PHYSICS 272 Electric Magnetic Interactions Prof Yulia Pushkar









- Slides: 28

PHYSICS 272 Electric & Magnetic Interactions Prof. Yulia Pushkar ypushkar@purdue. edu Room: 70, Phone: 63279

Course Content This course deals with electric and magnetic interactions, which are central to the structure of matter, to chemical and biological phenomena, and to the design and operation of most modern technology. The main goal of this course is to have you engage in a process central to science: the attempt to model a broad range of physical phenomena using a small set of powerful fundamental principles. The specific focus of the course is an introduction to field theory, in terms of the classical theory of electricity and magnetism (E&M). The course also emphasizes the atomic structure of matter, especially the role of electrons and protons in matter.

Textbook The textbook is Matter & Interactions, vol II: Electric & Magnetic Interactions by R. Chabay & B. Sherwood (John Wiley & Sons 2007). We will cover almost all of the topics in this volume. Make sure it is the Third Edition. The new book comes with a free coupon for Web. Assign, the on-line homework service. Follow the instructions and get yourself registered.

General Information Room PHYS 144: Undergraduate office Room PHYS 12: Help center Room PHYS 290: Physics Library We will use Web. Assign for homework and lab assignments. You will be able to access your scores in CHIP. See “Important Links” on course web page for details! For questions concerning Web. Assign contact: V. K. Saxena: Office: PHYS 176, Phone: 49575

Activities and Responsibilities • In-class activities and responsibilities – You are responsible for attending all classes, and attendance will count toward your grade. – Bring a scientific calculator to class. – If you miss class, it is your responsibility to find out what you missed. Lectures slides will be available shortly after lecture concludes. • Homework – Homework and lab assignments will be posted on the web. See Web. Assign (and Calendar section) for due dates. • Outside class – Study assigned textbook sections. – An assignment to study sections of the textbook means: • Read the assigned textbook sections thoughtfully. • Do the "stop and think" activities. • Write brief solutions to the in-line "exercises" and keep them in a notebook.

Quizzes, Exams, Grades • Clicker Questions in Lecture: – Short multiple choice questions will be posed in lecture. The purpose is to assess your understanding. It will also be used to check attendance. We will start counting clicker questions towards your grade at lecture #4. – You have to purchase an i. Clicker ( http: //www. iclicker. com ) from the bookstore. – You must register your clicker ID in CHIP!!!! • Exams: – There will be two 1. 5 -hour exams and a 2 -hour final exam. All exams are closedbook, but relevant formulas and constants will be provided. • Grades: – The final grade will be determined on the following basis (Course Total = 700 points): • 200 points - final exam • 100 points each - two 1. 5 -hour exams (8 -9: 30 pm Feb 13, April 3) • 75 points - Web. Assign homework • 100 points – Labs • 25 points - Clicker Questions & Attendance • 100 points – Recitation Problems

Argonne National Laboratory

Synchrotron

Clicker Question 1 Set your clicker frequency to AB My major is – A)Engineering B)Science C)Liberal Arts D)Education E)Other

Clicker Question 2 Set your clicker frequency to AB The origin of word “electron” comes from A) current caring wire B) fossilized tree resin C) spring flower D) attraction between objects

The word electron was coined in 1894 by Johnstone Stoney (an Irish physicist) and is derived from the Latin electrum or the Greek elektron meaning amber (fossilized tree resin).

