Lecture Presentation Chapter 14 Chemical Kinetics 2012 Pearson





![Concentration and Rate • This means Rate [NH 4+] Rate [NO 2 ] Therefore, Concentration and Rate • This means Rate [NH 4+] Rate [NO 2 ] Therefore,](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-23.jpg)
![Rate Laws Rate = k[NH 4+] [NO 2 ] • The overall reaction order Rate Laws Rate = k[NH 4+] [NO 2 ] • The overall reaction order](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-25.jpg)
![Integrated Rate Laws For First order reaction: Rate = -∆[A]/∆t = -k[A] Using calculus Integrated Rate Laws For First order reaction: Rate = -∆[A]/∆t = -k[A] Using calculus](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-27.jpg)
![Integrated Rate Laws Manipulating this equation produces [A]t ln = kt [A]0 ln [A]t Integrated Rate Laws Manipulating this equation produces [A]t ln = kt [A]0 ln [A]t](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-28.jpg)
![First-Order Processes ln [A]t = kt + ln [A]0 Therefore, if a reaction is First-Order Processes ln [A]t = kt + ln [A]0 Therefore, if a reaction is](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-29.jpg)

![Second-Order Processes For Second order reaction: Rate = -∆[A]/∆t = -k[A]2 Similarly, integrating the Second-Order Processes For Second order reaction: Rate = -∆[A]/∆t = -k[A]2 Similarly, integrating the](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-34.jpg)
![Second-Order Processes 1 1 = kt + [A]t [A]0 So if a process is Second-Order Processes 1 1 = kt + [A]t [A]0 So if a process is](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-35.jpg)
![Second-Order Processes • Plotting ln [NO 2] vs. t yields the graph that is Second-Order Processes • Plotting ln [NO 2] vs. t yields the graph that is](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-37.jpg)
![Second-Order Processes 1 [NO 2] • Graphing ln vs. t, however, gives this plot Second-Order Processes 1 [NO 2] • Graphing ln vs. t, however, gives this plot](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-38.jpg)
![Zero Order Reaction • Rate = -∆[A]/∆t = k • The integrated rate law Zero Order Reaction • Rate = -∆[A]/∆t = k • The integrated rate law](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-40.jpg)
![Half-Life For a first-order process, this becomes 0. 5 [A]0 ln = kt 1/2 Half-Life For a first-order process, this becomes 0. 5 [A]0 ln = kt 1/2](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-42.jpg)
![Half-Life For a second-order process, 1 1 = kt 1/2 + 0. 5 [A]0 Half-Life For a second-order process, 1 1 = kt 1/2 + 0. 5 [A]0](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-43.jpg)









![Fast Initial Step • Because Ratef = Rater, k 1[NO] [Br 2] = k Fast Initial Step • Because Ratef = Rater, k 1[NO] [Br 2] = k](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-70.jpg)
![Fast Initial Step Substituting this expression for [NOBr 2] in the rate law for Fast Initial Step Substituting this expression for [NOBr 2] in the rate law for](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-71.jpg)

- Slides: 76

Lecture Presentation Chapter 14 Chemical Kinetics © 2012 Pearson Education, Inc. Dr. Subhash Goel South GA State College Douglas, GA 31533

Kinetics • In kinetics we study the rate at which a chemical process occurs. • Besides information about the speed at which reactions occur, kinetics also sheds light on the reaction mechanism (exactly how the reaction occurs). Chemical Kinetics © 2012 Pearson Education, Inc.

Factors That Affect Reaction Rates • Physical state of the reactants. – In order to react, molecules must come in contact with each other. – The more homogeneous the mixture of reactants, the faster the molecules can react. Chemical Kinetics © 2012 Pearson Education, Inc.

Factors That Affect Reaction Rates • Concentration of reactants – As the concentration of reactants increases, so does the likelihood that reactant molecules will collide. • Temperature – At higher temperatures, reactant molecules have more kinetic energy, move faster, and collide more often and with greater energy. Chemical Kinetics © 2012 Pearson Education, Inc.

Factors That Affect Reaction Rates • Presence of a catalyst. – Catalysts speed up reactions by changing the mechanism of the reaction. – Catalysts are not consumed during the course of the reaction. Chemical Kinetics © 2012 Pearson Education, Inc.

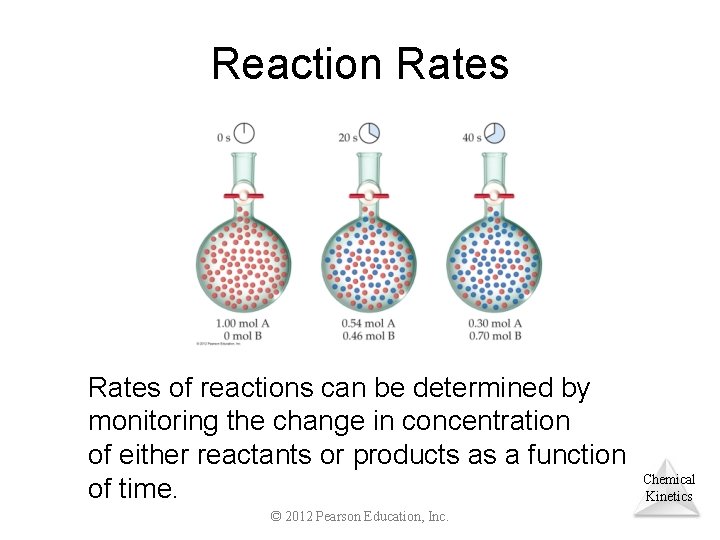
Reaction Rates of reactions can be determined by monitoring the change in concentration of either reactants or products as a function of time. © 2012 Pearson Education, Inc. Chemical Kinetics

Rate of Reaction from Figure Shown in Previous Slide Problem: Calculate the average rate at which A disappears over the time interval from 20 s to 40 s. Problem: Calculate the average rate of appearance of B over the time interval from 0 s to 40 s. Chemical Kinetics

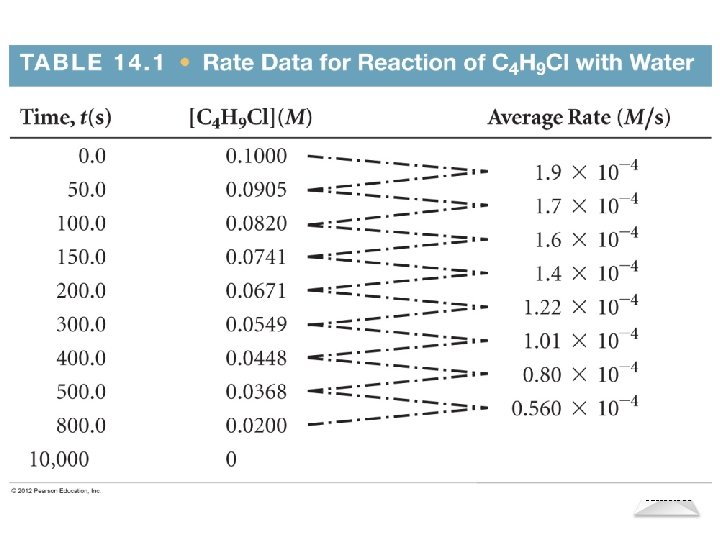
Reaction Rates Consider following reaction: C 4 H 9 Cl(aq) + H 2 O(l) C 4 H 9 OH(aq) + HCl(aq) In this reaction, the concentration of butyl chloride, C 4 H 9 Cl, was measured at various times as shown in Table on next slide Chemical Kinetics © 2012 Pearson Education, Inc.

Chemical Kinetics

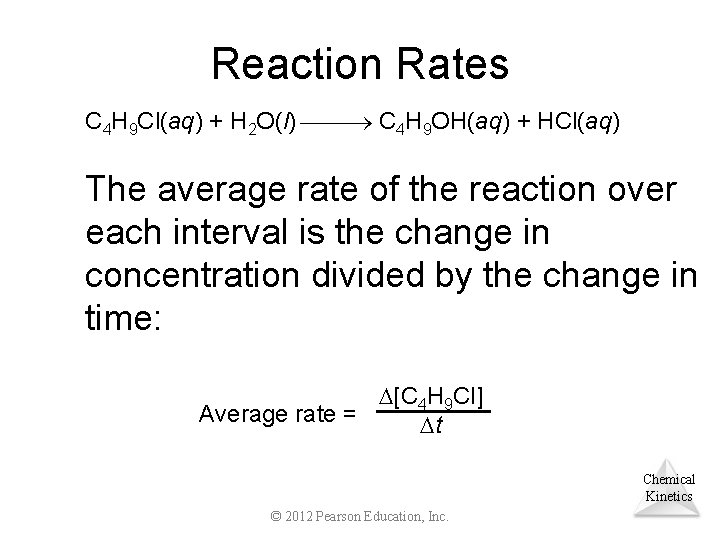
Reaction Rates C 4 H 9 Cl(aq) + H 2 O(l) C 4 H 9 OH(aq) + HCl(aq) The average rate of the reaction over each interval is the change in concentration divided by the change in time: [C 4 H 9 Cl] Average rate = t Chemical Kinetics © 2012 Pearson Education, Inc.

