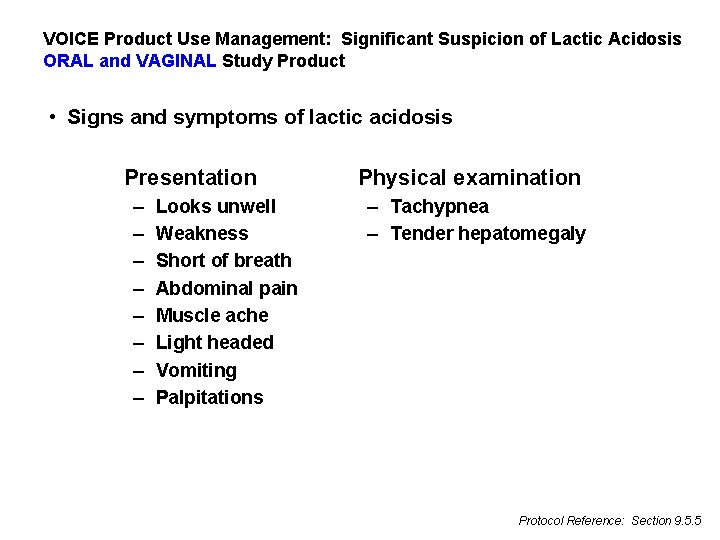
VOICE Product Use Management: Significant Suspicion of Lactic Acidosis ORAL and VAGINAL Study Product • Signs and symptoms of lactic acidosis Presentation – – – – Looks unwell Weakness Short of breath Abdominal pain Muscle ache Light headed Vomiting Palpitations Physical examination – Tachypnea – Tender hepatomegaly Protocol Reference: Section 9. 5. 5

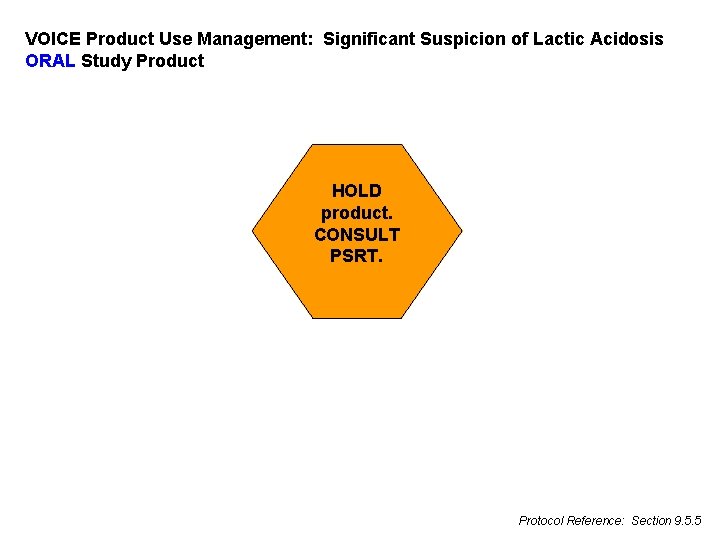
VOICE Product Use Management: Significant Suspicion of Lactic Acidosis ORAL Study Product HOLD product. CONSULT PSRT. Protocol Reference: Section 9. 5. 5

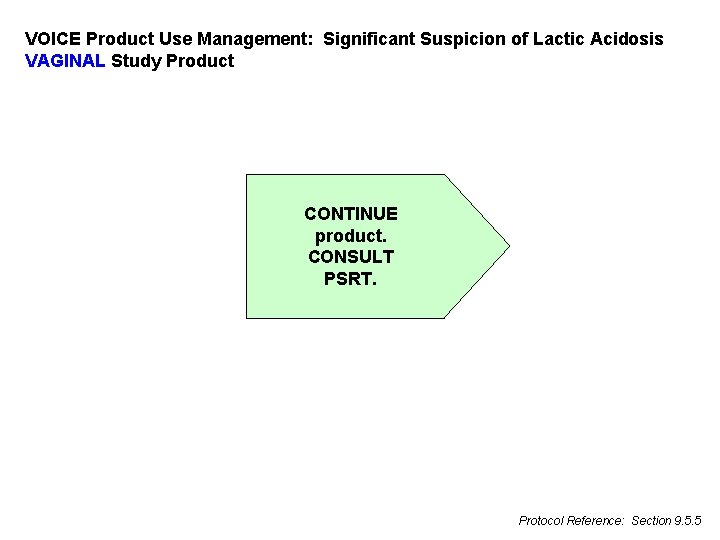
VOICE Product Use Management: Significant Suspicion of Lactic Acidosis VAGINAL Study Product CONTINUE product. CONSULT PSRT. Protocol Reference: Section 9. 5. 5

