Electronegativity and Polarity Chem 332 ODette How do



- Slides: 11

Electronegativity and Polarity Chem 332 – O’Dette

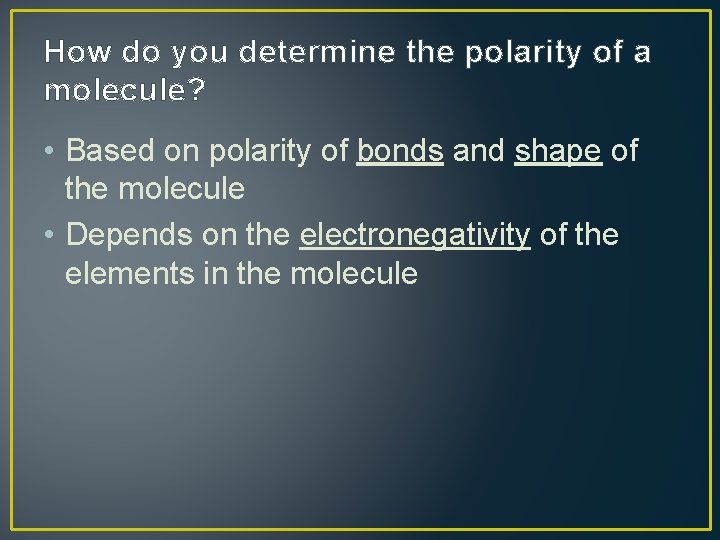
How do you determine the polarity of a molecule? • Based on polarity of bonds and shape of the molecule • Depends on the electronegativity of the elements in the molecule

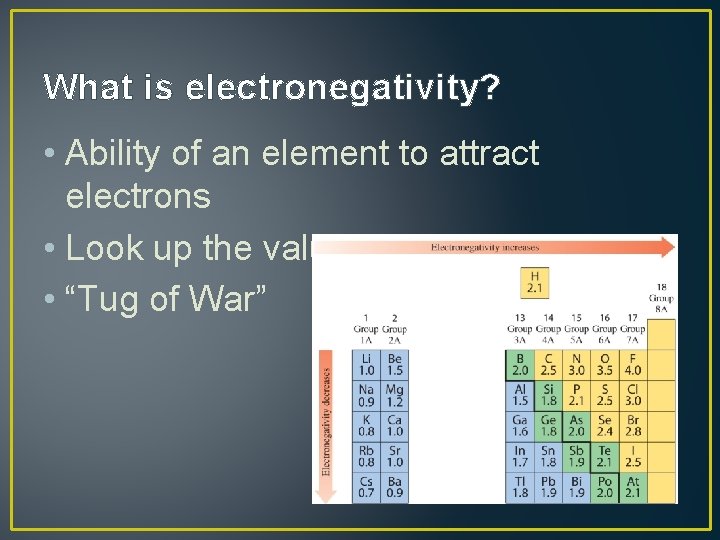
What is electronegativity? • Ability of an element to attract electrons • Look up the values in a table • “Tug of War”

Polarity of Bonds Type of Bond Nonpolar Polar Covalent Ionic Electronegativity Difference Between 0 – 0. 4 Atoms 0. 5 – 1. 8 1. 9 – 3. 3

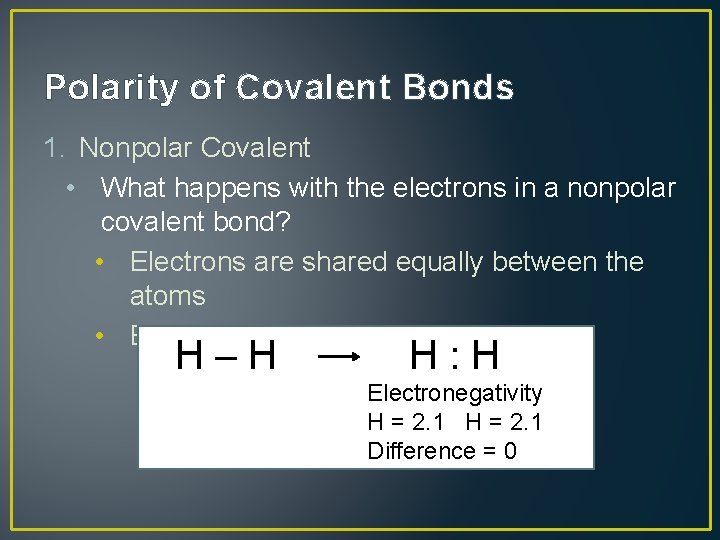
Polarity of Covalent Bonds 1. Nonpolar Covalent • What happens with the electrons in a nonpolar covalent bond? • Electrons are shared equally between the atoms • EX: H 2 H–H H: H Electronegativity H = 2. 1 Difference = 0

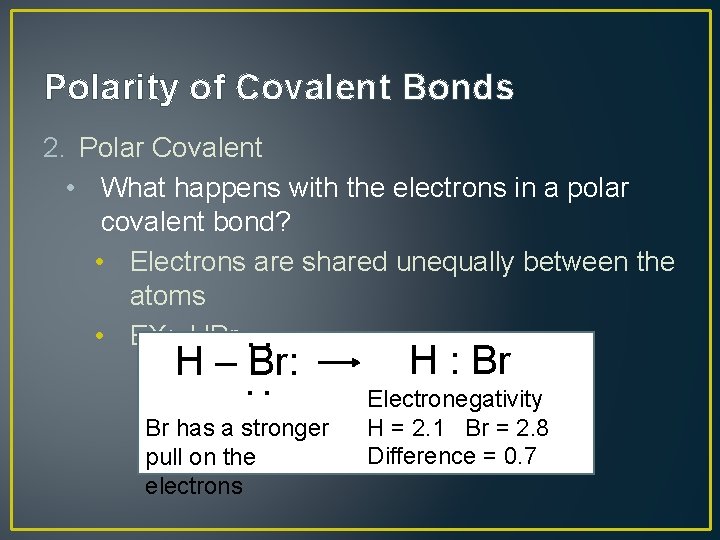
Polarity of Covalent Bonds 2. Polar Covalent • What happens with the electrons in a polar covalent bond? • Electrons are shared unequally between the atoms • EX: HBr : : H – Br: Br has a stronger pull on the electrons H : Br Electronegativity H = 2. 1 Br = 2. 8 Difference = 0. 7

