Matter and Change Matter Phys v Chem Periodic


















- Slides: 28

Matter and Change!

Matter Phys v. Chem Periodic Tbl Sig Figs Problems 10 10 10 20 20 20 30 30 30 40 40 40 50 50 50

Topic 1 – 10 Points QUESTION: • Define matter. (at least 2 of the 3) ANSWER: • mass, volume, exists as a solid, liquid, gas, or plasma

Topic 1 – 20 Points QUESTION: Which state of matter has the lowest amount of energy? • ANSWER: • Solid

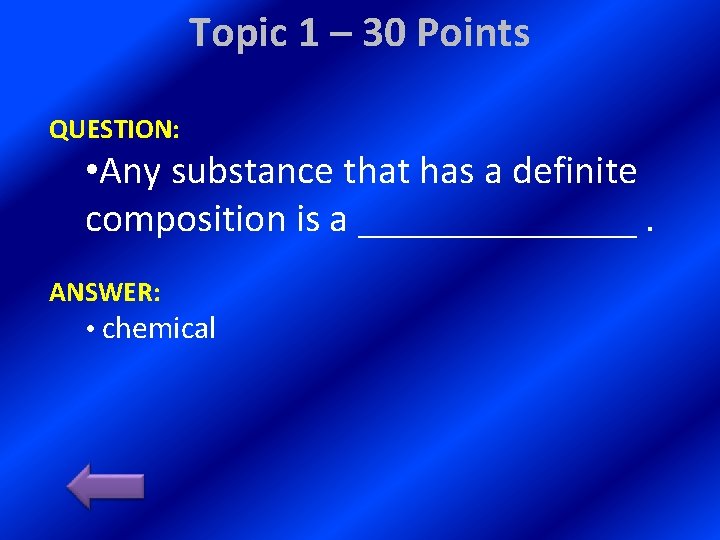
Topic 1 – 30 Points QUESTION: • Any substance that has a definite composition is a _______. ANSWER: • chemical

Topic 1 – 40 Points QUESTION: • Is oxygen gas (O 2) a compound, element, homogeneous mixture, or heterogeneous mixture? ANSWER: • element

Topic 1 – 50 Points QUESTION: • The smallest unit of an element. ANSWER: • an atom

Topic 2 – 10 Points QUESTION: • Changing matter without changing its chemical identity. ANSWER: • Physical

Topic 2 – 20 Points QUESTION: • Burning wood is an example of _____. ANSWER: • Chemical change

Topic 2 – 30 Points QUESTION: • List the five things that indicate a chemical change has occurred ANSWER: • smell, color, gas production, heat production, precipitate formation

Topic 2 – 40 Points QUESTION: • Hot glass cracking in cold water is an example of _______. ANSWER: • Physical Change

Topic 2 – 50 Points QUESTION: • The physical change from a solid to a gas is called _______. ANSWER: • sublimation

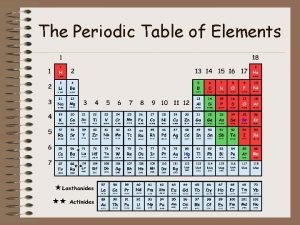
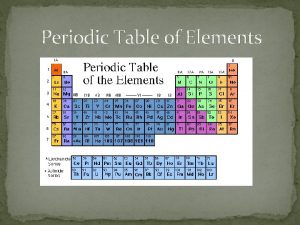
Topic 3 – 10 Points QUESTION: • The vertical columns are also known as _______. ANSWER: • groups or families

Topic 3 – 20 Points QUESTION: • Column 1 is known as the _________. ANSWER: • Alkali metals

Topic 3 – 30 Points QUESTION: • Between metals and non-metals is a group called _____. ANSWER: • Metalloids

Topic 3 – 40 Points QUESTION: • The horizontal rows are also known as ______. ANSWER: • Periods

Topic 3 – 50 Points QUESTION: • List four general properties of metals. ANSWER: • Luster, heat and electrical conductors, ductile, malleable

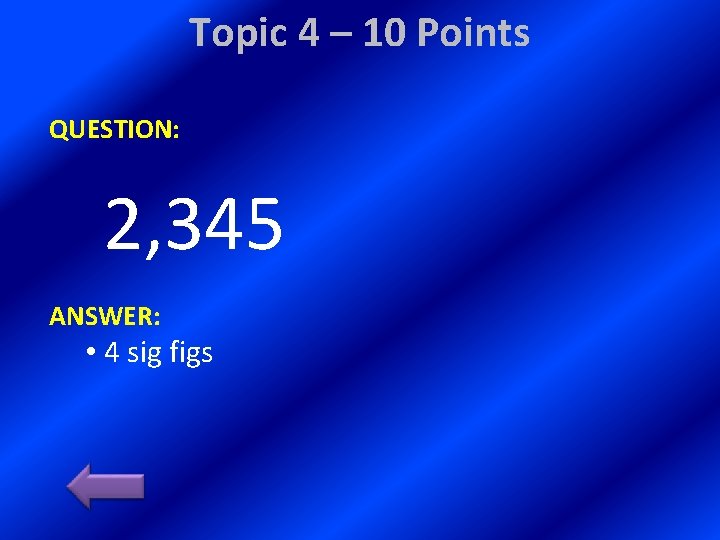
Topic 4 – 10 Points QUESTION: 2, 345 ANSWER: • 4 sig figs

