Chem 332 ODette PERIODIC TRENDS WHY UNDERSTAND PERIODIC







- Slides: 15

Chem 332 – O’Dette PERIODIC TRENDS

WHY UNDERSTAND PERIODIC TRENDS? � You can make predictions about the chemical behavior of the elements � Today we are going to focus on the following trends: � Valence Electrons � Atomic Size � Ionization Energy � Electronegativity

VALENCE ELECTRONS � The term valence means highest or outermost level � Atoms are most stable when they have a full or noble gas configuration � Known as the octet rule, all elements want to have 8 valence electrons

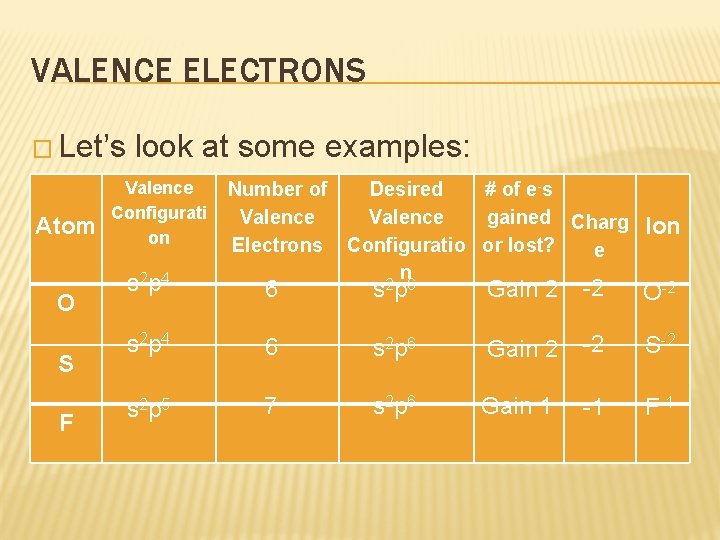
VALENCE ELECTRONS � Let’s Atom O S F look at some examples: Valence Configurati on s 2 p 4 Number of Desired # of e-s Valence gained Charg Electrons Configuratio or lost? e n Ion 6 s 2 p 6 Gain 2 -2 O-2 s 2 p 4 6 s 2 p 6 Gain 2 -2 S-2 s 2 p 5 7 s 2 p 6 Gain 1 -1 F-1

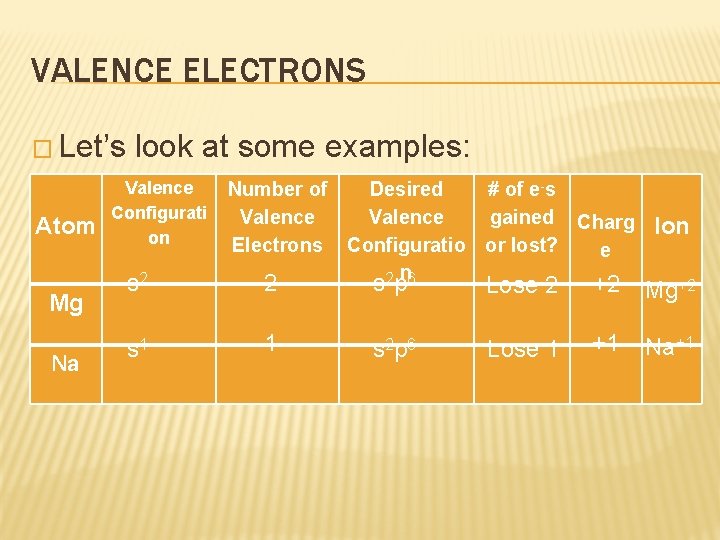
VALENCE ELECTRONS � Let’s Atom Mg Na look at some examples: Valence Configurati on s 2 s 1 Number of Desired # of e-s Valence gained Charg Electrons Configuratio or lost? e 2 n 6 Ion 2 sp Lose 2 +2 Mg+2 1 s 2 p 6 Lose 1 +1 Na+1

LEARNING CHECK � With a partner, determine the number of valence electrons and ion charge of the following elements: � Al �N � Ar Valence Electrons 3 Ion Charge 5 8 -3 0 +3 You should be able to predict the number of valence electrons and ion charge for any elements in Groups 1 -2 & 13 -18!!!!

