Study Product Return ReSupply and ReIssue MTN003 StudySpecific








































- Slides: 50

Study Product Return Re-Supply and Re-Issue MTN-003 Study-Specific Training

Overview of Presentation n n n Some Definitions Review of Visit Flow Study Product Return Study Product Hold/Discontinuation Study Product Re-Supply Study Product Re-Issue Some Examples for Discussion

Definitions n Study Product Return Refers to participants bringing their unused study product to follow-up visits for counting at the site pharmacy n Returned product may either be retained in the pharmacy or re-issued to the participant n

Definitions n Study Product Re-Supply n Refers to dispensing new supplies of study product n n Tablets re-supplied in quantities of 30 Gel usually re-supplied in quantities of 30 but may be re-supplied in quantities of 10 or 20

Definitions n Study Product Re-Issue n Refers to providing participants with their own previously returned study product

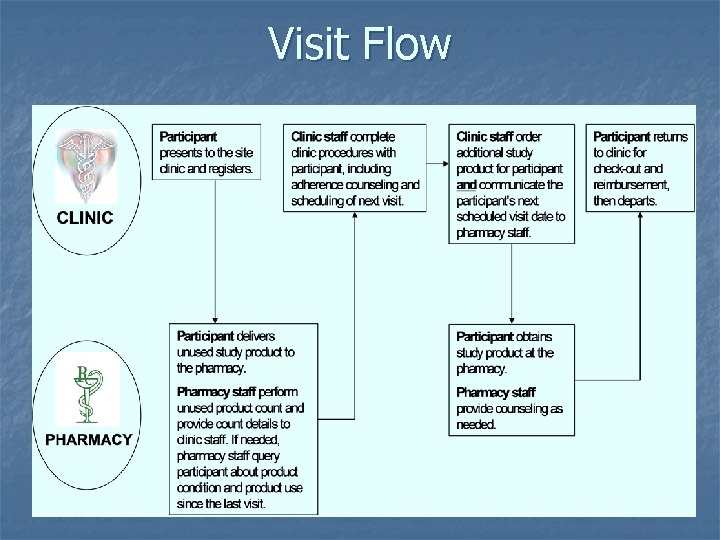
Visit Flow

Study Product Return Participants instructed to bring all unused product to all visits n Pharmacy staff count returned product and determine quantity: n Expected to be returned n Actually returned n Available for re-issue n

Product Eligible for Re-Issue n Pharmacy staff will determine based on factors such as: Physical condition n Expiry Dates n Oral: 30 days after bottle is opened n Gel: 60 days after dispensed n Indicated by “do not use after” date n Key counseling message: Use re-issued product first


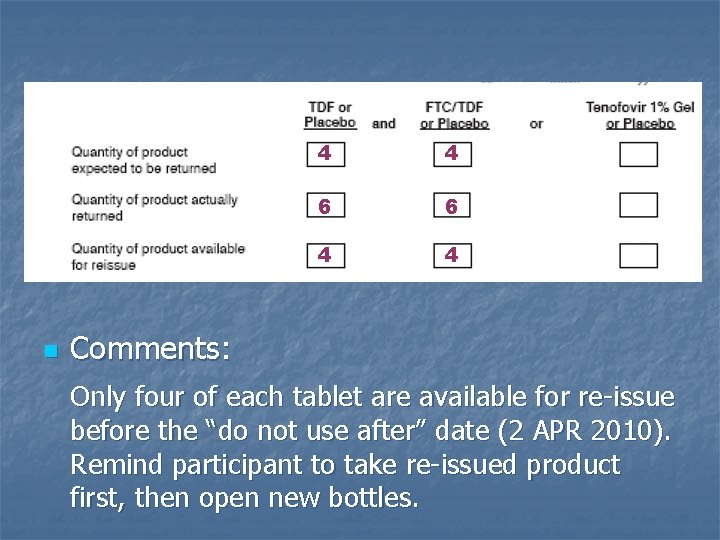
n 4 4 6 6 4 4 Comments: Only four of each tablet are available for re-issue before the “do not use after” date (2 APR 2010). Remind participant to take re-issued product first, then open new bottles.