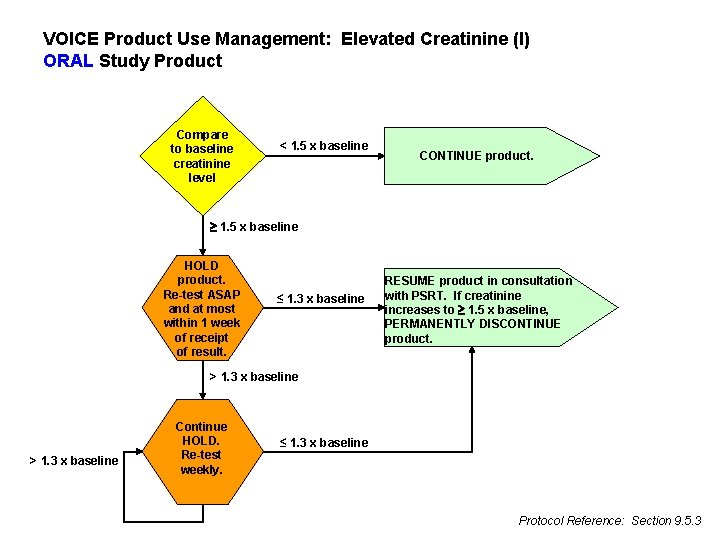
VOICE Product Use Management: Elevated Creatinine (I) ORAL Study Product Compare to baseline creatinine level < 1. 5 x baseline CONTINUE product. 1. 5 x baseline HOLD product. Re-test ASAP and at most within 1 week of receipt of result. ≤ 1. 3 x baseline RESUME product in consultation with PSRT. If creatinine increases to 1. 5 x baseline, PERMANENTLY DISCONTINUE product. > 1. 3 x baseline Continue HOLD. Re-test weekly. ≤ 1. 3 x baseline Protocol Reference: Section 9. 5. 3

VOICE Product Use Management: Elevated Creatinine (II) ORAL Study Product less than grade 1 Assess severity grade 1 or 2 CONTINUE product. Repeat testing 2 weeks after receipt of result. grade 3 or 4 Follow grade-specific guidance in protocol Section 9. 4 Protocol Reference: None

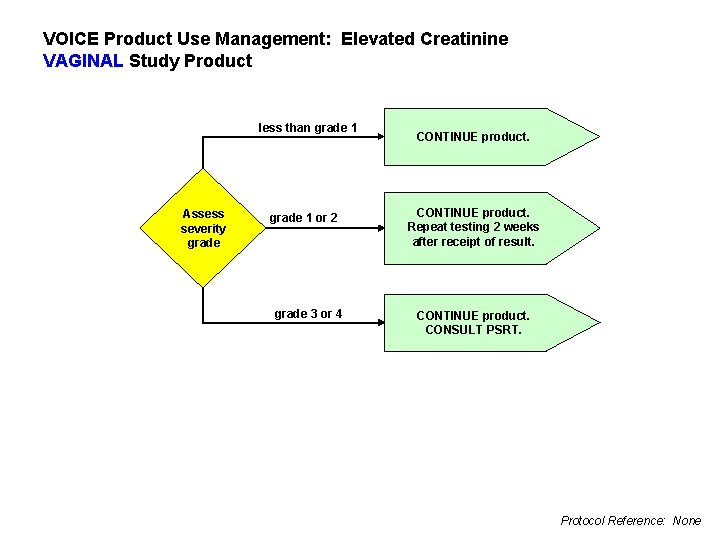
VOICE Product Use Management: Elevated Creatinine VAGINAL Study Product less than grade 1 Assess severity grade 1 or 2 grade 3 or 4 CONTINUE product. Repeat testing 2 weeks after receipt of result. CONTINUE product. CONSULT PSRT. Protocol Reference: None

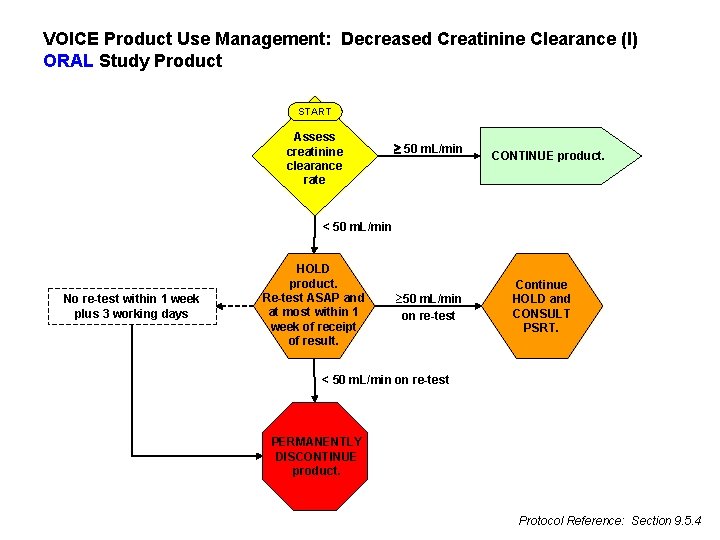
VOICE Product Use Management: Decreased Creatinine Clearance (I) ORAL Study Product START Assess creatinine clearance rate 50 m. L/min CONTINUE product. < 50 m. L/min No re-test within 1 week plus 3 working days HOLD product. Re-test ASAP and at most within 1 week of receipt of result. ³ 50 m. L/min on re-test Continue HOLD and CONSULT PSRT. < 50 m. L/min on re-test PERMANENTLY DISCONTINUE product. Protocol Reference: Section 9. 5. 4