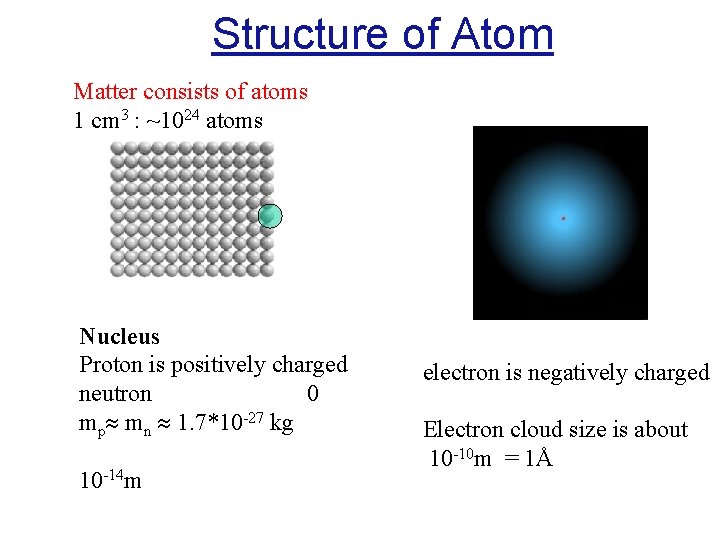
Structure of Atom Matter consists of atoms 1 cm 3 : ~1024 atoms . Nucleus Proton is positively charged neutron 0 mp mn 1. 7*10 -27 kg 10 -14 m electron is negatively charged Electron cloud size is about 10 -10 m = 1Å

Point Charges • Two types: positive and negative • Like charges: repel • Opposite charges: attract • Charge is quantized in units of e Millikan’s oil drop experiment (1910 -1913) • Point charge: Size is small compared to the distance between it and other objects of interest • Electric charge is an intrinsic property of the fundamental particles that everything is made of

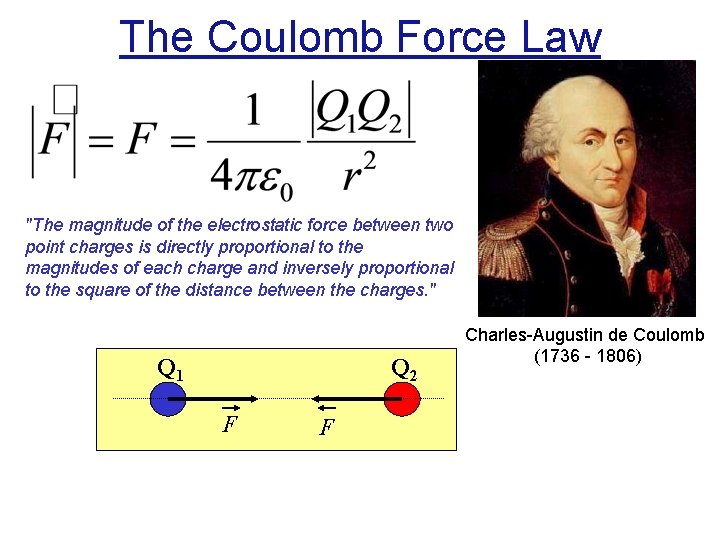
The Coulomb Force Law "The magnitude of the electrostatic force between two point charges is directly proportional to the magnitudes of each charge and inversely proportional to the square of the distance between the charges. " Q 1 Q 2 F F Charles-Augustin de Coulomb (1736 - 1806)

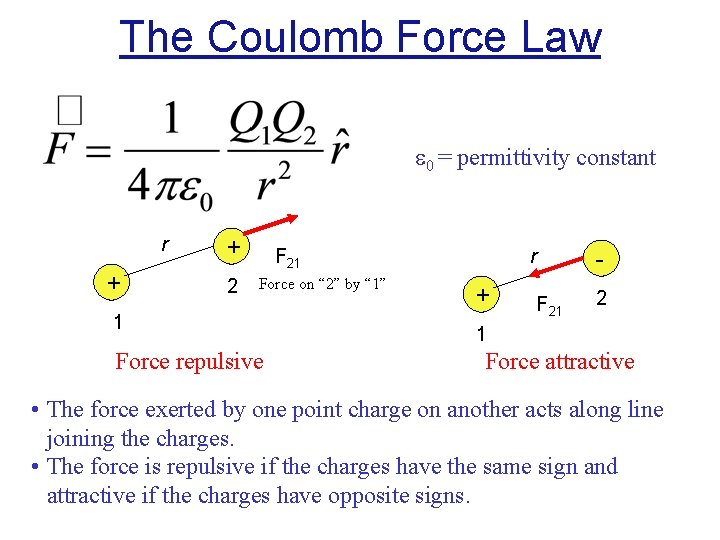
The Coulomb Force Law 0 = permittivity constant r + + 2 F 21 Force on “ 2” by “ 1” 1 Force repulsive + r - F 21 2 1 Force attractive • The force exerted by one point charge on another acts along line joining the charges. • The force is repulsive if the charges have the same sign and attractive if the charges have opposite signs.