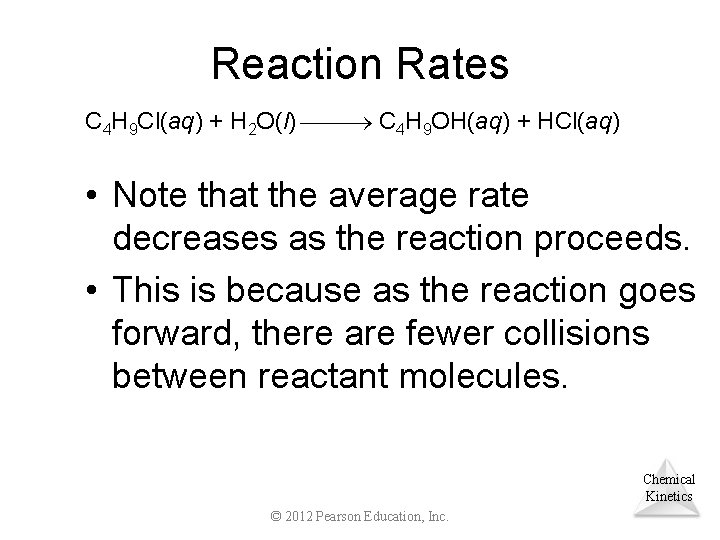
Reaction Rates C 4 H 9 Cl(aq) + H 2 O(l) C 4 H 9 OH(aq) + HCl(aq) • Note that the average rate decreases as the reaction proceeds. • This is because as the reaction goes forward, there are fewer collisions between reactant molecules. Chemical Kinetics © 2012 Pearson Education, Inc.

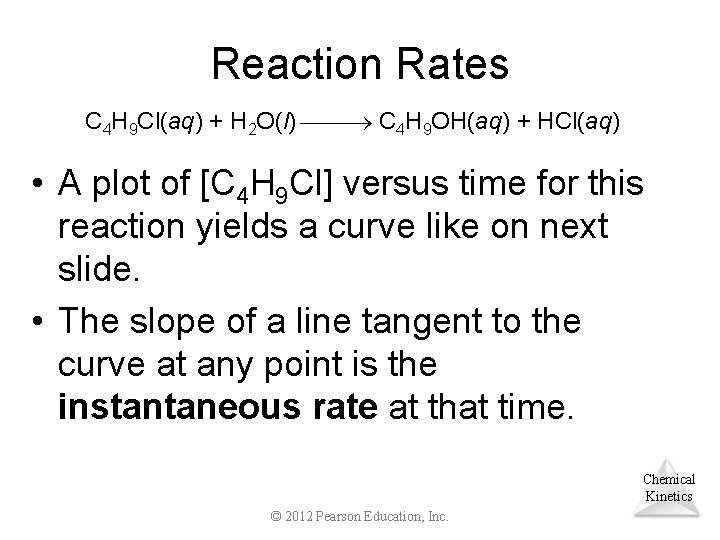
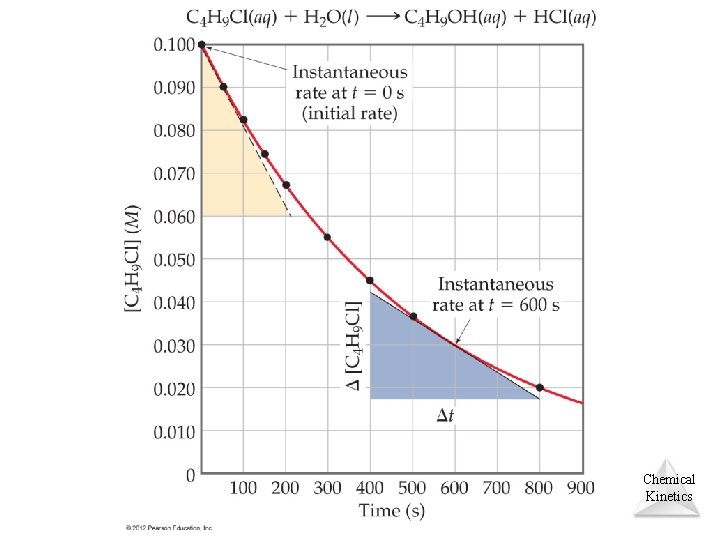
Reaction Rates C 4 H 9 Cl(aq) + H 2 O(l) C 4 H 9 OH(aq) + HCl(aq) • A plot of [C 4 H 9 Cl] versus time for this reaction yields a curve like on next slide. • The slope of a line tangent to the curve at any point is the instantaneous rate at that time. Chemical Kinetics © 2012 Pearson Education, Inc.

Chemical Kinetics

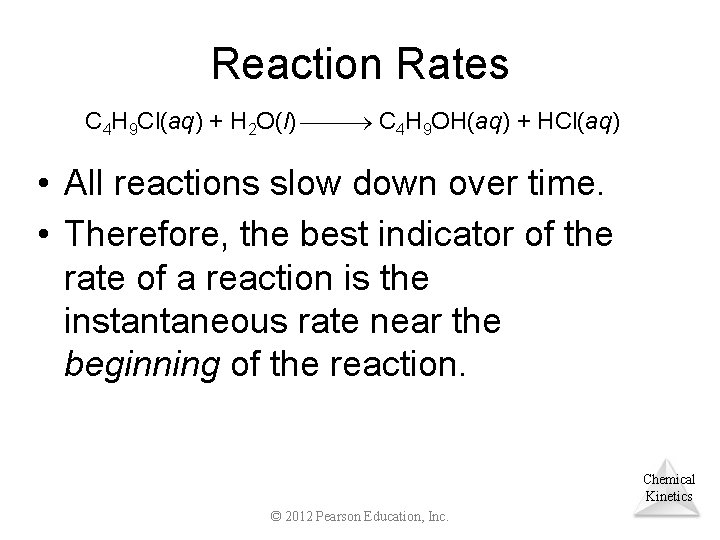
Reaction Rates C 4 H 9 Cl(aq) + H 2 O(l) C 4 H 9 OH(aq) + HCl(aq) • All reactions slow down over time. • Therefore, the best indicator of the rate of a reaction is the instantaneous rate near the beginning of the reaction. Chemical Kinetics © 2012 Pearson Education, Inc.

Exercise: Calculating an Instantaneous Rate of Reaction Using Figure on previous slide calculate the instantaneous rate of disappearance of C 4 H 9 Cl at t = 0 s (the initial rate). Chemical Kinetics

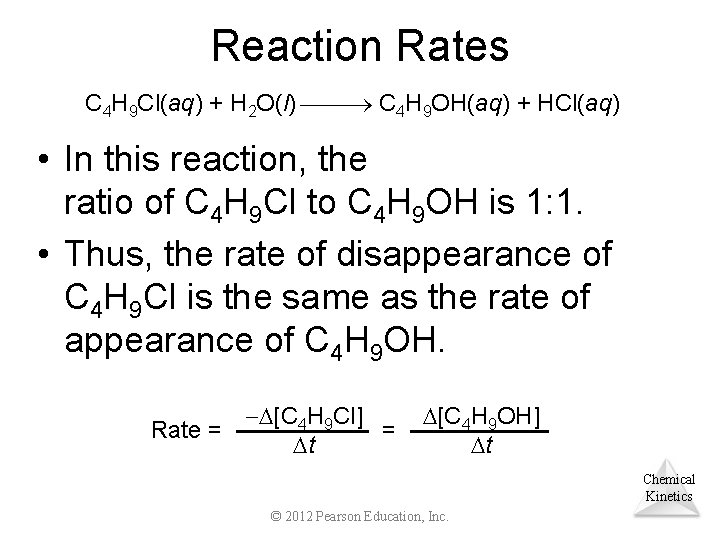
Reaction Rates C 4 H 9 Cl(aq) + H 2 O(l) C 4 H 9 OH(aq) + HCl(aq) • In this reaction, the ratio of C 4 H 9 Cl to C 4 H 9 OH is 1: 1. • Thus, the rate of disappearance of C 4 H 9 Cl is the same as the rate of appearance of C 4 H 9 OH. Rate = [C 4 H 9 Cl] = t [C 4 H 9 OH] t Chemical Kinetics © 2012 Pearson Education, Inc.