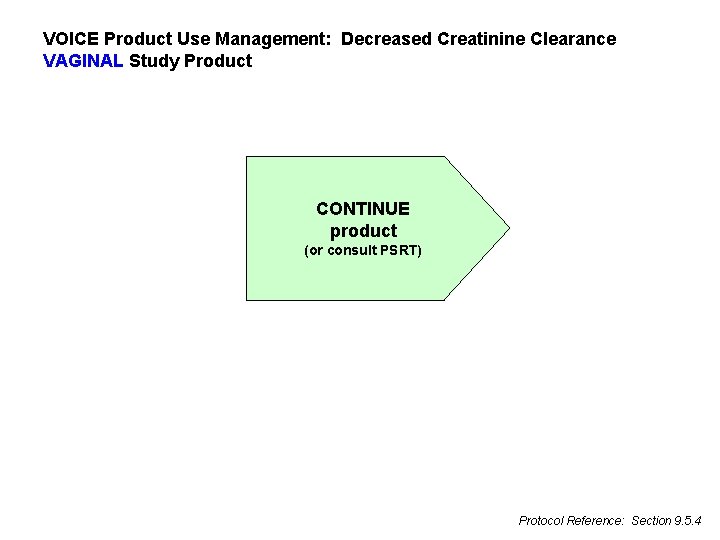
VOICE Product Use Management: Decreased Creatinine Clearance VAGINAL Study Product CONTINUE product (or consult PSRT) Protocol Reference: Section 9. 5. 4

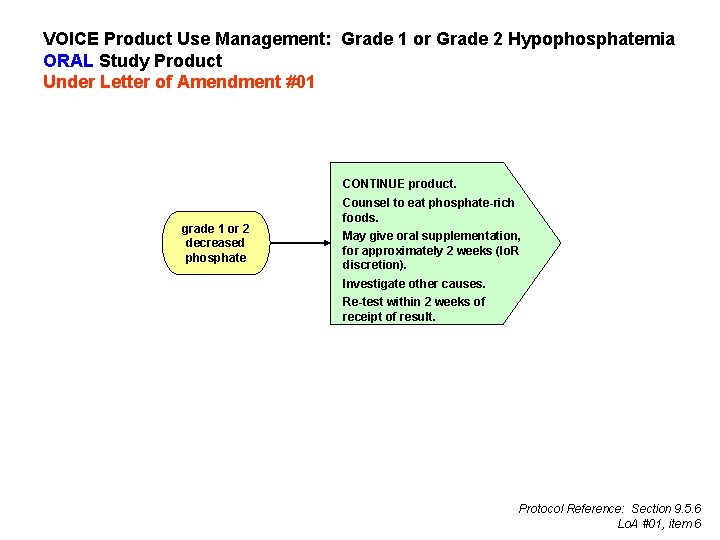
VOICE Product Use Management: Grade 1 or Grade 2 Hypophosphatemia ORAL Study Product Under Letter of Amendment #01 CONTINUE product. grade 1 or 2 decreased phosphate Counsel to eat phosphate-rich foods. May give oral supplementation, for approximately 2 weeks (Io. R discretion). Investigate other causes. Re-test within 2 weeks of receipt of result. Protocol Reference: Section 9. 5. 6 Lo. A #01, item 6