ATOMIC SIZE � Problem: There are no fixed boundaries to atoms. How do we determine the size? � We use the atomic radius!!! � Atomic radius = the distance from the outermost (valence) electrons to the nucleus

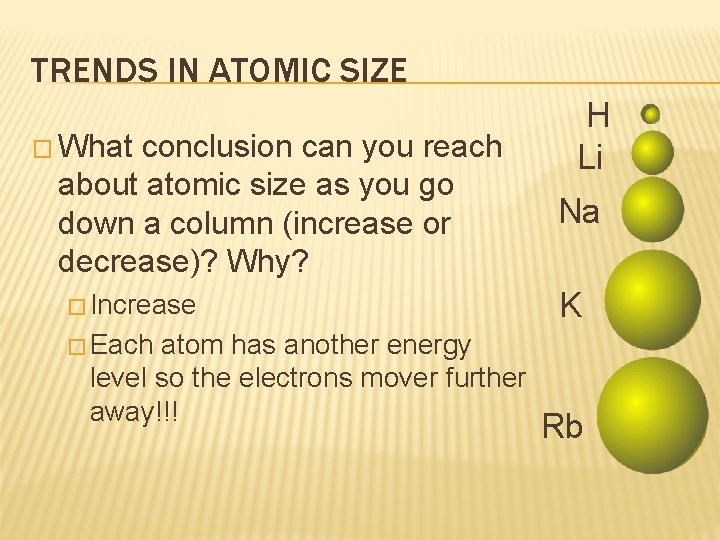
TRENDS IN ATOMIC SIZE � What conclusion can you reach about atomic size as you go down a column (increase or decrease)? Why? � Increase atom has another energy level so the electrons mover further away!!! H Li Na K � Each Rb

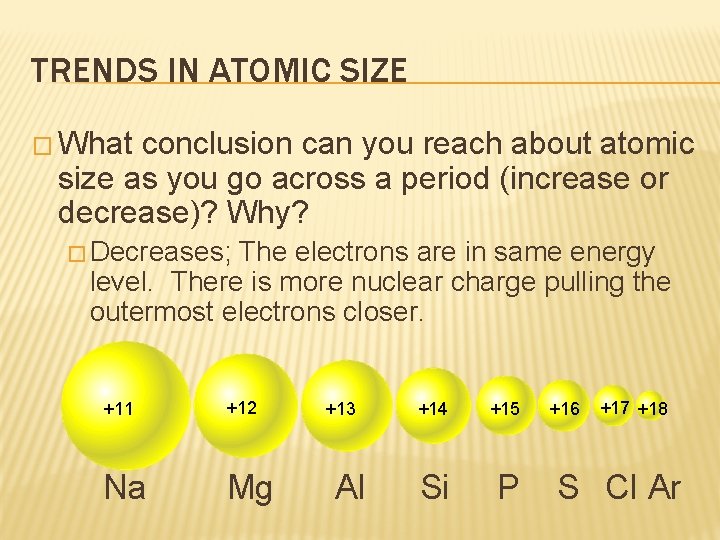
TRENDS IN ATOMIC SIZE � What conclusion can you reach about atomic size as you go across a period (increase or decrease)? Why? � Decreases; The electrons are in same energy level. There is more nuclear charge pulling the outermost electrons closer. +11 +12 Na Mg +13 Al +14 +15 Si P +16 +17 +18 S Cl Ar

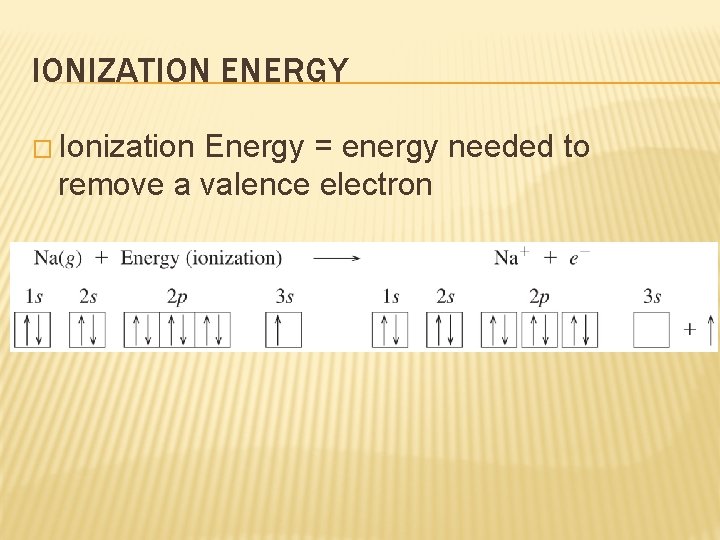
IONIZATION ENERGY � Ionization Energy = energy needed to remove a valence electron

IONIZATION ENERGY � Which group on the periodic table would be most willing to give up their electrons? � Alkali Metals � Why? � They are bigger, there is not a strong pull on the electrons by the nucleus

IONIZATION ENERGY � Which group on the periodic table would be least willing to give up their electrons? � Noble Gases � Why? � They have stable configurations, s 2 p 6.