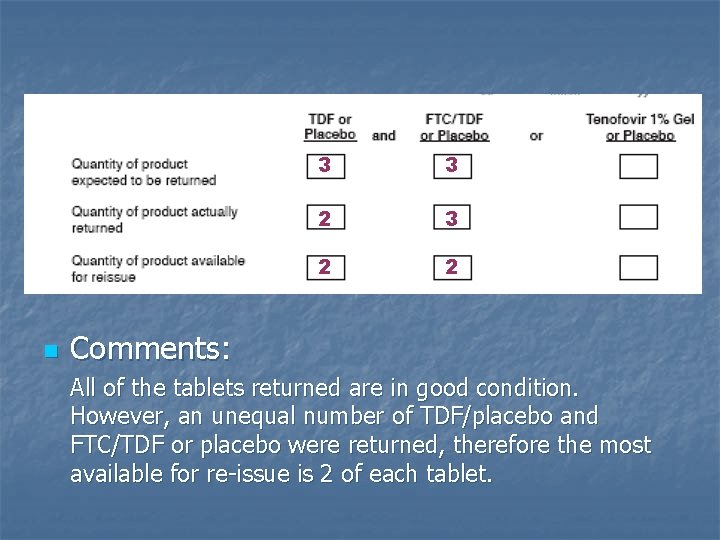
n 3 3 2 2 Comments: All of the tablets returned are in good condition. However, an unequal number of TDF/placebo and FTC/TDF or placebo were returned, therefore the most available for re-issue is 2 of each tablet.

4 0 0 n Comments: Four gels were expected to be returned, but none were actually returned. Participant stated she did not accidentally leave at home, but suspects that her sister may have used some of her gels.

1 4 2 n Comments: Of 4 gels returned, 2 were damaged. Therefore only 2 applicators are available for re-issue

Study Product Return n Clinic staff use information recorded on the Study Product Returns Form to Guide adherence counseling n Guide the ordering of study product to be resupplied and re-issued n

Study Product Re-Supply n n At each follow-up visit, authorized clinic staff must assess participant eligibility to continue product use For those NOT eligible, product use is temporarily held or permanently discontinued Document fully in participant’s clinic records Inform pharmacy



Study Product Retrieval n n For participants NOT eligible to continue product use, product retrieval may be required, if participant did not bring all unused product to her visit Retrieval may be done by Participant returning product n Study staff conducting outreach to retrieve product from participant n

Study Product Retrieval n See protocol Section 6. 6 for retrieval requirements 24 hours for HIV (oral & vaginal) 24 hours for Grade 3+ liver or renal toxicity (oral) otherwise 5 -7 days working days (oral & vaginal) n Inform Protocol Safety Review Team if product not retrieved within timeframe

Study Product Re-Supply n For participants eligible to continue product use, authorized clinic staff determine quantity of product needed until next visit n n n Usually 30 -day supply Up to 60 -day supply permitted at Io. R discretion More than 60 -day supply requires approval of DAIDS Medical Officer (see SSP Section 9)



Study Product Request Slip n n For signatures, follow local regulations on: n Staff authorized to “prescribe” vs “re-supply” n How often an authorized prescriber must sign n NB: Authorized prescriber must sign for all resumptions Document authorizations on staff roster and maintain current listings of staff in each category

Study Product Re-Issue n n n For participants eligible to continue product use, authorized clinic staff determine quantity of product needed until next visit Total amount = re-supply + re-issue To determine total amount, consider n Number of days until next visit n Standard re-supply quantities (30 tablets/bottle; 10 gels/carton) n Quantity available for re-issue

Ideal Quantity n Unless specific concerns are identified by the Io. R or designee, aim to provide the larger of these two quantities Quantity needed for daily use through the next scheduled visit date, plus 7 days or n Quantity needed for daily use through the next target visit date, plus 7 days n

Minimum Quantity n n At a minimum, participants must be provided with enough study product for daily use until their next scheduled study visit Ideal is ideal, minimum is required

Study Product Re-Supply n n For example, if next visit is targeted to take place in 28 days, but is scheduled to take place in 29 days, aim to provide a 36 -day supply (29+7=36) That is, ideally re-supply 30 and re-issue 6 if available from the participant’s returns At a minimum, a 29 -day supply is required Remember Expiry: Instruct and counsel participant to use re-issued product first