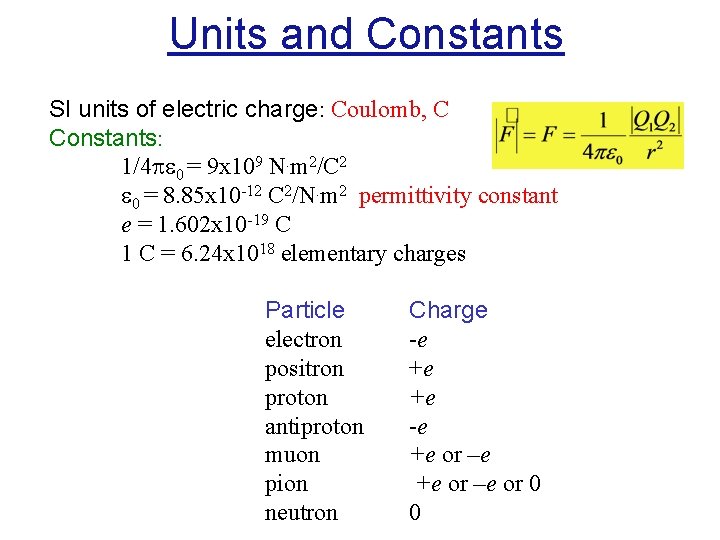
Units and Constants SI units of electric charge: Coulomb, C Constants: 1/4 0 = 9 x 109 N. m 2/C 2 0 = 8. 85 x 10 -12 C 2/N. m 2 permittivity constant e = 1. 602 x 10 -19 C 1 C = 6. 24 x 1018 elementary charges Particle electron positron proton antiproton muon pion neutron Charge -e +e +e -e +e or –e or 0 0

