Jeopardy States of Matter Atoms Compounds Mixtures Elements





















































- Slides: 53

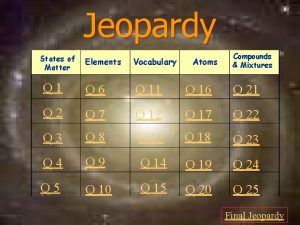
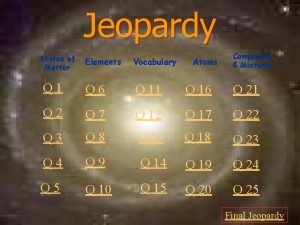
Jeopardy States of Matter Atoms Compounds & Mixtures Elements Vocabulary Q 1 Q 6 Q 11 Q 16 Q 21 Q 2 Q 7 Q 12 Q 17 Q 22 Q 3 Q 8 Q 13 Q 18 Q 23 Q 4 Q 9 Q 14 Q 19 Q 24 Q 5 Q 10 Q 15 Q 20 Q 25 Final Jeopardy

Question 1 State of matter in which particles are spread very far apart.

Answer 1 What is – gas?

Question 2 The state of matter found in the stars.

Answer 2 What is – plasma ?

Question 3 State of matter which fills whatever container it is placed in.

Answer 3 What is – gas ?

Question 4 State of matter in which particles are tightly packed together. .

Answer 4 What is – a solid ?

Question 5 State of matter which has a definite volume but not a definite shape.

Answer 5 What is – a liquid ?

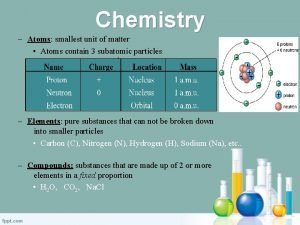
Question 6 The elements are displayed in this chart.

Answer 6 What is – the Periodic Table ?

Question 7 The atomic number gives us this information.

Answer 7 What is – the number of protons ?

Question 8 The number of known elements.

Answer 8 What is – 118 ?

Question 9 The smallest unit of an element.

Answer 9 What is – the atom ?

Question 10 The symbol for the element oxygen. .

Answer 10 What is – o ?

Question 11 The term for the amount of matter something contains.

Answer 11 What is –mass ?

Question 12 The amount of space matter takes up.

Answer 12 What is – volume ?

Question 13 A change in the appearance only of matter.

Answer 13 What is – physical change?

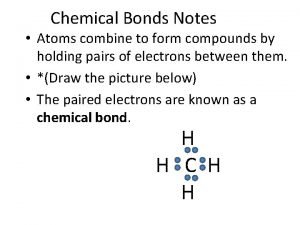
Question 14 Two or more atoms held together by a chemical bond.

Answer 14 What is – a molecule ?

Question 15 A change that forms a brand new substance with new properties.

Answer 15 What is – a chemical change ?

Question 16 The center of an atom.

Answer 16 What is – the nucleus ?

Question 17 A positive particle found in the nucleus.

Answer 17 What is – the proton ?

Question 18 The negative particle that orbits the atom.

Answer 18 What is – an electron ?

Question 19 The neutral particle found in the nucleus.

Answer 19 What is – the neutron ?

Question 20 The name given to the outermost electron shell

Answer 20 What is – valence?

Question 21 The type of bond with the transferring of electrons.

Answer 21 What is an ionic bond ?

Question 22 Change needed to create a compound of 2 or more atoms.

Answer 22 What is – chemical change ?

Question 23 Maximum number of electrons in the second shell.

Answer 23 What is 8 ?

Question 24 A mixture in which one substance dissolves in another.

Answer 24 What is – a solution ?

Question 25 A mixture in which substances are not dissolved.

Answer 25 What is – suspension ?