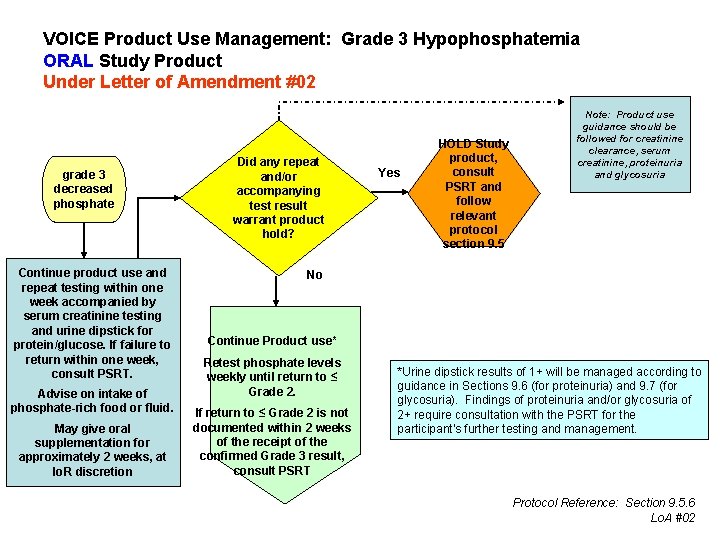
VOICE Product Use Management: Grade 3 Hypophosphatemia ORAL Study Product Under Letter of Amendment #02 grade 3 decreased phosphate Continue product use and repeat testing within one week accompanied by serum creatinine testing and urine dipstick for protein/glucose. If failure to return within one week, consult PSRT. Advise on intake of phosphate-rich food or fluid. May give oral supplementation for approximately 2 weeks, at Io. R discretion Did any repeat and/or accompanying test result warrant product hold? Yes HOLD Study product, consult PSRT and follow relevant protocol section 9. 5 Note: Product use guidance should be followed for creatinine clearance, serum creatinine, proteinuria and glycosuria No Continue Product use* Retest phosphate levels weekly until return to ≤ Grade 2. If return to ≤ Grade 2 is not documented within 2 weeks of the receipt of the confirmed Grade 3 result, consult PSRT *Urine dipstick results of 1+ will be managed according to guidance in Sections 9. 6 (for proteinuria) and 9. 7 (for glycosuria). Findings of proteinuria and/or glycosuria of 2+ require consultation with the PSRT for the participant’s further testing and management. Protocol Reference: Section 9. 5. 6 Lo. A #02

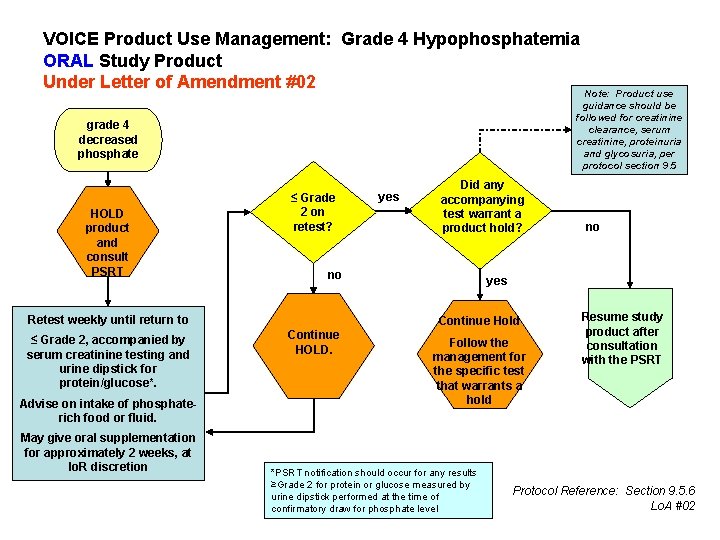
VOICE Product Use Management: Grade 4 Hypophosphatemia ORAL Study Product Under Letter of Amendment #02 Note: Product use guidance should be followed for creatinine clearance, serum creatinine, proteinuria and glycosuria, per protocol section 9. 5 grade 4 decreased phosphate HOLD product and consult PSRT ≤ Grade 2 on retest? Advise on intake of phosphaterich food or fluid. May give oral supplementation for approximately 2 weeks, at Io. R discretion Did any accompanying yes test warrant a product hold? no Retest weekly until return to ≤ Grade 2, accompanied by serum creatinine testing and urine dipstick for protein/glucose*. yes Continue Hold Continue HOLD. no Follow the management for the specific test that warrants a hold *PSRT notification should occur for any results ≥Grade 2 for protein or glucose measured by urine dipstick performed at the time of confirmatory draw for phosphate level Resume study product after consultation with the PSRT Protocol Reference: Section 9. 5. 6 Lo. A #02

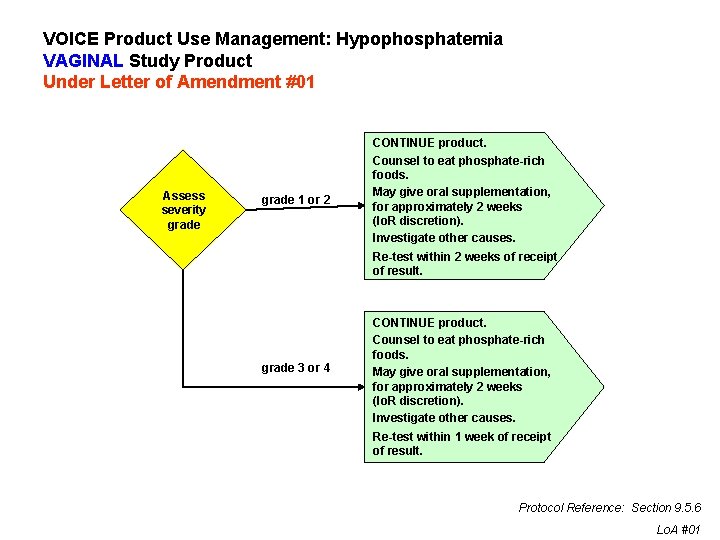
VOICE Product Use Management: Hypophosphatemia VAGINAL Study Product Under Letter of Amendment #01 Assess severity grade 1 or 2 CONTINUE product. Counsel to eat phosphate-rich foods. May give oral supplementation, for approximately 2 weeks (Io. R discretion). Investigate other causes. Re-test within 2 weeks of receipt of result. grade 3 or 4 CONTINUE product. Counsel to eat phosphate-rich foods. May give oral supplementation, for approximately 2 weeks (Io. R discretion). Investigate other causes. Re-test within 1 week of receipt of result. Protocol Reference: Section 9. 5. 6 Lo. A #01