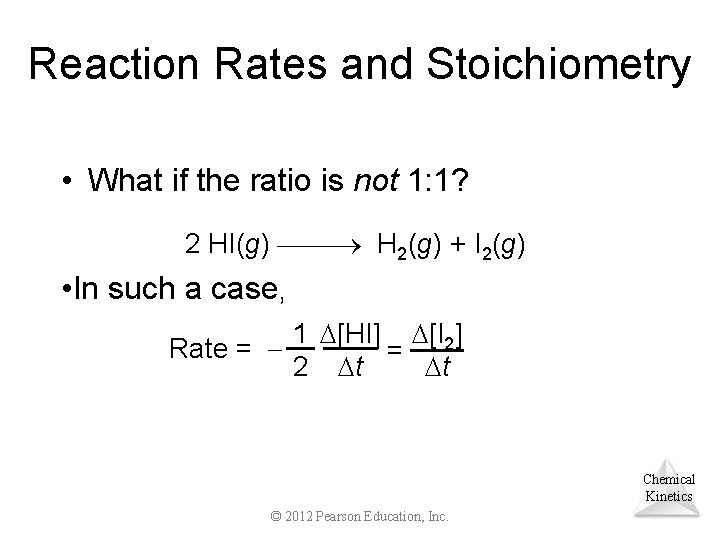
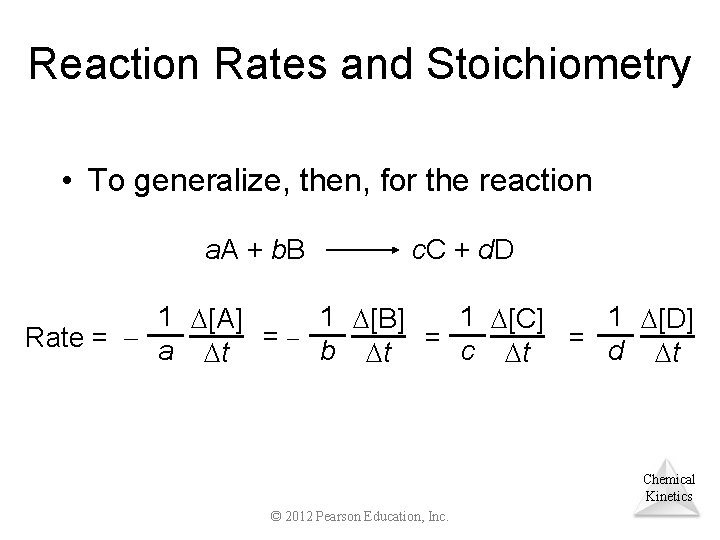
Reaction Rates and Stoichiometry • What if the ratio is not 1: 1? 2 HI(g) H 2(g) + I 2(g) • In such a case, 1 [HI] [I 2] Rate = = 2 t t Chemical Kinetics © 2012 Pearson Education, Inc.

Reaction Rates and Stoichiometry • To generalize, then, for the reaction a. A + b. B c. C + d. D 1 [A] 1 [B] 1 [C] 1 [D] = b = c = d Rate = a t t Chemical Kinetics © 2012 Pearson Education, Inc.

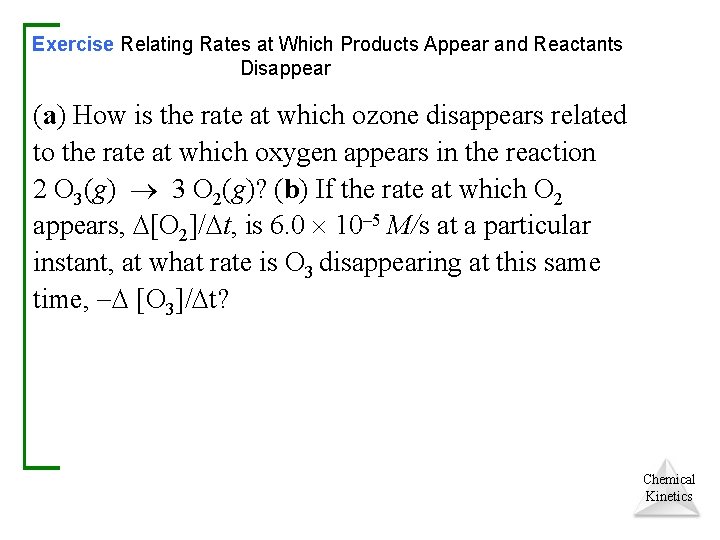
Exercise Relating Rates at Which Products Appear and Reactants Disappear (a) How is the rate at which ozone disappears related to the rate at which oxygen appears in the reaction 2 O 3(g) 3 O 2(g)? (b) If the rate at which O 2 appears, [O 2]/ t, is 6. 0 10– 5 M/s at a particular instant, at what rate is O 3 disappearing at this same time, [O 3]/ t? Chemical Kinetics

Concentration and Rate One can gain information about the rate of a reaction by seeing how the rate changes with changes in concentration. Chemical Kinetics © 2012 Pearson Education, Inc.

Chemical Kinetics

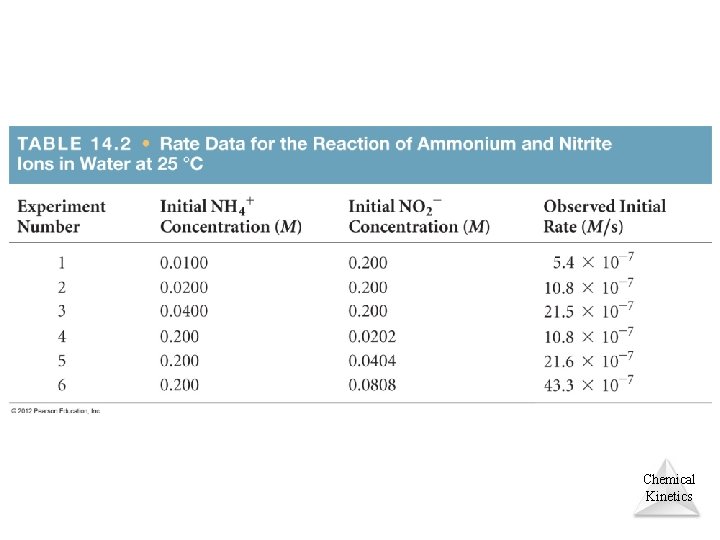
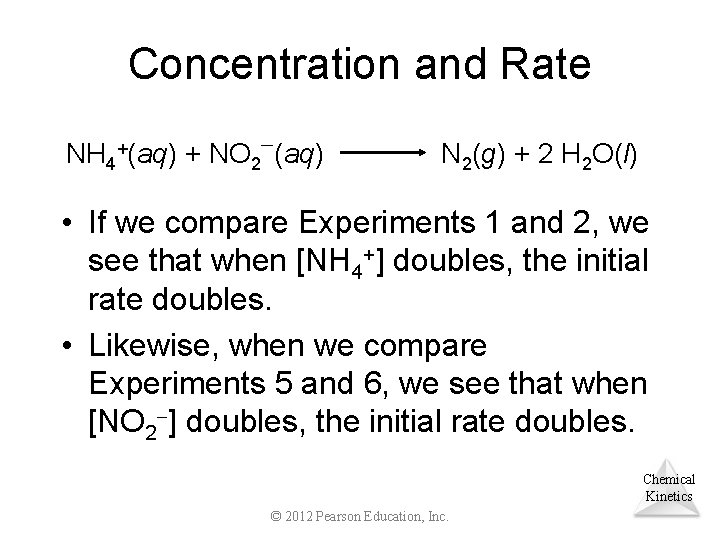
Concentration and Rate NH 4+(aq) + NO 2 (aq) N 2(g) + 2 H 2 O(l) • If we compare Experiments 1 and 2, we see that when [NH 4+] doubles, the initial rate doubles. • Likewise, when we compare Experiments 5 and 6, we see that when [NO 2 ] doubles, the initial rate doubles. Chemical Kinetics © 2012 Pearson Education, Inc.
![Concentration and Rate This means Rate NH 4 Rate NO 2 Therefore Concentration and Rate • This means Rate [NH 4+] Rate [NO 2 ] Therefore,](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-23.jpg)
Concentration and Rate • This means Rate [NH 4+] Rate [NO 2 ] Therefore, [NO 2 ] Rate [NH 4 which, when written as an equation, becomes + Rate = k [NH 4 ] [NO 2 ] • This equation is called the rate law, and k is the rate constant. Chemical +] Kinetics © 2012 Pearson Education, Inc.

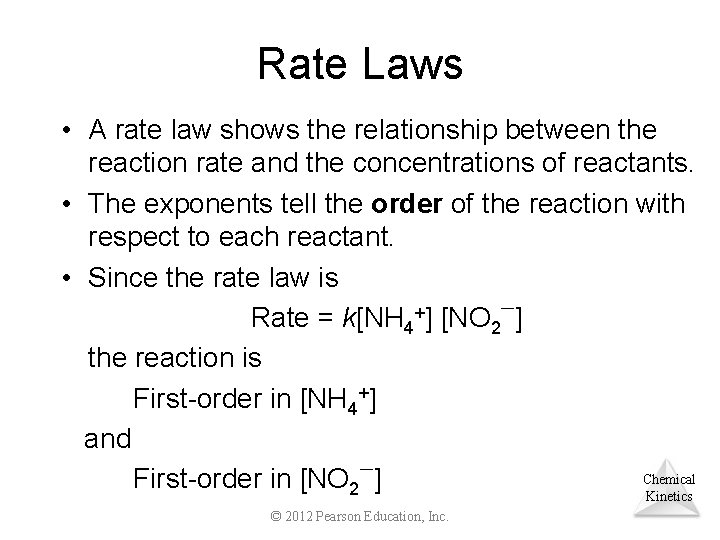
Rate Laws • A rate law shows the relationship between the reaction rate and the concentrations of reactants. • The exponents tell the order of the reaction with respect to each reactant. • Since the rate law is Rate = k[NH 4+] [NO 2 ] the reaction is First-order in [NH 4+] and Chemical First-order in [NO 2 ] Kinetics © 2012 Pearson Education, Inc.
![Rate Laws Rate kNH 4 NO 2 The overall reaction order Rate Laws Rate = k[NH 4+] [NO 2 ] • The overall reaction order](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-25.jpg)
Rate Laws Rate = k[NH 4+] [NO 2 ] • The overall reaction order can be found by adding the exponents on the reactants in the rate law. • This reaction is second-order overall. Chemical Kinetics © 2012 Pearson Education, Inc.