Topic 4 – 20 Points QUESTION: 67, 000 ANSWER: • 2 sig figs

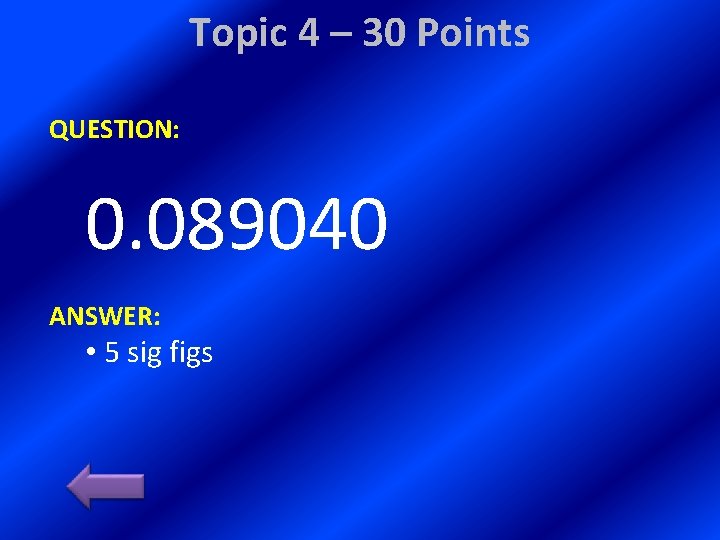
Topic 4 – 30 Points QUESTION: 0. 089040 ANSWER: • 5 sig figs

Topic 4 – 40 Points QUESTION: Problem: 1818. 2 lb x 3. 23 ft 5872. 786 lbft Daily Double Calc says: What is the answer? ANSWER: • 5870 lbft

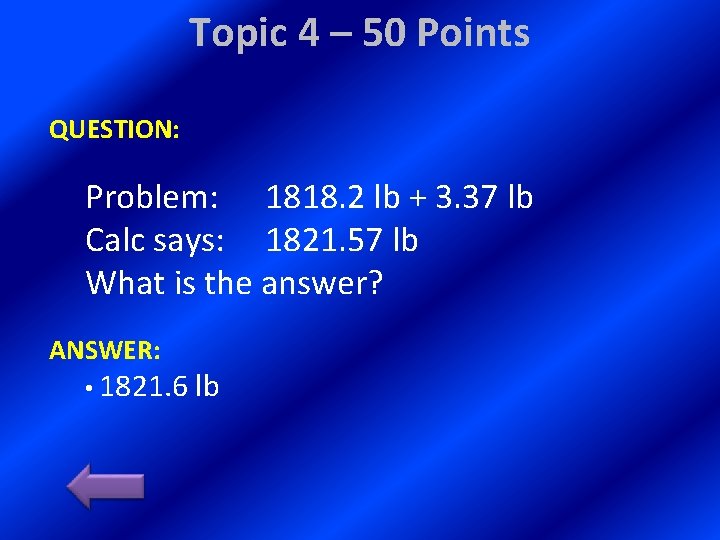
Topic 4 – 50 Points QUESTION: Problem: 1818. 2 lb + 3. 37 lb Calc says: 1821. 57 lb What is the answer? ANSWER: • 1821. 6 lb

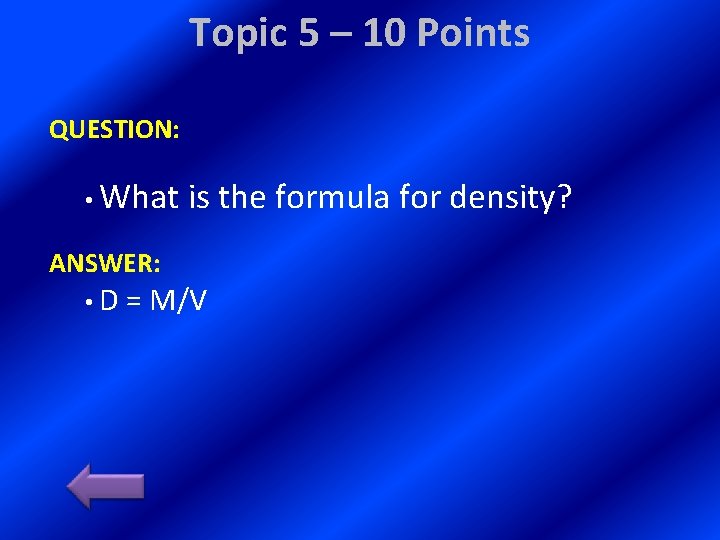
Topic 5 – 10 Points QUESTION: • What ANSWER: is the formula for density? • D = M/V