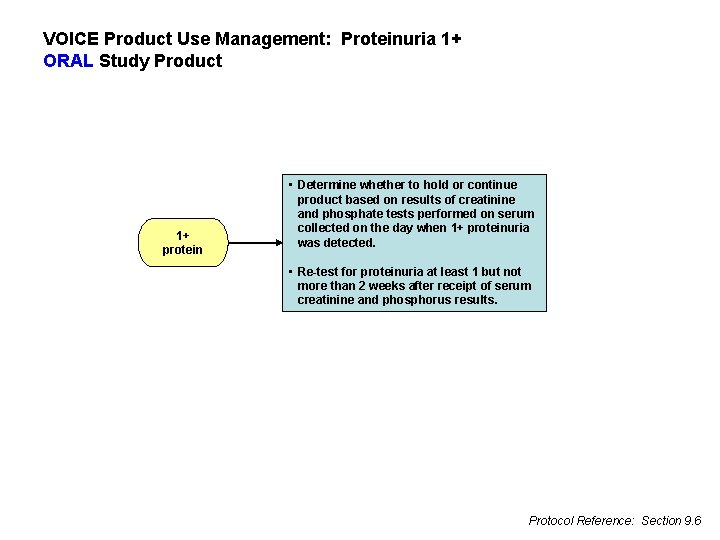
VOICE Product Use Management: Proteinuria 1+ ORAL Study Product 1+ protein • Determine whether to hold or continue product based on results of creatinine and phosphate tests performed on serum collected on the day when 1+ proteinuria was detected. • Re-test for proteinuria at least 1 but not more than 2 weeks after receipt of serum creatinine and phosphorus results. Protocol Reference: Section 9. 6

VOICE Product Use Management: Proteinuria 2+ ORAL Study Product 2+ protein HOLD product pending results of creatinine and phosphate tests performed on serum collected on the day when 2+ proteinuria was detected. RESUME product. Creatinine or phosphate results require product hold? no Investigate proteinuria per local standard of care and discretion of the Io. R. If proteinuria recurs at 2+ or greater, PERMANENTLY DISCONTINUE product. yes Continue HOLD per guidelines for management of creatinine and phosphate results. Protocol Reference: Section 9. 6

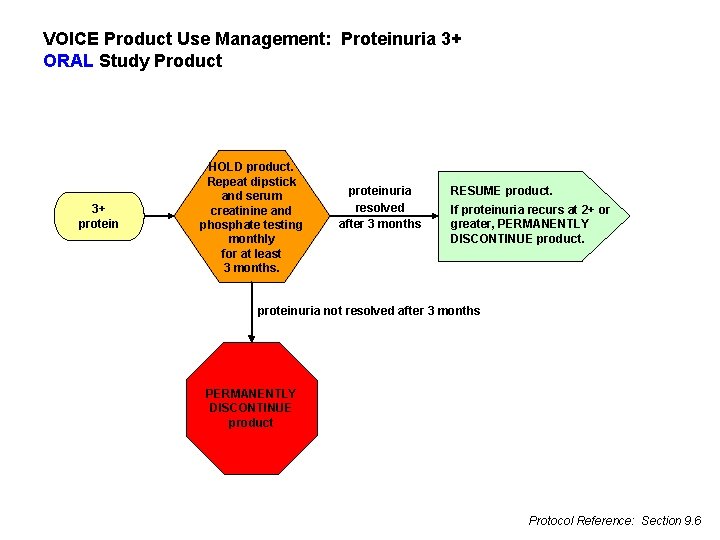
VOICE Product Use Management: Proteinuria 3+ ORAL Study Product 3+ protein HOLD product. Repeat dipstick and serum creatinine and phosphate testing monthly for at least 3 months. proteinuria resolved after 3 months RESUME product. If proteinuria recurs at 2+ or greater, PERMANENTLY DISCONTINUE product. proteinuria not resolved after 3 months PERMANENTLY DISCONTINUE product Protocol Reference: Section 9. 6