Exercise Determining a Rate Law from Initial Rate Data The initial rate of a reaction A + B C was measured for several different starting concentrations of A and B, and the results are as follows: Using these data, determine (a) the rate law for the reaction, (b) the rate constant, (c) the rate of the reaction when [A] = 0. 050 M and [B] = 0. 100 M. Chemical Kinetics
![Integrated Rate Laws For First order reaction Rate At kA Using calculus Integrated Rate Laws For First order reaction: Rate = -∆[A]/∆t = -k[A] Using calculus](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-27.jpg)
Integrated Rate Laws For First order reaction: Rate = -∆[A]/∆t = -k[A] Using calculus to integrate the rate law for a firstorder process gives us where [A]t ln = kt [A]0 is the initial concentration of A, and [A]t is the concentration of A at some time, t, during the course of the reaction. © 2012 Pearson Education, Inc. Chemical Kinetics
![Integrated Rate Laws Manipulating this equation produces At ln kt A0 ln At Integrated Rate Laws Manipulating this equation produces [A]t ln = kt [A]0 ln [A]t](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-28.jpg)
Integrated Rate Laws Manipulating this equation produces [A]t ln = kt [A]0 ln [A]t ln [A]0 = kt ln [A]t = kt + ln [A]0 which is in the form y = mx + b © 2012 Pearson Education, Inc. Chemical Kinetics
![FirstOrder Processes ln At kt ln A0 Therefore if a reaction is First-Order Processes ln [A]t = kt + ln [A]0 Therefore, if a reaction is](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-29.jpg)
First-Order Processes ln [A]t = kt + ln [A]0 Therefore, if a reaction is first-order, a plot of ln [A] vs. t will yield a straight line, and the slope of the line will be k. Chemical Kinetics © 2012 Pearson Education, Inc.

Problem The decomposition of a certain insecticide in water at 12 C follows first-order kinetics with a rate constant of 1. 45 yr 1. A quantity of this insecticide is washed into a lake on June 1, leading to a concentration of 5. 0 10 7 g/cm 3. Assume that the average temperature of the lake is 12 ºC. (a) What is the concentration of the insecticide on June 1 of the following year? (b) How long will it take for the insecticide concentration to decrease to 3. 0 10– 7 g/cm 3? Chemical Kinetics

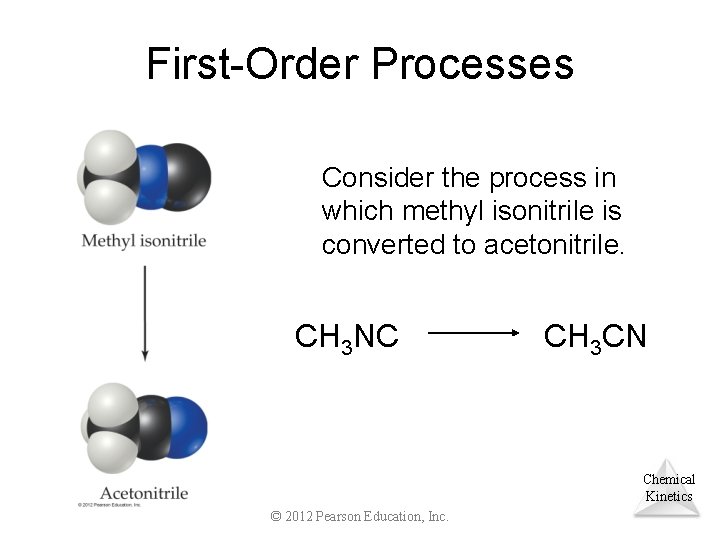
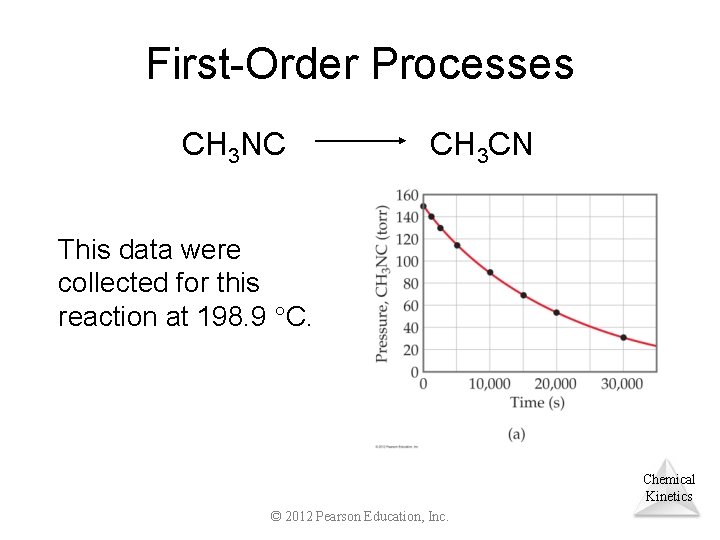
First-Order Processes Consider the process in which methyl isonitrile is converted to acetonitrile. CH 3 NC CH 3 CN Chemical Kinetics © 2012 Pearson Education, Inc.

First-Order Processes CH 3 NC CH 3 CN This data were collected for this reaction at 198. 9 C. Chemical Kinetics © 2012 Pearson Education, Inc.

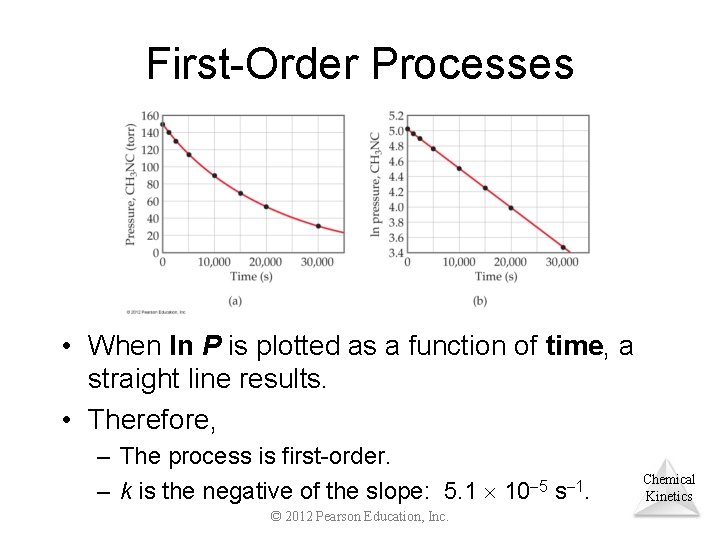
First-Order Processes • When ln P is plotted as a function of time, a straight line results. • Therefore, – The process is first-order. – k is the negative of the slope: 5. 1 10 5 s 1. © 2012 Pearson Education, Inc. Chemical Kinetics
![SecondOrder Processes For Second order reaction Rate At kA2 Similarly integrating the Second-Order Processes For Second order reaction: Rate = -∆[A]/∆t = -k[A]2 Similarly, integrating the](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-34.jpg)
Second-Order Processes For Second order reaction: Rate = -∆[A]/∆t = -k[A]2 Similarly, integrating the rate law for a process that is second-order in reactant A, we get 1 1 = kt + [A]t [A]0 also in the form y = mx + b © 2012 Pearson Education, Inc. Chemical Kinetics
![SecondOrder Processes 1 1 kt At A0 So if a process is Second-Order Processes 1 1 = kt + [A]t [A]0 So if a process is](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-35.jpg)
Second-Order Processes 1 1 = kt + [A]t [A]0 So if a process is second-order in A, a 1 plot of [A] vs. t yields a straight line, and the slope of that line is k. Chemical Kinetics © 2012 Pearson Education, Inc.