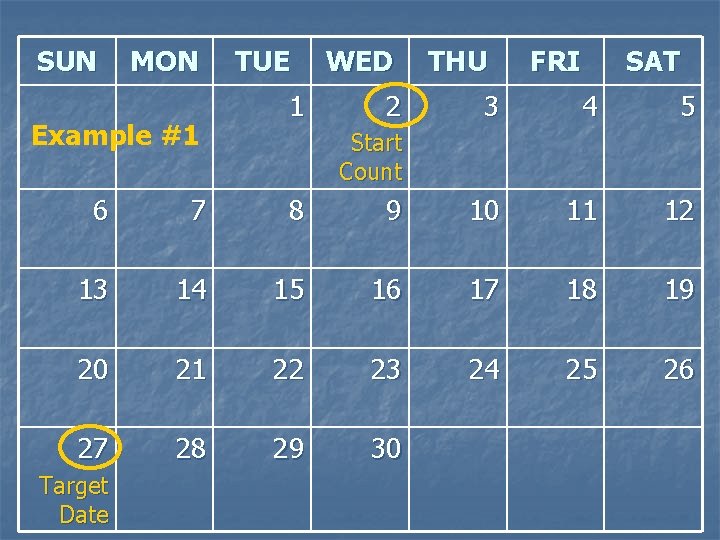
Example #1 n n Today’s visit date = 1 SEP 09 Unused Product Returns slip indicates 2 TDF and 2 TDF/FTC tablets available for re-issue n Target date for next visit is Sunday, 27 SEP 09 n Scheduled date for next visit is Friday, 25 SEP 09 n n What is the ideal quantity of product to provide to the participant at today’s visit? How much would you re-supply and how much would you re-issue?

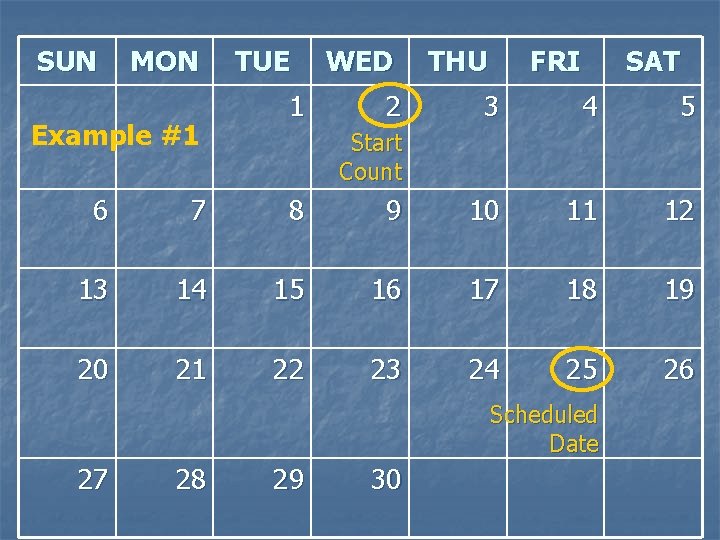
Example #1 n Today’s visit date = 1 SEP 09 n The next visit’s Target Date is 27 SEP 09 What is the number of days from today to the target date? 1. 2. 3. 4. 24 25 26 28

SUN MON Example #1 TUE 1 WED 2 THU FRI SAT 3 4 5 Start Count 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 Target Date

Example #1 n Today’s visit date = 1 SEP 09 n The next visit’s Scheduled Date is 25 SEP 09 What is the number of days from today to the scheduled date? 1. 2. 3. 4. 23 24 26 27

SUN MON Example #1 TUE 1 WED 2 THU FRI SAT 3 4 5 Start Count 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 Scheduled Date 27 28 29 30

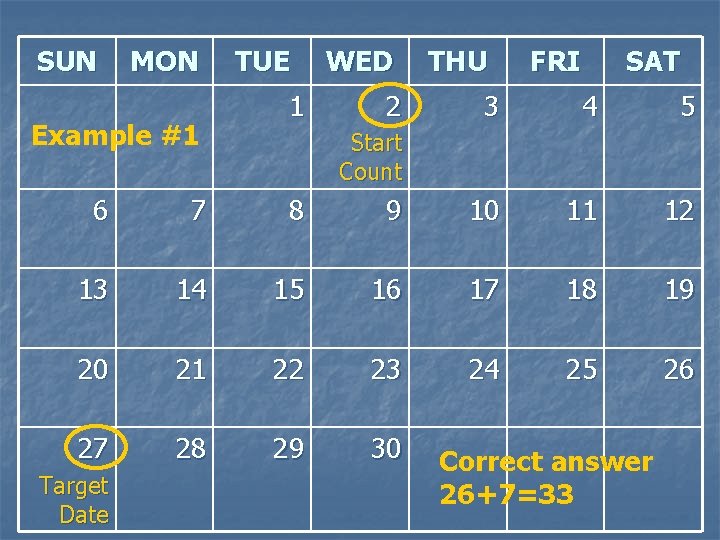
Example #1 n n n Today’s visit date = 1 SEP 09 The next visit’s Target Date is 27 SEP 09 (26 days from today) The next visit’s Scheduled Date is 25 SEP 09 days from today) What is the ideal quantity of product to provide to the participant at today’s visit? 1. 2. 3. 4. 31 33 37 30 (24