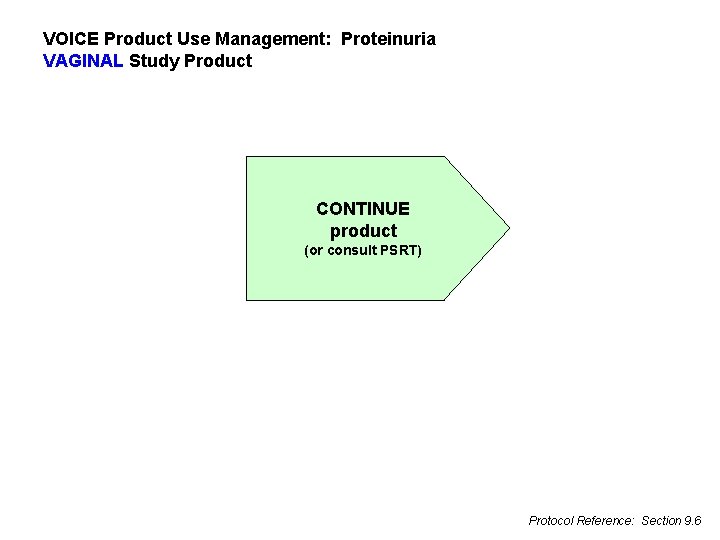
VOICE Product Use Management: Proteinuria VAGINAL Study Product CONTINUE product (or consult PSRT) Protocol Reference: Section 9. 6

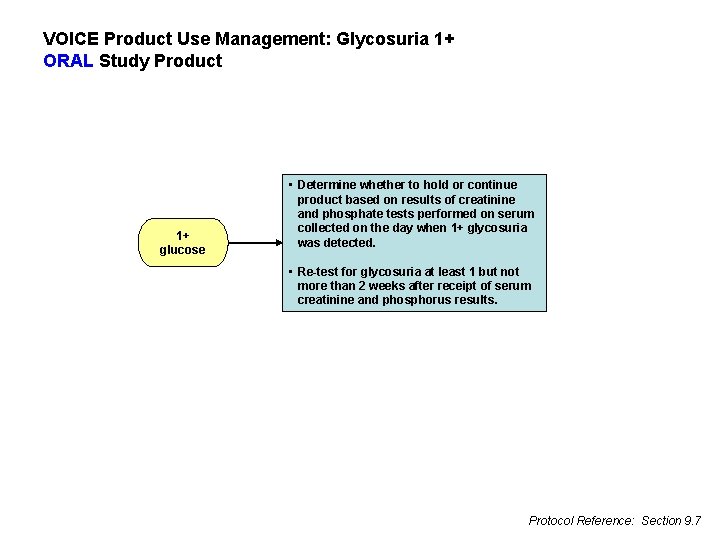
VOICE Product Use Management: Glycosuria 1+ ORAL Study Product 1+ glucose • Determine whether to hold or continue product based on results of creatinine and phosphate tests performed on serum collected on the day when 1+ glycosuria was detected. • Re-test for glycosuria at least 1 but not more than 2 weeks after receipt of serum creatinine and phosphorus results. Protocol Reference: Section 9. 7

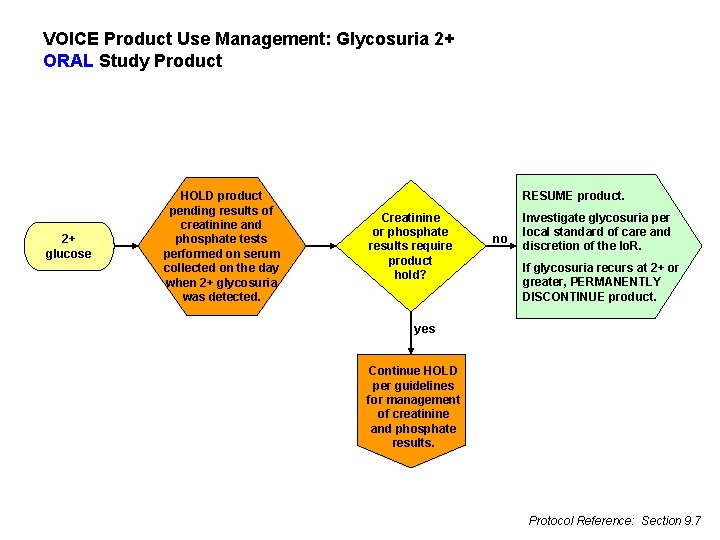
VOICE Product Use Management: Glycosuria 2+ ORAL Study Product 2+ glucose HOLD product pending results of creatinine and phosphate tests performed on serum collected on the day when 2+ glycosuria was detected. RESUME product. Creatinine or phosphate results require product hold? no Investigate glycosuria per local standard of care and discretion of the Io. R. If glycosuria recurs at 2+ or greater, PERMANENTLY DISCONTINUE product. yes Continue HOLD per guidelines for management of creatinine and phosphate results. Protocol Reference: Section 9. 7