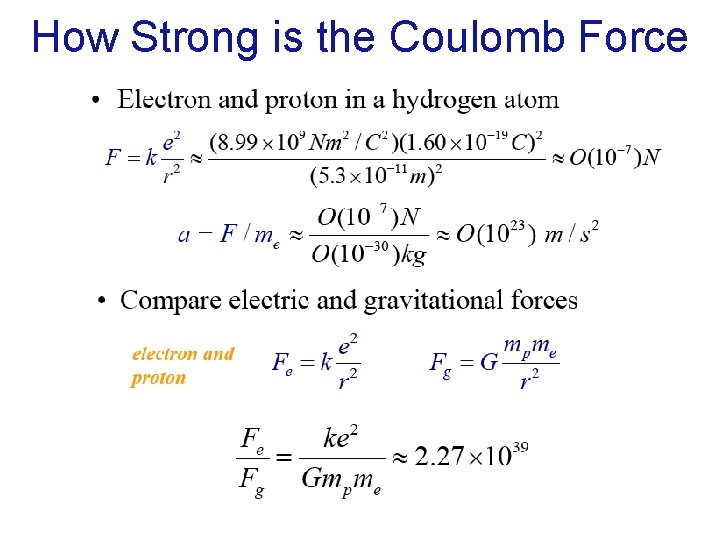
How Strong is the Coulomb Force

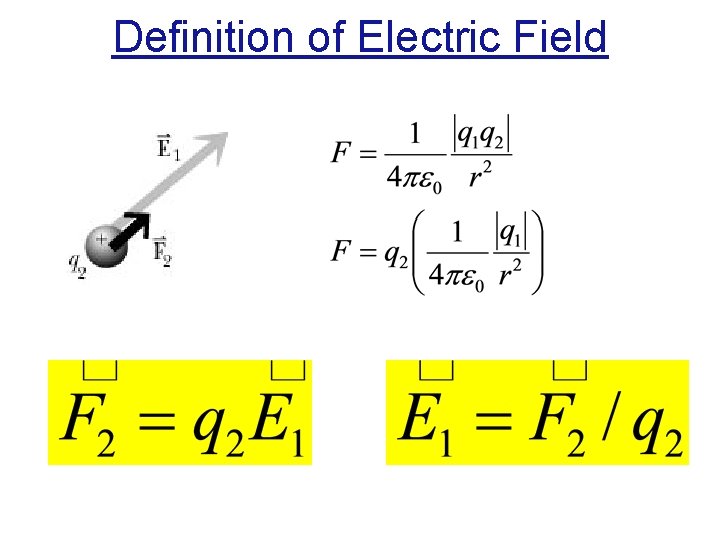
Definition of Electric Field

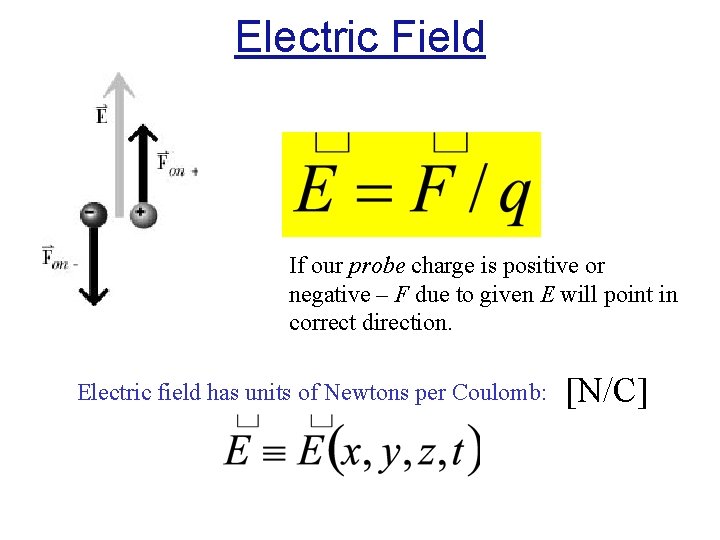
Electric Field If our probe charge is positive or negative – F due to given E will point in correct direction. Electric field has units of Newtons per Coulomb: [N/C]

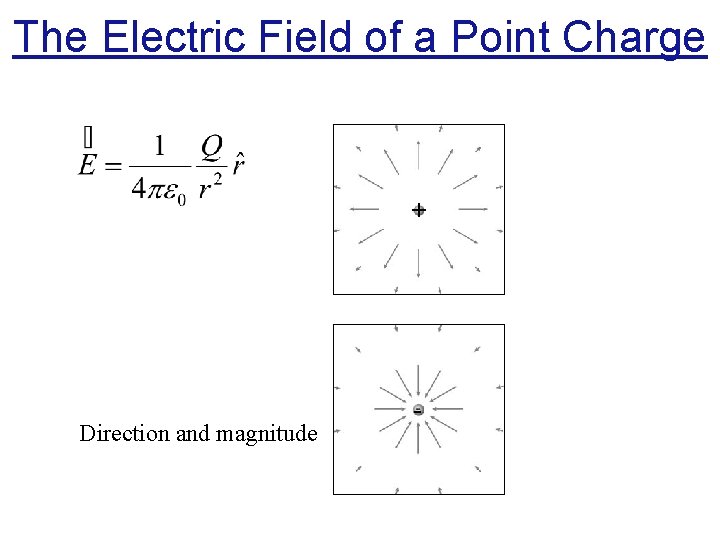
The Electric Field of a Point Charge + Direction and magnitude -