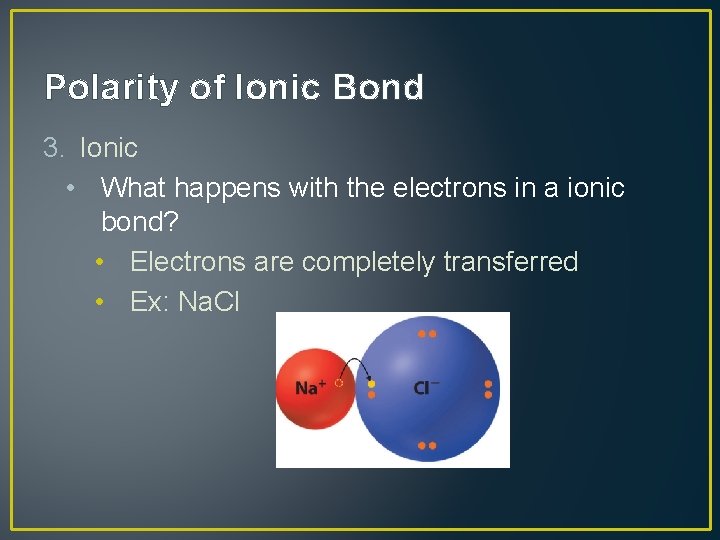
Polarity of Ionic Bond 3. Ionic • What happens with the electrons in a ionic bond? • Electrons are completely transferred • Ex: Na. Cl


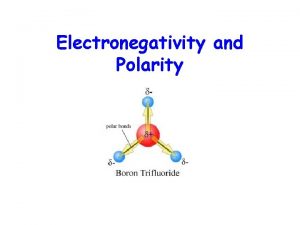
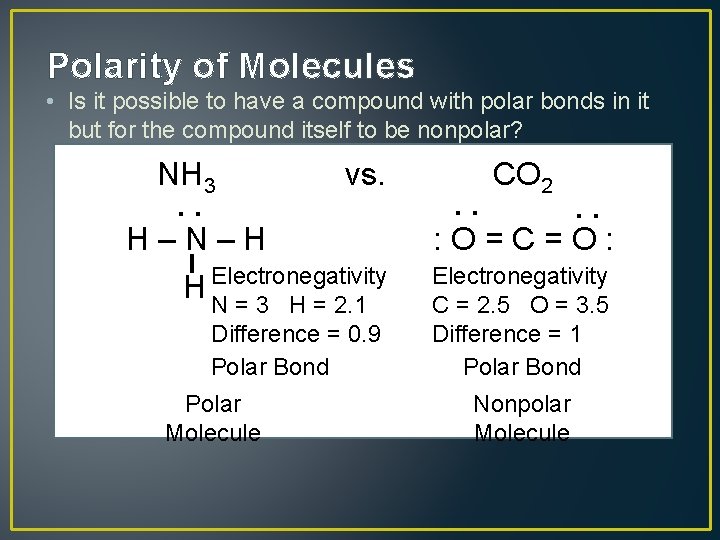
Polarity of Molecules • Is it possible to have a compound with polar bonds in it but for the compound itself to be nonpolar? NH 3 vs. Polar Molecule : Difference = 0. 9 Polar Bond : : H–N–H Electronegativity H N = 3 H = 2. 1 CO 2 : O=C=O: Electronegativity C = 2. 5 O = 3. 5 Difference = 1 Polar Bond Nonpolar Molecule

Polarity of Molecules • Even if the bonds between atoms are polar, the overall molecule polarity is nonpolar if 1. There are no lone pairs on central atom 2. Elements surrounding central atom are the same

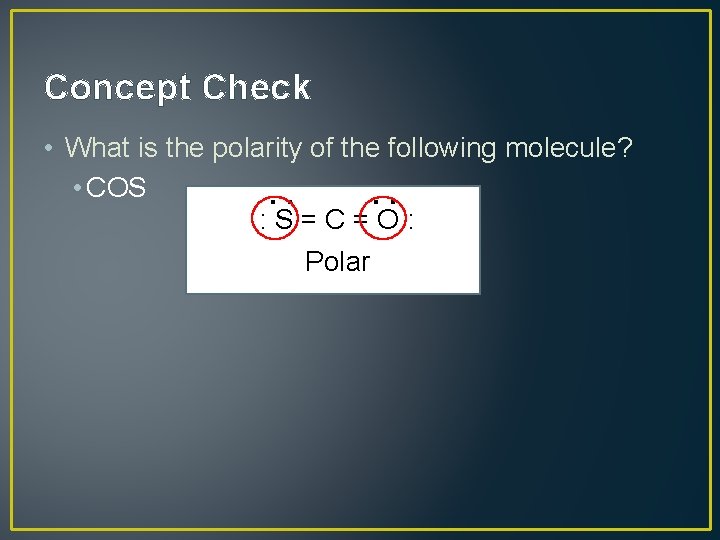
Concept Check : : • What is the polarity of the following molecule? • COS : S=C=O: Polar