Final Jeopardy The symbol for the element which has 61 neutrons. (((((

Final Jeopardy Answer What is – Ag (silver)?
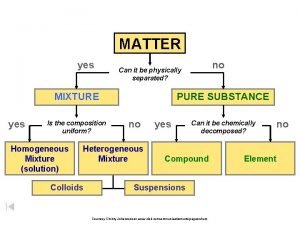
 Classifying elements compounds and mixtures
Classifying elements compounds and mixtures Elements, compounds and mixtures worksheet answer
Elements, compounds and mixtures worksheet answer Elements, compounds and mixtures worksheet with answers
Elements, compounds and mixtures worksheet with answers Is a rock a element compound or mixture
Is a rock a element compound or mixture Difference between mixture and compund
Difference between mixture and compund Elements compounds and mixtures ks3
Elements compounds and mixtures ks3 Elements and compounds graphic organizer
Elements and compounds graphic organizer Is sterling silver a pure substance
Is sterling silver a pure substance Elements compounds and mixtures oh my worksheet
Elements compounds and mixtures oh my worksheet Elements compounds and mixtures quiz
Elements compounds and mixtures quiz Compound substance
Compound substance Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 6 section 1 atoms elements and compounds answer key
Chapter 6 section 1 atoms elements and compounds answer key Chapter 6 section 1 atoms elements and compounds
Chapter 6 section 1 atoms elements and compounds Properties of matter jeopardy
Properties of matter jeopardy States of matter jeopardy
States of matter jeopardy Jeopardy states of matter
Jeopardy states of matter Compared to atoms of metals, atoms of nonmetals generally
Compared to atoms of metals, atoms of nonmetals generally Is sugar a pure substance
Is sugar a pure substance Compounds vs mixtures
Compounds vs mixtures Compounds vs mixtures
Compounds vs mixtures Do atoms combine to form compounds
Do atoms combine to form compounds In covalent compounds atoms become chemically stable
In covalent compounds atoms become chemically stable A mechanical mixture
A mechanical mixture How is matter classified
How is matter classified Ionic covalent and metallic venn diagram
Ionic covalent and metallic venn diagram Are atoms the smallest unit of matter
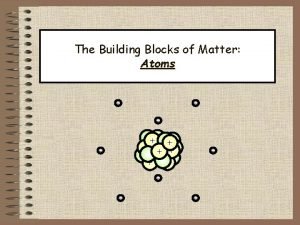
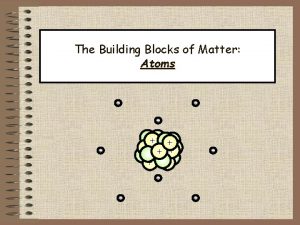
Are atoms the smallest unit of matter Atoms building blocks of matter
Atoms building blocks of matter Atoms the building blocks of matter
Atoms the building blocks of matter Smallest building blocks of matter
Smallest building blocks of matter Collision theory states that
Collision theory states that Chapter 3 atoms the building blocks of matter
Chapter 3 atoms the building blocks of matter Which subatomic particle has the least mass
Which subatomic particle has the least mass Atoms vs elements
Atoms vs elements Www.chem.purdue/gchelp/atoms/elements.html
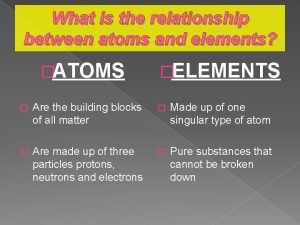
Www.chem.purdue/gchelp/atoms/elements.html What is the relationship between atoms and elements
What is the relationship between atoms and elements Phases of matter foldable
Phases of matter foldable Four phases of matter
Four phases of matter Four states of matter
Four states of matter 5 states of matter
5 states of matter Which state of matter has the most thermal energy
Which state of matter has the most thermal energy Changing state
Changing state Phet states of matter basics
Phet states of matter basics 5 states of matter
5 states of matter Venn diagram of solid liquid gas
Venn diagram of solid liquid gas The kinetic theory of matter states that
The kinetic theory of matter states that 11 free states
11 free states Northern and southern states
Northern and southern states Section 1 composition of matter
Section 1 composition of matter Chapter 12 states of matter study guide
Chapter 12 states of matter study guide Chapter 10 review states of matter section 4
Chapter 10 review states of matter section 4 States of matter: basics
States of matter: basics States of matter foldable
States of matter foldable Thermal energy vs heat
Thermal energy vs heat