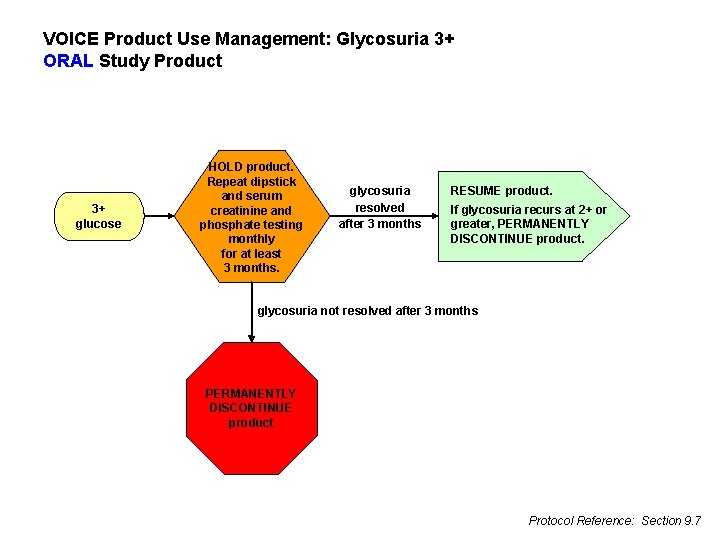
VOICE Product Use Management: Glycosuria 3+ ORAL Study Product 3+ glucose HOLD product. Repeat dipstick and serum creatinine and phosphate testing monthly for at least 3 months. glycosuria resolved after 3 months RESUME product. If glycosuria recurs at 2+ or greater, PERMANENTLY DISCONTINUE product. glycosuria not resolved after 3 months PERMANENTLY DISCONTINUE product Protocol Reference: Section 9. 7

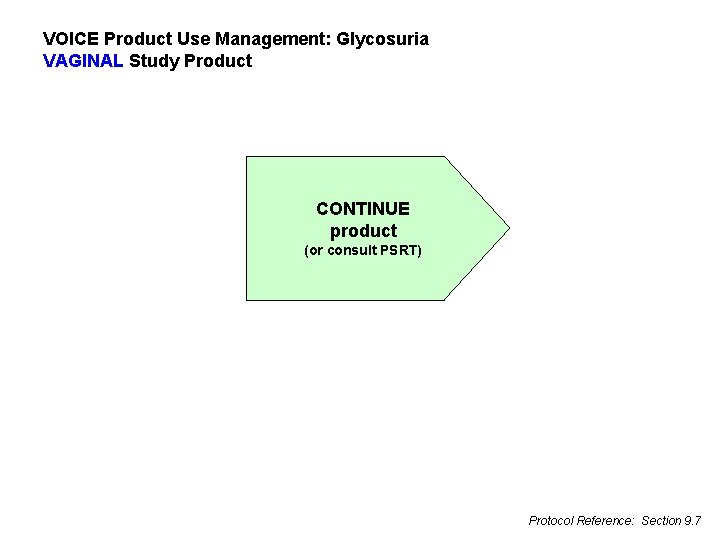
VOICE Product Use Management: Glycosuria VAGINAL Study Product CONTINUE product (or consult PSRT) Protocol Reference: Section 9. 7

VOICE Product Use Management: Vertebral Compression Fracture ORAL Study Product Compare height to baseline no change or decrease < 1. 5 in (3. 8 cm) CONTINUE product. decrease 1. 5 in (3. 8 cm) Repeat height measurement decrease 1. 5 in (3. 8 cm) not confirmed decrease 1. 5 in (3. 8 cm) confirmed Order vertebral radiography. compression fracture not confirmed compression fracture confirmed PERMANENTLY DISCONTINUE product. Protocol Reference: Section 9. 13

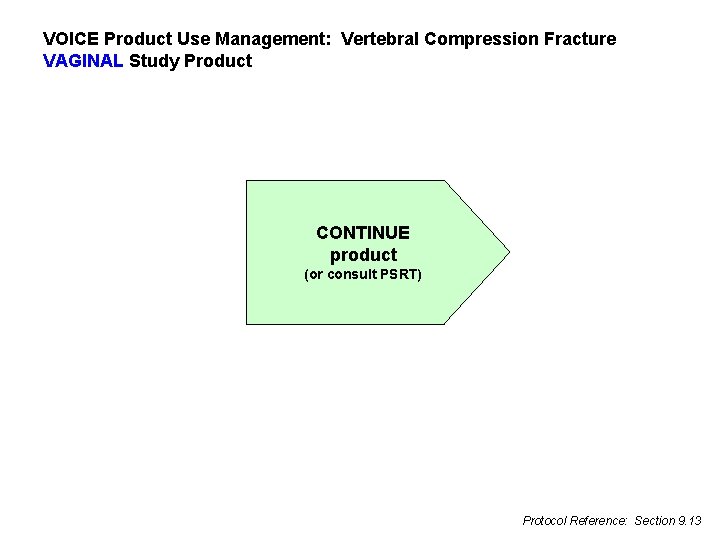
VOICE Product Use Management: Vertebral Compression Fracture VAGINAL Study Product CONTINUE product (or consult PSRT) Protocol Reference: Section 9. 13