Topic 5 – 20 Points QUESTION: • If there is a sample of gold with a volume of 10 cm 3 and a mass of 100 g, what is the density? ANSWER: • 10 g/cm 3

Topic 5 – 30 Points QUESTION: • I have 35 m. L of water and the density of water is 1. 0 g/m. L, what is the mass of the water? ANSWER: • 35 g

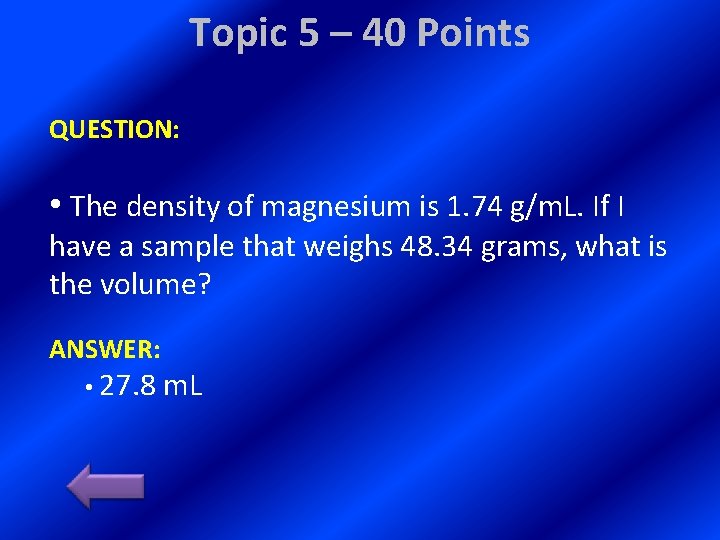
Topic 5 – 40 Points QUESTION: • The density of magnesium is 1. 74 g/m. L. If I have a sample that weighs 48. 34 grams, what is the volume? ANSWER: • 27. 8 m. L

Topic 5 – 50 Points QUESTION: • Convert 2, 780 μm into km and express the answer in scientific notation. ANSWER: • 2. 78 x 10 -6

Final Jeopardy Question: You are given a piece of gold foil with a length of 7. 32 cm and a width of 14. 9 mm. You weigh the foil at. 30 g. The density of gold is 19. 3 g/m. L. What is the thickness of the foil? Answer: . 30 / 19. 3 = 0. 016 cm 3 0. 016 / (7. 32 x 1. 49) = 0. 0015 cm
 Chapter 6 the periodic table
Chapter 6 the periodic table The periodic table and periodic law chapter 6
The periodic table and periodic law chapter 6 The periodic table and periodic law chapter 6
The periodic table and periodic law chapter 6 Periodic tremds
Periodic tremds 15/999 mass street periodic table, o 8
15/999 mass street periodic table, o 8 Uiuc phys 102
Uiuc phys 102 Uiuc physics 101
Uiuc physics 101 Ucsd physics
Ucsd physics Percent difference formula
Percent difference formula Purdue physics 172 past exams
Purdue physics 172 past exams Phys 241 lecture quizzes
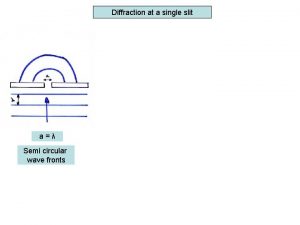
Phys 241 lecture quizzes Single slit envelope
Single slit envelope Optics topics
Optics topics Phys 212 equation sheet
Phys 212 equation sheet Phys 398 uiuc
Phys 398 uiuc Si units for charge
Si units for charge What does att phys mean in medical terms
What does att phys mean in medical terms Aquatic root word
Aquatic root word Mastering physics login
Mastering physics login Phys 214
Phys 214 Phys 172
Phys 172 Phys 121 umd
Phys 121 umd Eosc 111 ubc
Eosc 111 ubc Quarter wave plate jones matrix
Quarter wave plate jones matrix Physics 101 uiuc
Physics 101 uiuc Phys 225
Phys 225 Phys 140
Phys 140 Phys 244
Phys 244 Purdue phys 241
Purdue phys 241