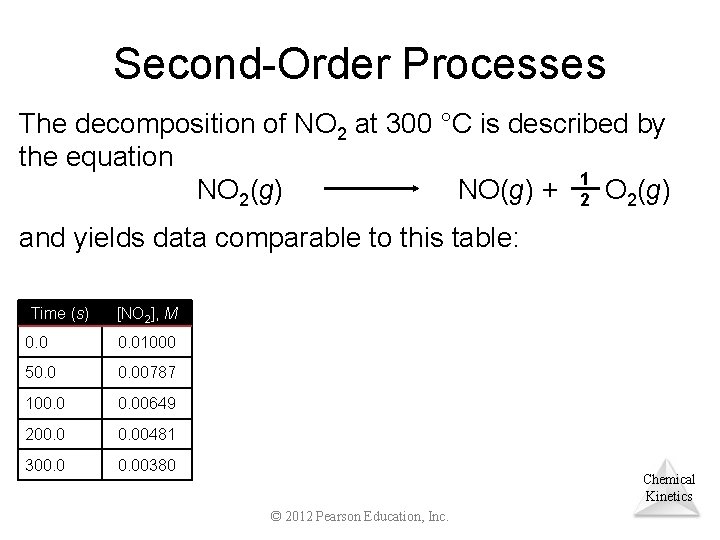
Second-Order Processes The decomposition of NO 2 at 300 °C is described by the equation NO 2(g) NO(g) + 12 O 2(g) and yields data comparable to this table: Time (s) [NO 2], M 0. 01000 50. 00787 100. 00649 200. 00481 300. 00380 Chemical Kinetics © 2012 Pearson Education, Inc.
![SecondOrder Processes Plotting ln NO 2 vs t yields the graph that is Second-Order Processes • Plotting ln [NO 2] vs. t yields the graph that is](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-37.jpg)
Second-Order Processes • Plotting ln [NO 2] vs. t yields the graph that is not a straight line, so the process is not firstorder in [A]. Time (s) [NO 2], M ln [NO 2] 0. 01000 4. 610 50. 00787 4. 845 100. 00649 5. 038 200. 00481 5. 337 300. 00380 5. 573 Chemical Kinetics © 2012 Pearson Education, Inc.
![SecondOrder Processes 1 NO 2 Graphing ln vs t however gives this plot Second-Order Processes 1 [NO 2] • Graphing ln vs. t, however, gives this plot](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-38.jpg)
Second-Order Processes 1 [NO 2] • Graphing ln vs. t, however, gives this plot Fig. 14. 9(b). Time (s) [NO 2], M 1/[NO 2] 0. 01000 100 50. 00787 127 100. 00649 154 200. 00481 208 300. 00380 263 • Because this is a straight line, the process is secondorder in [A]. Chemical Kinetics © 2012 Pearson Education, Inc.

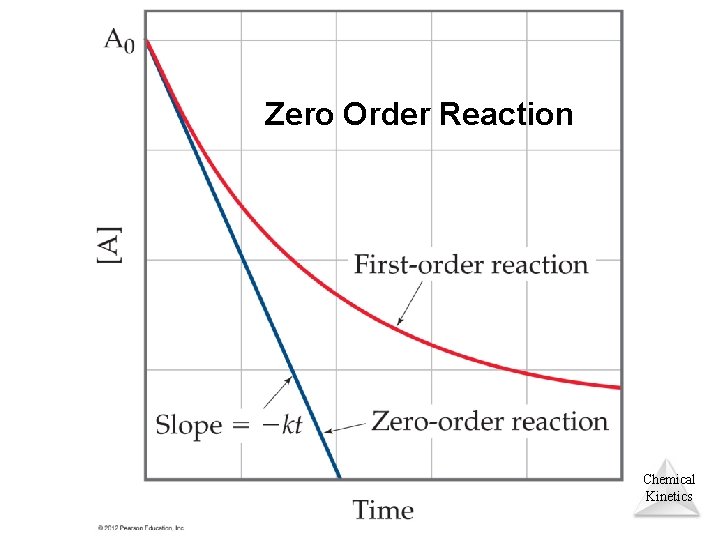
Zero Order Reaction Chemical Kinetics
![Zero Order Reaction Rate At k The integrated rate law Zero Order Reaction • Rate = -∆[A]/∆t = k • The integrated rate law](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-40.jpg)
Zero Order Reaction • Rate = -∆[A]/∆t = k • The integrated rate law for zero order reaction is [A]t = -kt + [A]0 Chemical Kinetics

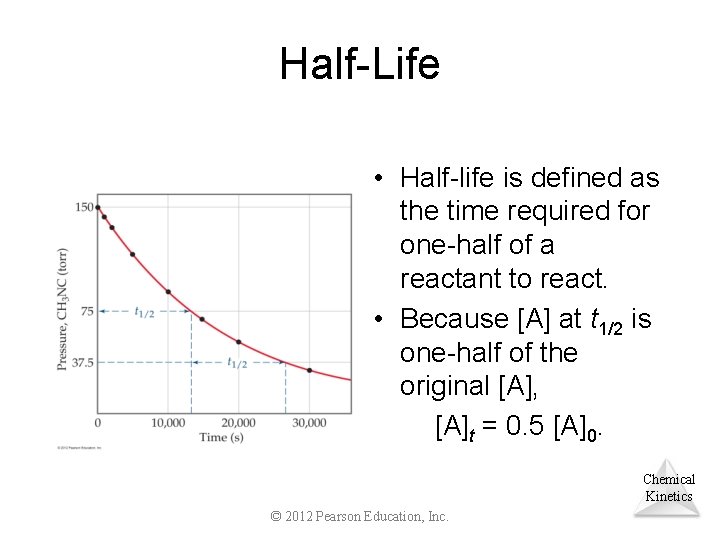
Half-Life • Half-life is defined as the time required for one-half of a reactant to react. • Because [A] at t 1/2 is one-half of the original [A], [A]t = 0. 5 [A]0. Chemical Kinetics © 2012 Pearson Education, Inc.
![HalfLife For a firstorder process this becomes 0 5 A0 ln kt 12 Half-Life For a first-order process, this becomes 0. 5 [A]0 ln = kt 1/2](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-42.jpg)
Half-Life For a first-order process, this becomes 0. 5 [A]0 ln = kt 1/2 [A]0 ln 0. 5 = kt 1/2 0. 693 = t 1/2 k Note: For a first-order process, then, the half-life does not depend on [A]0. © 2012 Pearson Education, Inc. Chemical Kinetics
![HalfLife For a secondorder process 1 1 kt 12 0 5 A0 Half-Life For a second-order process, 1 1 = kt 1/2 + 0. 5 [A]0](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-43.jpg)
Half-Life For a second-order process, 1 1 = kt 1/2 + 0. 5 [A]0 2 1 = kt 1/2 + [A]0 2 1 = kt 1/2 [A]0 0 1 = t 1/2 k[A]0 © 2012 Pearson Education, Inc. Chemical Kinetics

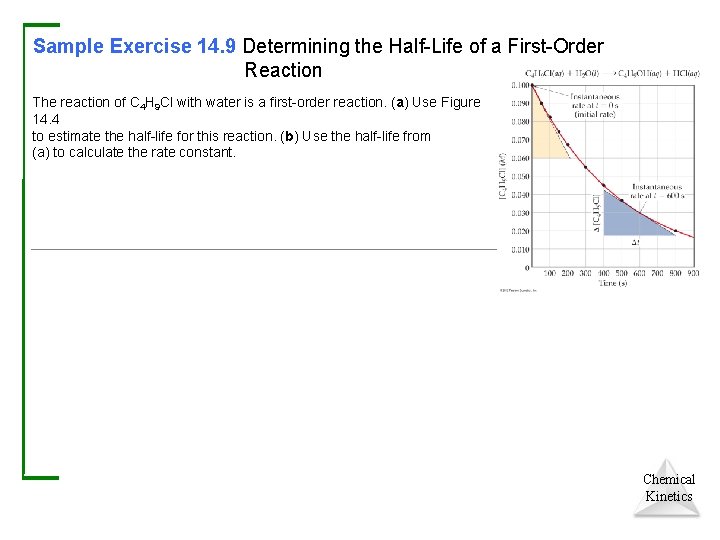
Sample Exercise 14. 9 Determining the Half-Life of a First-Order Reaction The reaction of C 4 H 9 Cl with water is a first-order reaction. (a) Use Figure 14. 4 to estimate the half-life for this reaction. (b) Use the half-life from (a) to calculate the rate constant. Chemical Kinetics

Temperature and Rate • Generally, as temperature increases, so does the reaction rate. • This is because k is temperature-dependent. Chemical Kinetics © 2012 Pearson Education, Inc.

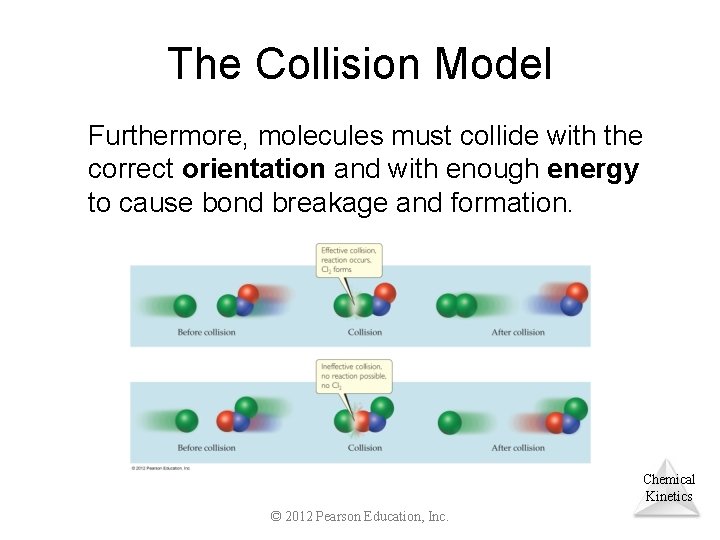
The Collision Model • In a chemical reaction, bonds are broken and new bonds are formed. • Molecules can only react if they collide with each other. Chemical Kinetics © 2012 Pearson Education, Inc.