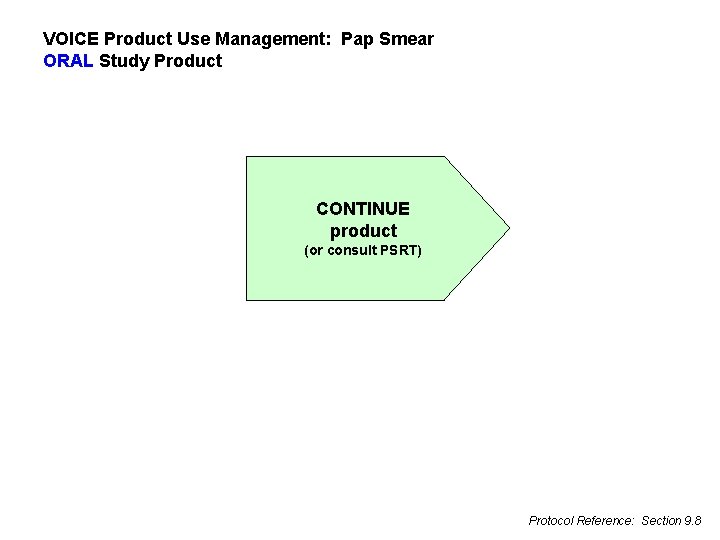
VOICE Product Use Management: Pap Smear ORAL Study Product CONTINUE product (or consult PSRT) Protocol Reference: Section 9. 8

VOICE Product Use Management: Pap Smear VAGINAL Study Product • HOLD product if H-SIL or more severe abnormality identified. • HOLD product for lower grade abnormalities if local standard of care requires clinical colposcopy and/or biopsy to assess the abnormality. • Initiate hold on the day of the procedure (or 1 -2 days before, if participant advised to avoid sex on these days). • Continue hold until clinically acceptable resolution of the biopsy and/or treatment has occurred, in the judgment of the Io. R. • Obtain medical records documenting the evaluation/biopsy/ treatment. • Assuming adequate treatment is confirmed, perform pelvic exam to confirm healing of the cervix. • Assuming no contraindications are identified on pelvic exam, RESUME product use. Protocol Reference: Section 9. 8

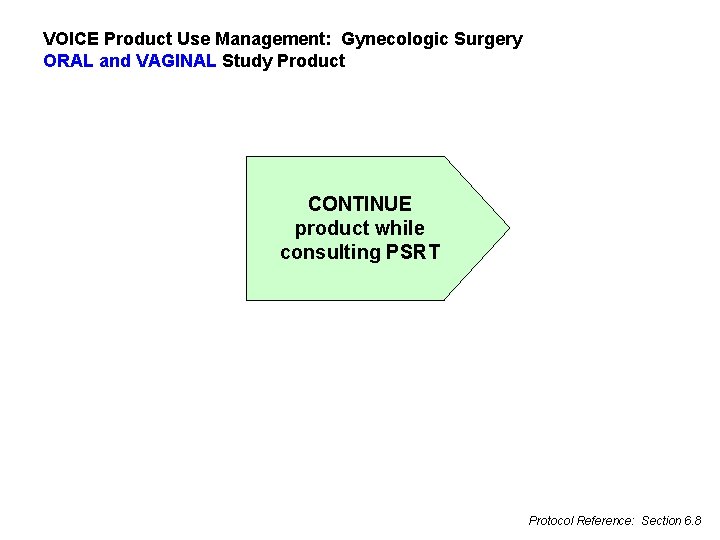
VOICE Product Use Management: Gynecologic Surgery ORAL and VAGINAL Study Product CONTINUE product while consulting PSRT Protocol Reference: Section 6. 8

VOICE Product Use Management: ORAL and VAGINAL Study Product Participant Non-Compliance or Other Safety Concern HOLD product if participant is unable or unwilling to comply with required study procedures or otherwise might be put at undue risk to her safety and wellbeing by continuing product use. CONSULT PSRT on all such holds for guidance on resuming product use, continuing the temporary hold, or progressing to permanent discontinuation. If product is temporarily held or permanently discontinued for this reason, but the underlying reason later resolves, CONSULT PSRT on resuming product at that time. Protocol Reference: Section 9. 3

VOICE Product Use Management: ORAL and VAGINAL Study Product Participant Non-Compliance or Other Safety Concern: CO-ENROLLMENT If co-enrollment in another study is identified, obtain as much information as possible about the other study from the participant and the other study team. HOLD product upon identification of co-enrollment unless the other study is known to not involve a study product and/or confirmation is available from the other study team that the participant is not using another study product. CONSULT the PSRT on further management of the participant. Schedule the participant to return when a response from the PSRT is expected. Protocol Reference: Section 9. 3

What are your questions?