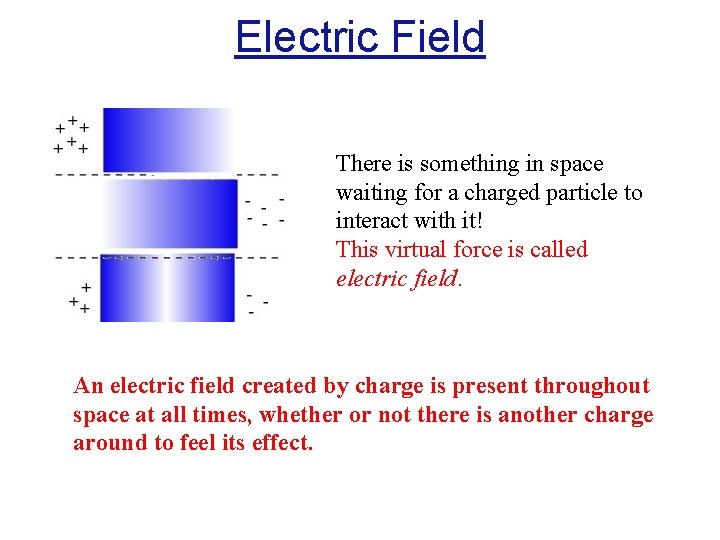
Electric Field There is something in space waiting for a charged particle to interact with it! This virtual force is called electric field. An electric field created by charge is present throughout space at all times, whether or not there is another charge around to feel its effect.

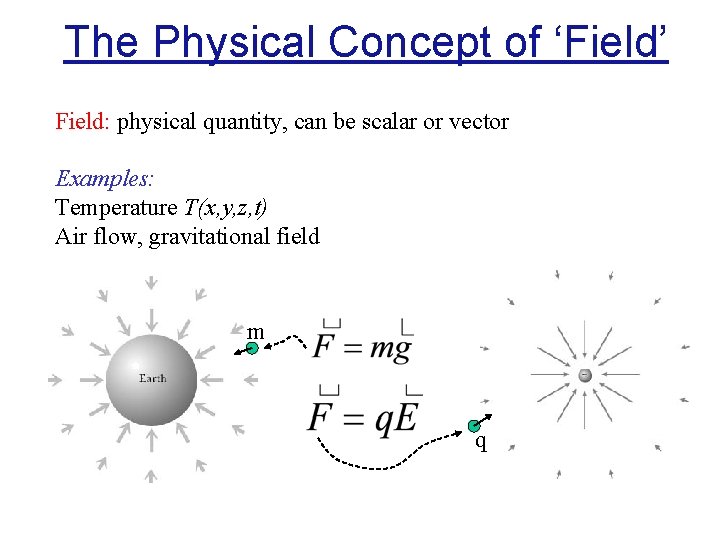
The Physical Concept of ‘Field’ Field: physical quantity, can be scalar or vector Examples: Temperature T(x, y, z, t) Air flow, gravitational field m q

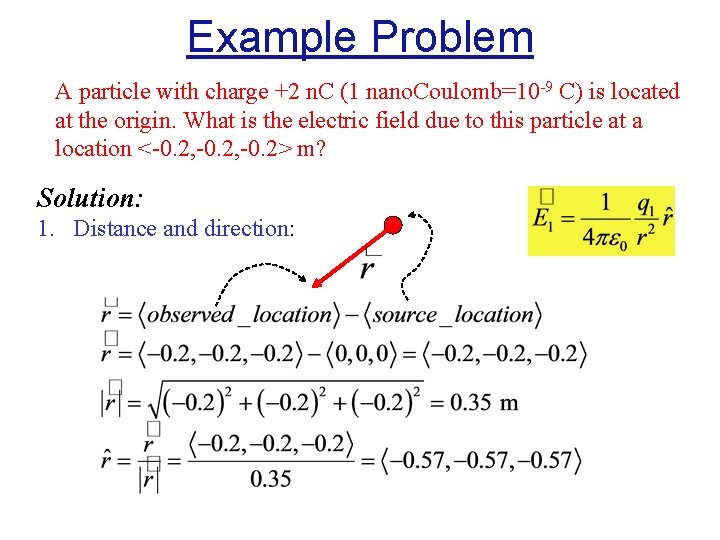
Example Problem A particle with charge +2 n. C (1 nano. Coulomb=10 -9 C) is located at the origin. What is the electric field due to this particle at a location <-0. 2, -0. 2> m? Solution: 1. Distance and direction:

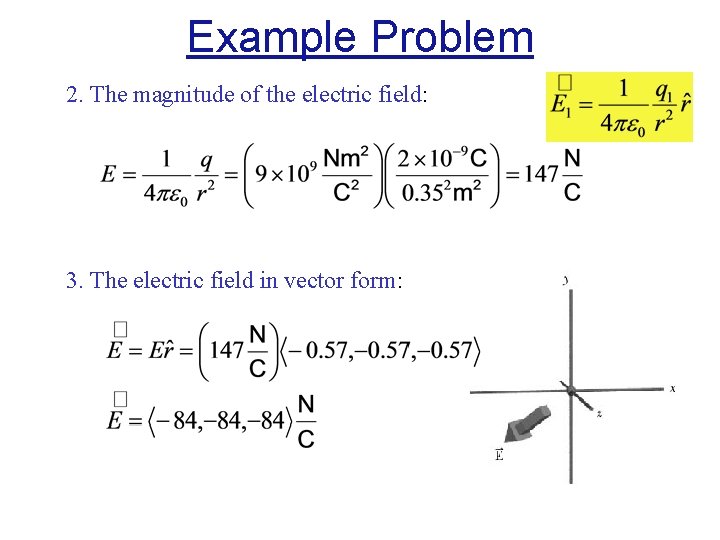
Example Problem 2. The magnitude of the electric field: 3. The electric field in vector form:

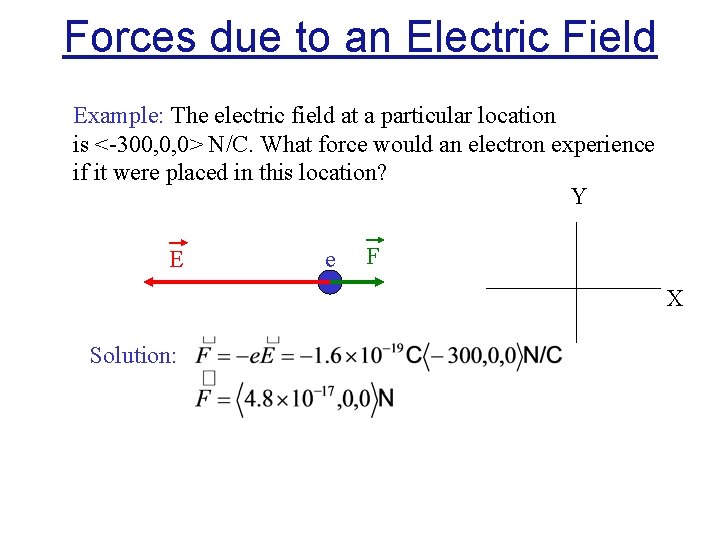
Forces due to an Electric Field Example: The electric field at a particular location is <-300, 0, 0> N/C. What force would an electron experience if it were placed in this location? Y E e F X Solution:

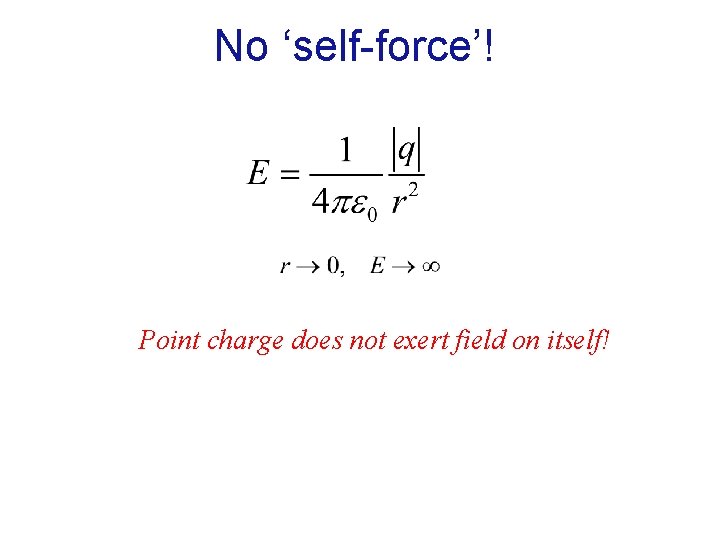
No ‘self-force’! Point charge does not exert field on itself!

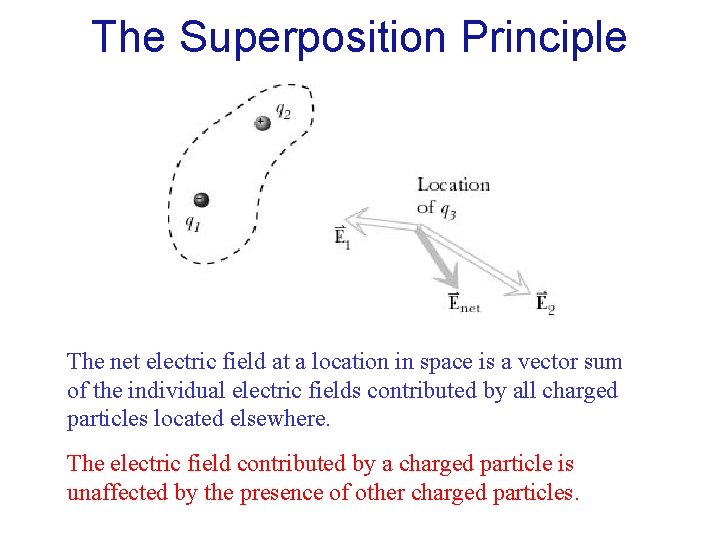
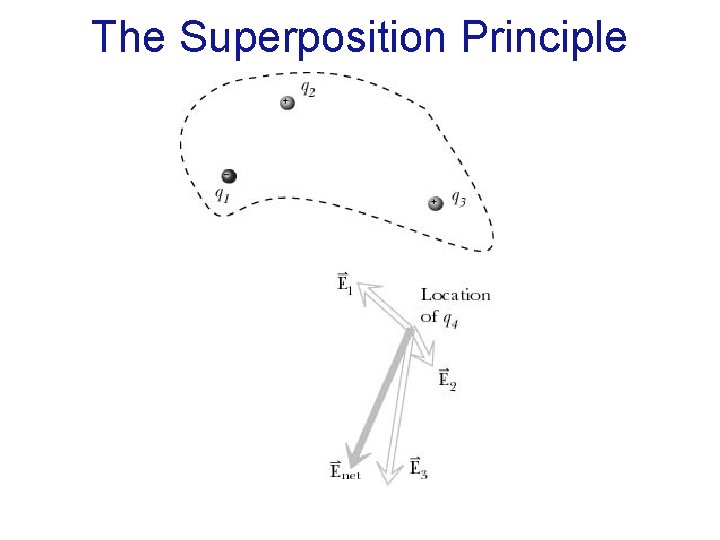
The Superposition Principle The net electric field at a location in space is a vector sum of the individual electric fields contributed by all charged particles located elsewhere. The electric field contributed by a charged particle is unaffected by the presence of other charged particles.

The Superposition Principle