The Collision Model Furthermore, molecules must collide with the correct orientation and with enough energy to cause bond breakage and formation. Chemical Kinetics © 2012 Pearson Education, Inc.

Activation Energy • In other words, there is a minimum amount of energy required for reaction: the activation energy, Ea. • Just as a ball cannot get over a hill if it does not roll up the hill with enough energy, a reaction cannot occur unless the molecules possess sufficient energy to get over the activation-energy barrier. Chemical Kinetics © 2012 Pearson Education, Inc.

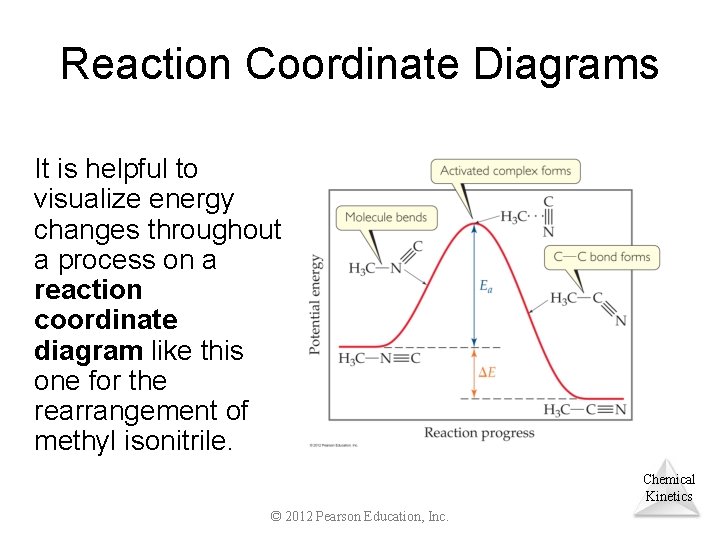
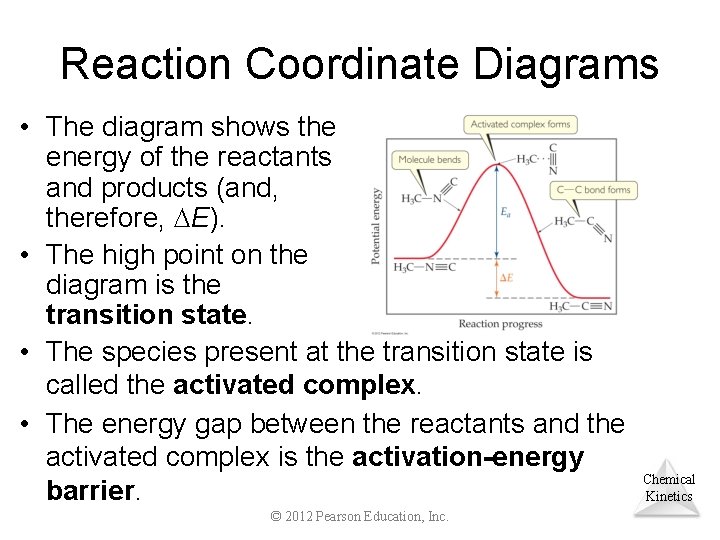
Reaction Coordinate Diagrams It is helpful to visualize energy changes throughout a process on a reaction coordinate diagram like this one for the rearrangement of methyl isonitrile. Chemical Kinetics © 2012 Pearson Education, Inc.

Reaction Coordinate Diagrams • The diagram shows the energy of the reactants and products (and, therefore, E). • The high point on the diagram is the transition state. • The species present at the transition state is called the activated complex. • The energy gap between the reactants and the activated complex is the activation-energy barrier. © 2012 Pearson Education, Inc. Chemical Kinetics

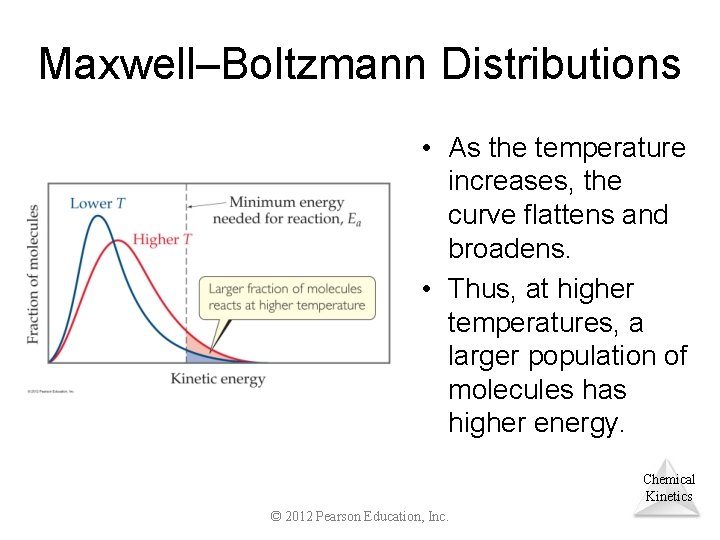
Maxwell–Boltzmann Distributions • Temperature is defined as a measure of the average kinetic energy of the molecules in a sample. • At any temperature there is a wide distribution of kinetic energies. Chemical Kinetics © 2012 Pearson Education, Inc.

Maxwell–Boltzmann Distributions • As the temperature increases, the curve flattens and broadens. • Thus, at higher temperatures, a larger population of molecules has higher energy. Chemical Kinetics © 2012 Pearson Education, Inc.

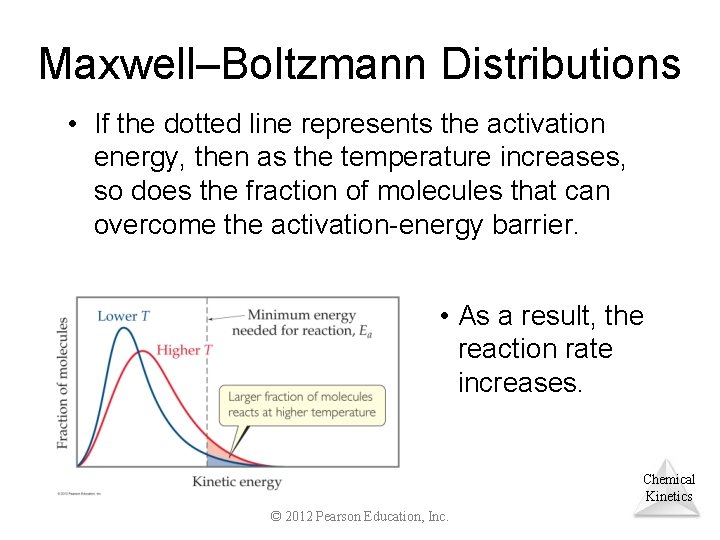
Maxwell–Boltzmann Distributions • If the dotted line represents the activation energy, then as the temperature increases, so does the fraction of molecules that can overcome the activation-energy barrier. • As a result, the reaction rate increases. Chemical Kinetics © 2012 Pearson Education, Inc.

Maxwell–Boltzmann Distributions This fraction of molecules can be found through the expression −Ea/RT f=e where R is the gas constant and T is the Kelvin temperature. Chemical Kinetics © 2012 Pearson Education, Inc.

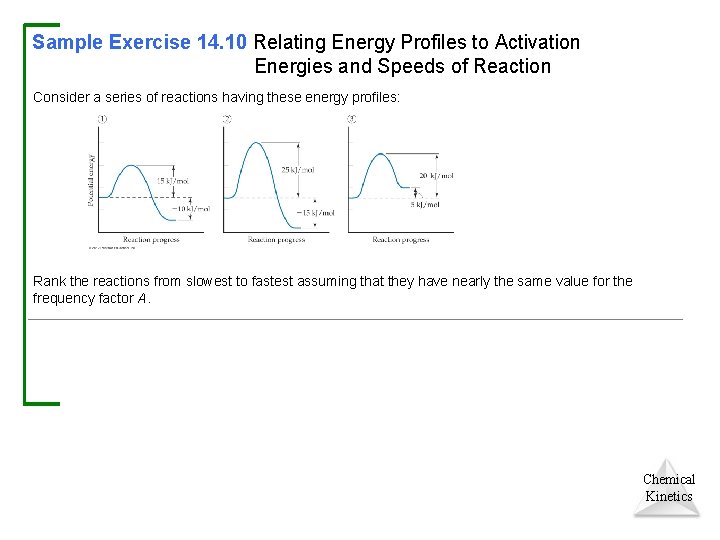
Sample Exercise 14. 10 Relating Energy Profiles to Activation Energies and Speeds of Reaction Consider a series of reactions having these energy profiles: Rank the reactions from slowest to fastest assuming that they have nearly the same value for the frequency factor A. Chemical Kinetics