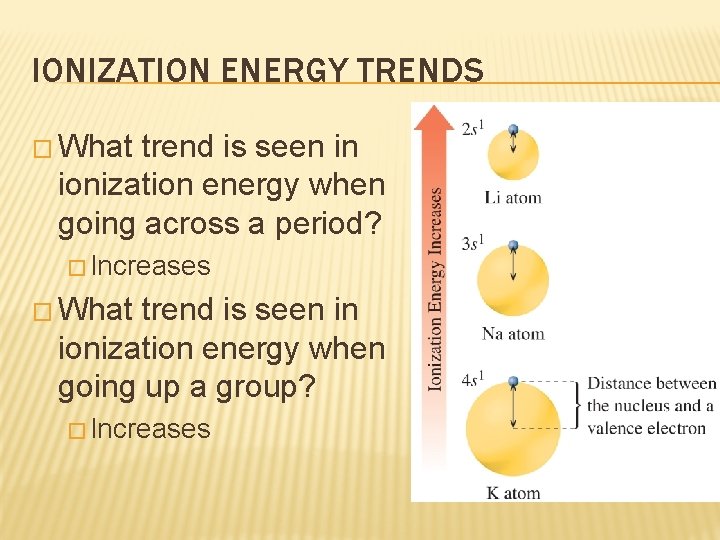
IONIZATION ENERGY TRENDS � What trend is seen in ionization energy when going across a period? � Increases � What trend is seen in ionization energy when going up a group? � Increases

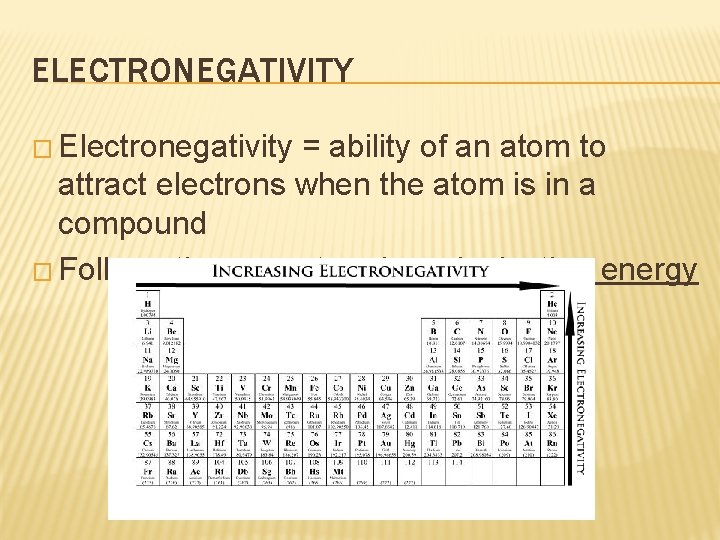
ELECTRONEGATIVITY � Electronegativity = ability of an atom to attract electrons when the atom is in a compound � Follows the same trends as ionization energy

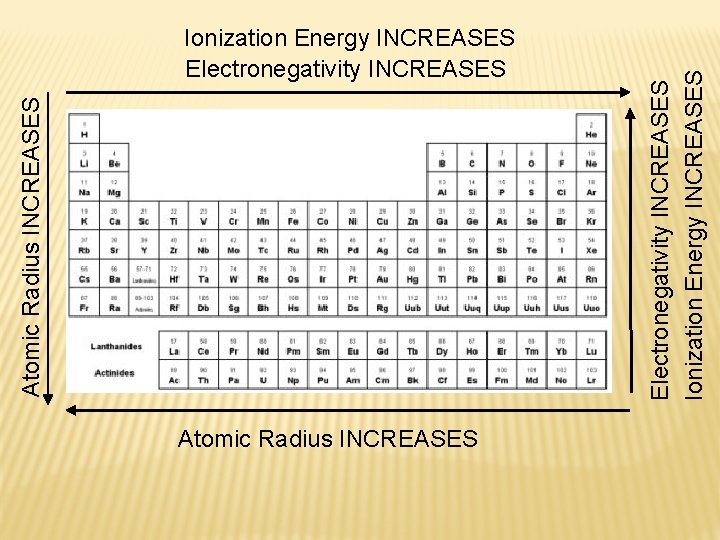
Atomic Radius INCREASES Electronegativity INCREASES Ionization Energy INCREASES Atomic Radius INCREASES Ionization Energy INCREASES Electronegativity INCREASES
 Odette
Odette Odette international
Odette international Odette skills
Odette skills Periodic trends in the periodic table
Periodic trends in the periodic table Alien periodic table periodic trends answers
Alien periodic table periodic trends answers To understand recursion you must understand recursion
To understand recursion you must understand recursion Andreas carlsson bye bye bye
Andreas carlsson bye bye bye Atomic radius trend in periodic table
Atomic radius trend in periodic table Atomic radius and electronegativity
Atomic radius and electronegativity Cheat periodic table
Cheat periodic table Ap chemistry chapter 7 atomic structure and periodicity
Ap chemistry chapter 7 atomic structure and periodicity Graphing periodic trends
Graphing periodic trends Oxidation trends periodic table
Oxidation trends periodic table Electronegativity meaning
Electronegativity meaning Zeff trend on periodic table
Zeff trend on periodic table Periodic trends activity worksheet
Periodic trends activity worksheet