SUN MON Example #1 TUE 1 WED 2 THU FRI SAT 3 4 5 Start Count 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 Target Date Correct answer 26+7=33

Example #1 Today’s visit date = 1 SEP 09 n Unused Product Returns Form indicates 2 TDF and 2 TDF/FTC tablets available for re-issue n The next visit’s Target Date is 27 SEP 09 n The next visit’s Scheduled Date is 25 SEP 09 n The ideal quantity of product to provide is 33 How much would you re-supply and how much would you re-issue? n 1. 2. 3. 4. Re-supply 2, Re-issue 30 Re-supply 31, Re-issue 2 Re-supply 30, Re-issue 2

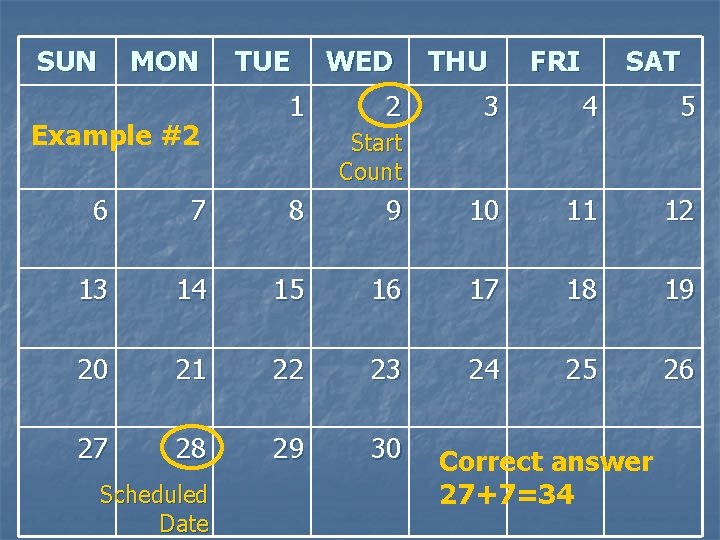
Example #2 n n Today’s visit date = 1 SEP 09 Unused Product Returns Form indicates 8 applicators available for re-issue n The next visit’s Target Date is Saturday, 26 SEP 09 n The next visit’s Scheduled Date is Monday, 28 SEP 09 n n What is the ideal quantity of product to provide to the participant at today’s visit? How much would you re-supply and how much would you re-issue?

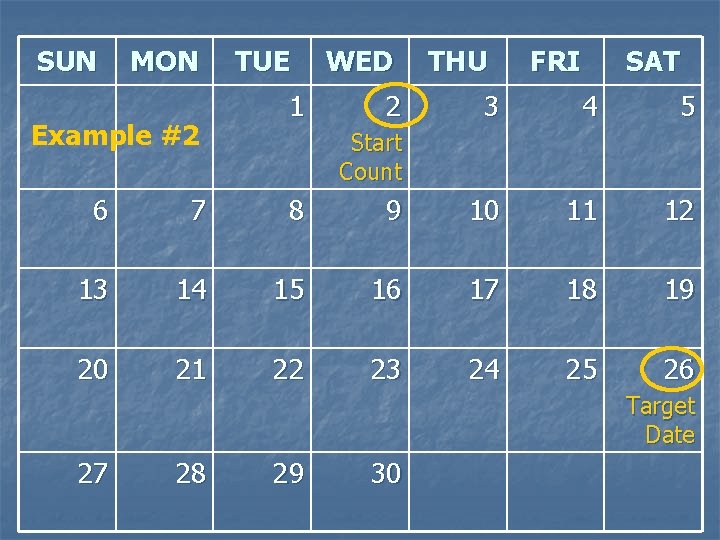
Example #2 n Today’s visit date = 1 SEP 09 n The next visit’s Target Date is 26 SEP 09 What is the number of days from today? 1. 2. 3. 4. 24 25 26 28

SUN MON Example #2 TUE 1 WED 2 THU FRI SAT 3 4 5 Start Count 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 Target Date 27 28 29 30