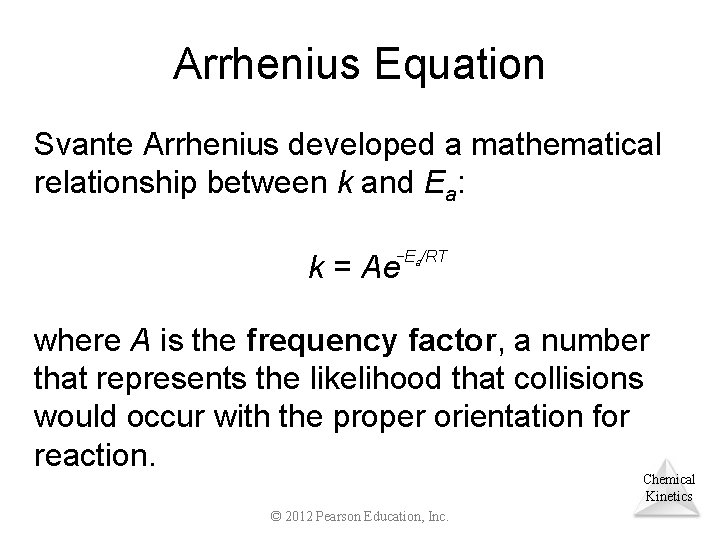
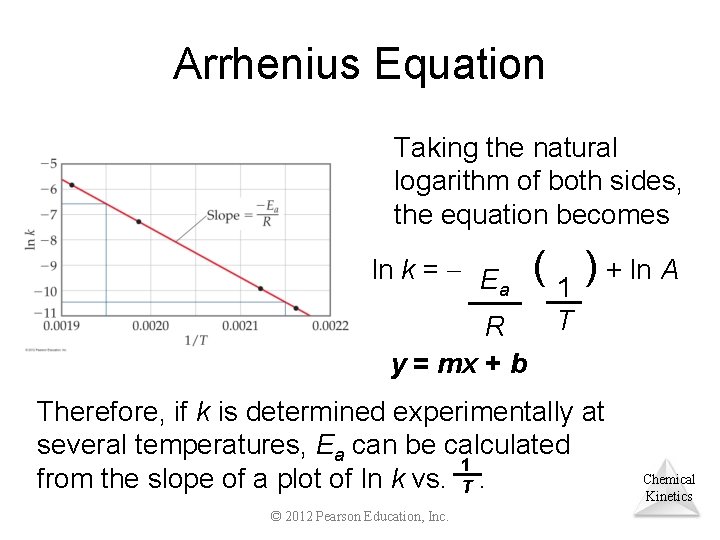
Arrhenius Equation Svante Arrhenius developed a mathematical relationship between k and Ea: −Ea/RT k = Ae where A is the frequency factor, a number that represents the likelihood that collisions would occur with the proper orientation for reaction. Chemical Kinetics © 2012 Pearson Education, Inc.

Arrhenius Equation Taking the natural logarithm of both sides, the equation becomes ln k = E a R y = mx + b ( 1 ) + ln A T Therefore, if k is determined experimentally at several temperatures, Ea can be calculated 1 from the slope of a plot of ln k vs. T. © 2012 Pearson Education, Inc. Chemical Kinetics

Reaction Mechanisms The sequence of events that describes the actual process by which reactants become products is called the reaction mechanism. Chemical Kinetics © 2012 Pearson Education, Inc.

Reaction Mechanisms • Reactions may occur all at once or through several discrete steps. • Each of these processes is known as an elementary reaction or elementary process. Chemical Kinetics © 2012 Pearson Education, Inc.

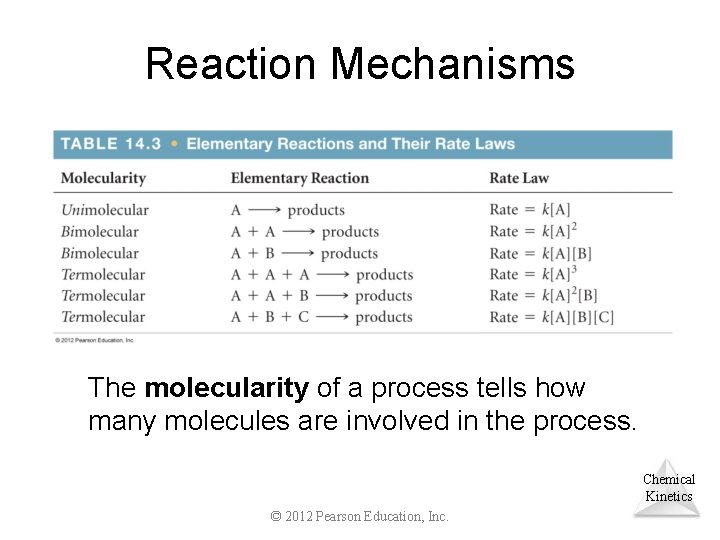
Reaction Mechanisms The molecularity of a process tells how many molecules are involved in the process. Chemical Kinetics © 2012 Pearson Education, Inc.

Exercise Determining Molecularity and Identifying Intermediates It has been proposed that the conversion of ozone into O 2 proceeds by a two-step mechanism: O 3(g) O 2(g) + O(g) O 3(g) + O(g) 2 O 2(g) (a) Describe the molecularity of each elementary reaction in this mechanism. (b) Write the equation for the overall reaction. (c) Identify the intermediate(s). Chemical Kinetics

Multistep Mechanisms • In a multistep process, one of the steps will be slower than all others. • The overall reaction cannot occur faster than this slowest, ratedetermining step. Chemical Kinetics © 2012 Pearson Education, Inc.

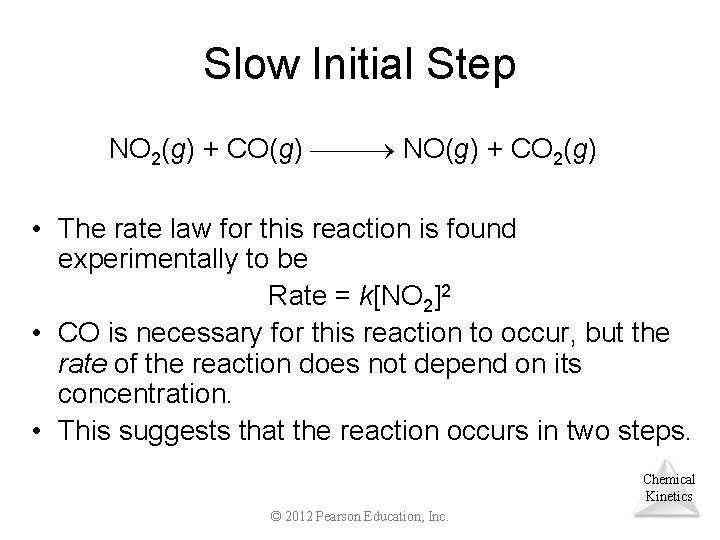
Slow Initial Step NO 2(g) + CO(g) NO(g) + CO 2(g) • The rate law for this reaction is found experimentally to be Rate = k[NO 2]2 • CO is necessary for this reaction to occur, but the rate of the reaction does not depend on its concentration. • This suggests that the reaction occurs in two steps. Chemical Kinetics © 2012 Pearson Education, Inc.

Slow Initial Step • A proposed mechanism for this reaction is Step 1: NO 2 + NO 2 NO 3 + NO (slow) Step 2: NO 3 + CO NO 2 + CO 2 (fast) • The NO 3 intermediate is consumed in the second step. • As CO is not involved in the slow, ratedetermining step, it does not appear in the rate law. Chemical Kinetics © 2012 Pearson Education, Inc.

Exercise Determining the Rate Law for a Multistep Mechanism The decomposition of nitrous oxide, N 2 O, is believed to occur by a two-step mechanism: N 2 O(g) N 2(g) + O(g) N 2 O(g) + O(g) N 2(g) + O 2(g) (slow) (fast) (a) Write the equation for the overall reaction. (b) Write the rate law for the overall reaction. Chemical Kinetics

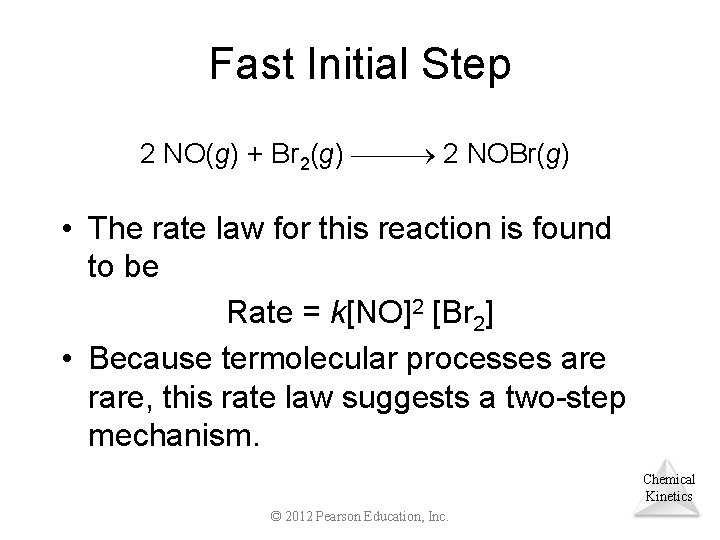
Fast Initial Step 2 NO(g) + Br 2(g) 2 NOBr(g) • The rate law for this reaction is found to be Rate = k[NO]2 [Br 2] • Because termolecular processes are rare, this rate law suggests a two-step mechanism. Chemical Kinetics © 2012 Pearson Education, Inc.

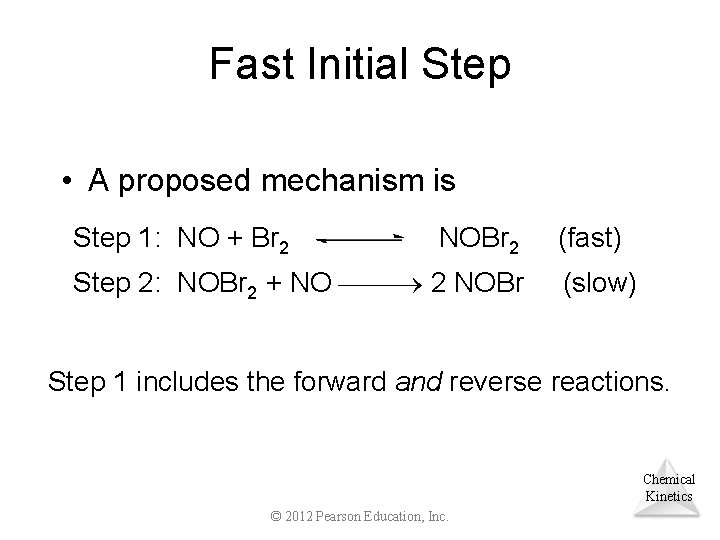
Fast Initial Step • A proposed mechanism is Step 1: NO + Br 2 NOBr 2 Step 2: NOBr 2 + NO 2 NOBr (fast) (slow) Step 1 includes the forward and reverse reactions. Chemical Kinetics © 2012 Pearson Education, Inc.

Fast Initial Step • The rate of the overall reaction depends upon the rate of the slow step. • The rate law for that step would be Rate = k 2[NOBr 2] [NO] • But how can we find [NOBr 2]? Chemical Kinetics © 2012 Pearson Education, Inc.

Fast Initial Step • NOBr 2 can react two ways: – With NO to form NOBr. – By decomposition to reform NO and Br 2. • The reactants and products of the first step are in equilibrium with each other. • Therefore, Ratef = Rater Chemical Kinetics © 2012 Pearson Education, Inc.
![Fast Initial Step Because Ratef Rater k 1NO Br 2 k Fast Initial Step • Because Ratef = Rater, k 1[NO] [Br 2] = k](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-70.jpg)
Fast Initial Step • Because Ratef = Rater, k 1[NO] [Br 2] = k 1[NOBr 2] • Solving for [NOBr 2], gives us k 1 [NO] [Br ] = [NOBr ] 2 2 k 1 Chemical Kinetics © 2012 Pearson Education, Inc.
![Fast Initial Step Substituting this expression for NOBr 2 in the rate law for Fast Initial Step Substituting this expression for [NOBr 2] in the rate law for](https://slidetodoc.com/presentation_image_h/acfdb58ae8352dd6ce81c5686159b4c7/image-71.jpg)
Fast Initial Step Substituting this expression for [NOBr 2] in the rate law for the rate-determining step, gives Rate = k 2 k 1 [NO] [Br 2] [NO] = k[NO]2 [Br 2] Chemical Kinetics © 2012 Pearson Education, Inc.

Sample Exercise 14. 15 Deriving the Rate Law for a Mechanism with a Fast Initial Step Show that the following mechanism for Equation 14. 24 also produces a rate law consistent with the experimentally observed one: Chemical Kinetics

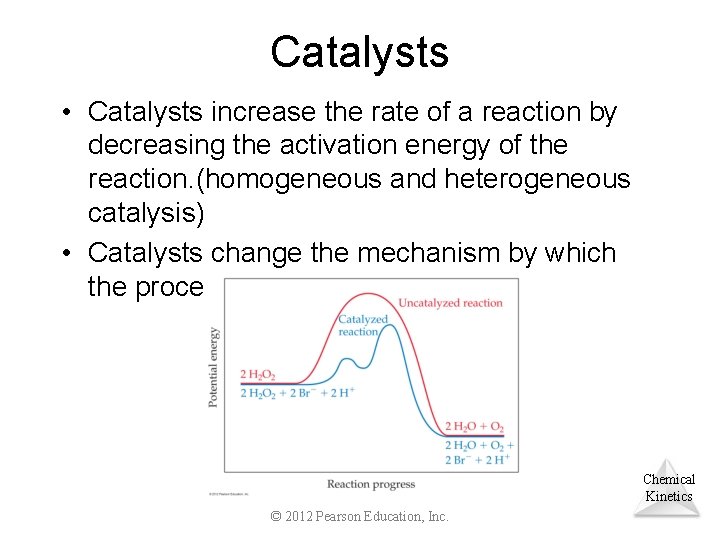
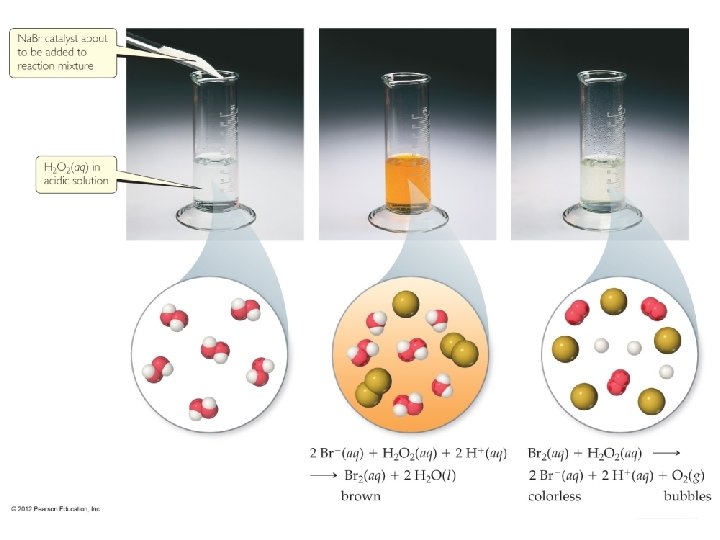
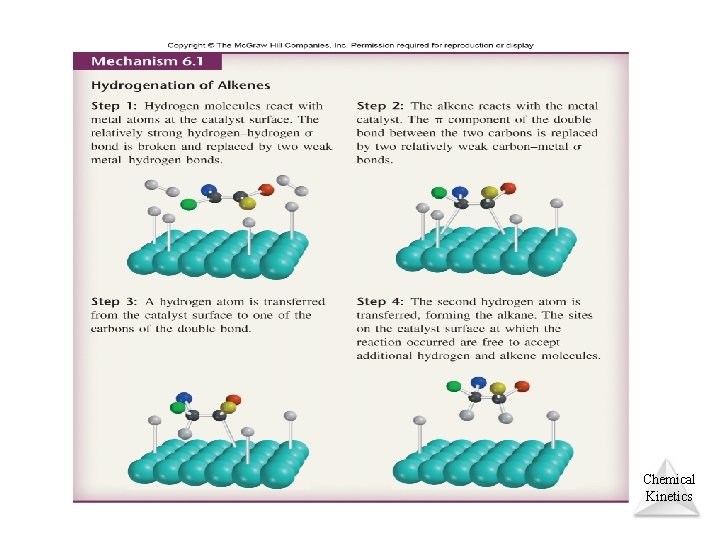
Catalysts • Catalysts increase the rate of a reaction by decreasing the activation energy of the reaction. (homogeneous and heterogeneous catalysis) • Catalysts change the mechanism by which the process occurs. Chemical Kinetics © 2012 Pearson Education, Inc.

Chemical Kinetics

Chemical Kinetics

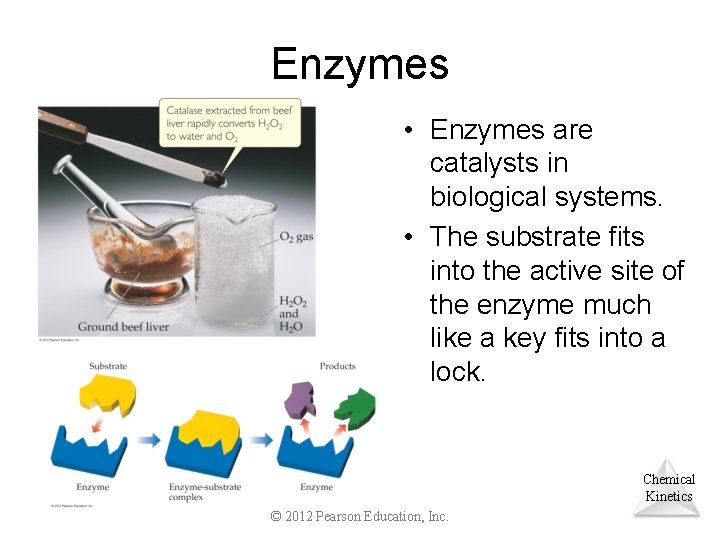
Enzymes • Enzymes are catalysts in biological systems. • The substrate fits into the active site of the enzyme much like a key fits into a lock. Chemical Kinetics © 2012 Pearson Education, Inc.