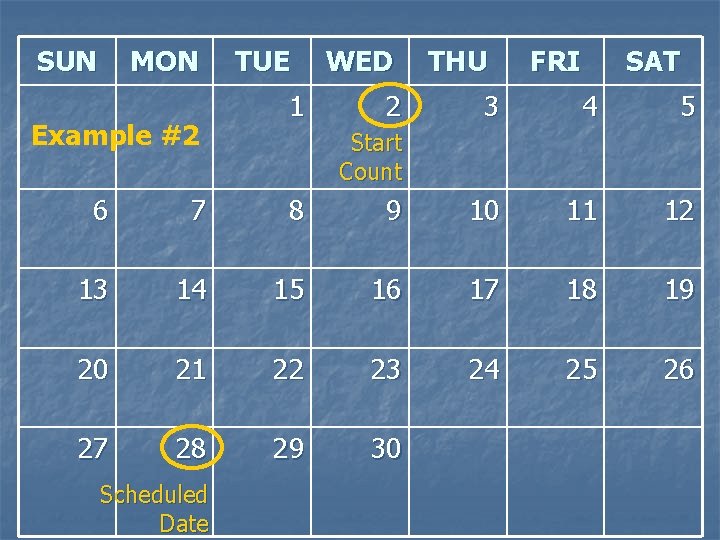
Example #2 n Today’s visit date = 1 SEP 09 n The next visit’s Scheduled Date is 28 SEP 09 What is the number of days from today? 1. 2. 3. 4. 23 24 26 27

SUN MON Example #2 TUE 1 WED 2 THU FRI SAT 3 4 5 Start Count 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 Scheduled Date

Example #2 n Today’s visit date = 1 SEP 09 n The next visit’s Target Date is 26 SEP 09 n The next visit’s Scheduled Date is 28 SEP 09 What is the ideal quantity of product to provide to the participant at today’s visit? 1. 2. 3. 4. 31 34 37 30

SUN MON Example #2 TUE 1 WED 2 THU FRI SAT 3 4 5 Start Count 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 Scheduled Date Correct answer 27+7=34

Example #2 n n Today’s visit date = 1 SEP 09 Unused Product Returns Form indicates 8 applicators available for re-issue n The next visit’s Target Date is 26 SEP 09 n The next visit’s Scheduled Date is 28 SEP 09 How much would you re-supply and how much would you re-issue? 1. 2. 3. 4. Re-supply 26, Re-issue 8 Re-supply 4, Re-issue 30 Re-supply 30, Re-issue 4 Re-supply 30, Re-issue 8

Example #3 n n Today’s visit date = 1 SEP 09 Participant does not return any unused applicators but reports that she has an unopened carton of gel at home that she forgot to bring with her to the visit Target date for next visit is Saturday, 26 SEP 09 25 ) (number of days from today = ___ Scheduled date for next visit is Monday, 28 SEP 09 27 ) (number of days from today = ___ n How will you handle this situation?

Example #4 n Unused Product Returns slip indicates 4 TDF and 4 TDF/FTC tablets available for re-issue n Target Date is in 28 days n Scheduled Date is in 37 days What is the ideal and minimum quantity to supply? 1. 2. Ideal 35, Minimum 37 Ideal 35, Minimum 30

Example #4 n n n Ideal 44, Minimum 37 Four of each tablet available for re-issue How might you handle this situation? n Consider re-scheduling participant n Up to 60 -day supply permitted at Io. R discretion n n More than 60 -day supply requires approval of DAIDS Medical Officer Can always consult the PSRT

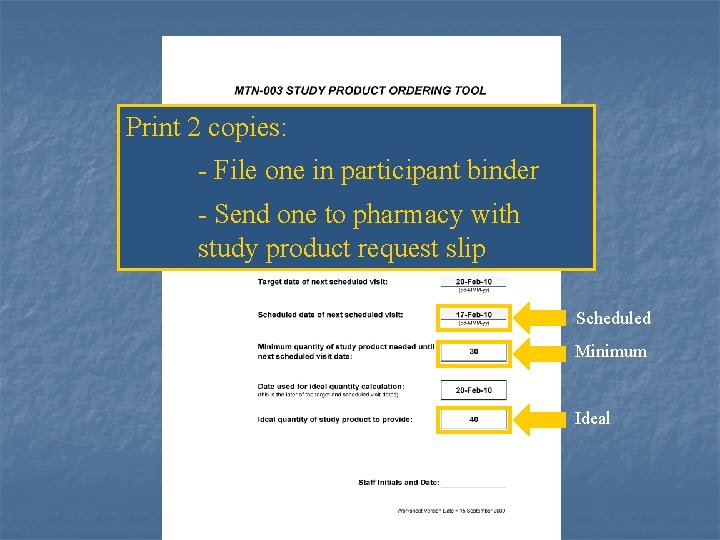
Print 2 copies: - File one in participant binder - Send one to pharmacy with study product request slip Scheduled Minimum Ideal

What Are Your Questions?