How heat Is Measured Some specific heat capacity
























- Slides: 48

How heat Is Measured

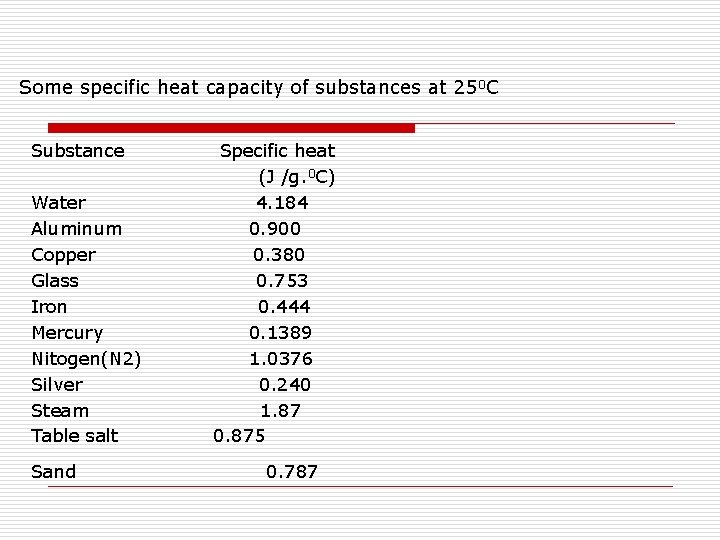
Some specific heat capacity of substances at 250 C Substance Water Aluminum Copper Glass Iron Mercury Nitogen(N 2) Silver Steam Table salt Sand Specific heat (J /g. 0 C) 4. 184 0. 900 0. 380 0. 753 0. 444 0. 1389 1. 0376 0. 240 1. 87 0. 875 0. 787

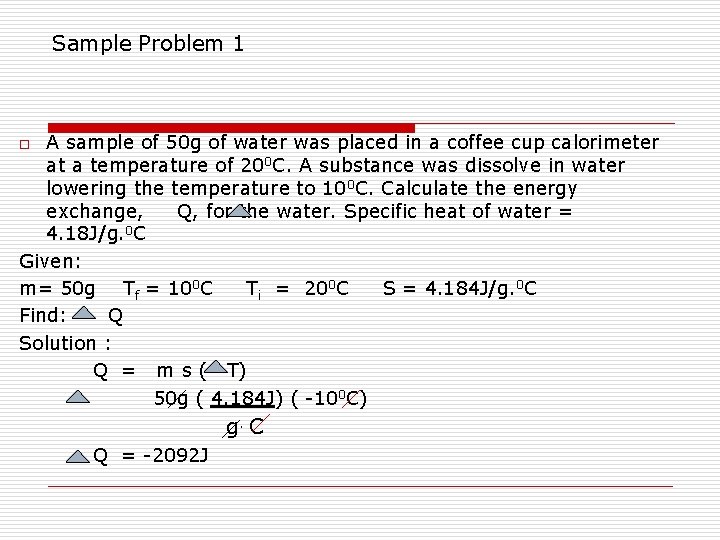
Sample Problem 1 A sample of 50 g of water was placed in a coffee cup calorimeter at a temperature of 200 C. A substance was dissolve in water lowering the temperature to 100 C. Calculate the energy exchange, Q, for the water. Specific heat of water = 4. 18 J/g. 0 C Given: m= 50 g Tf = 100 C Ti = 200 C S = 4. 184 J/g. 0 C Find: Q Solution : Q = m s ( T) 50 g ( 4. 184 J) ( -100 C) o g. C Q = -2092 J

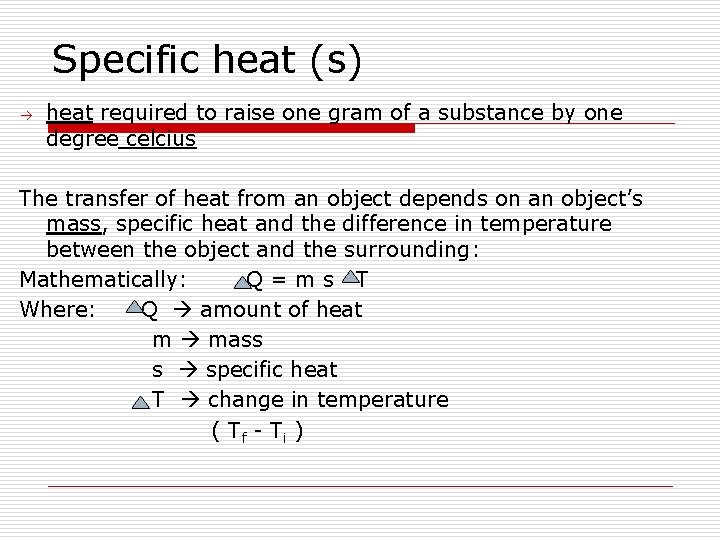
Specific heat (s) heat required to raise one gram of a substance by one degree celcius The transfer of heat from an object depends on an object’s mass, specific heat and the difference in temperature between the object and the surrounding: Mathematically: Q=ms T Where: Q amount of heat m mass s specific heat T change in temperature ( Tf - T i )

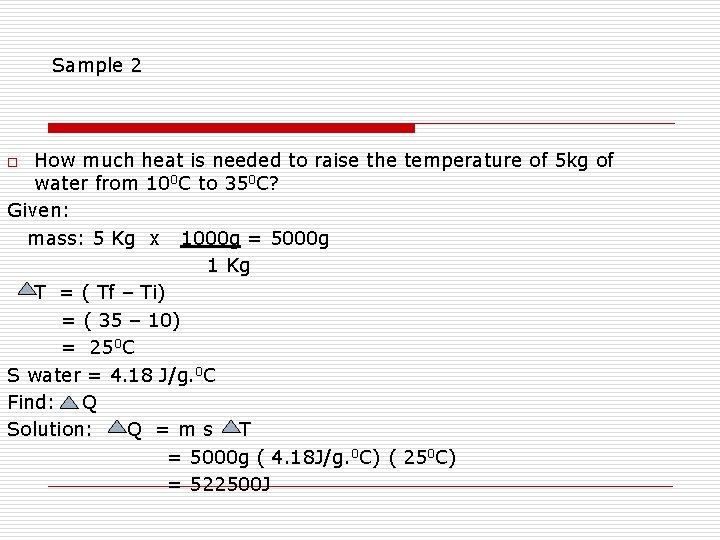
Sample 2 How much heat is needed to raise the temperature of 5 kg of water from 100 C to 350 C? Given: mass: 5 Kg x 1000 g = 5000 g 1 Kg T = ( Tf – Ti) = ( 35 – 10) = 250 C S water = 4. 18 J/g. 0 C Find: Q Solution: Q =ms T = 5000 g ( 4. 18 J/g. 0 C) ( 250 C) = 522500 J o

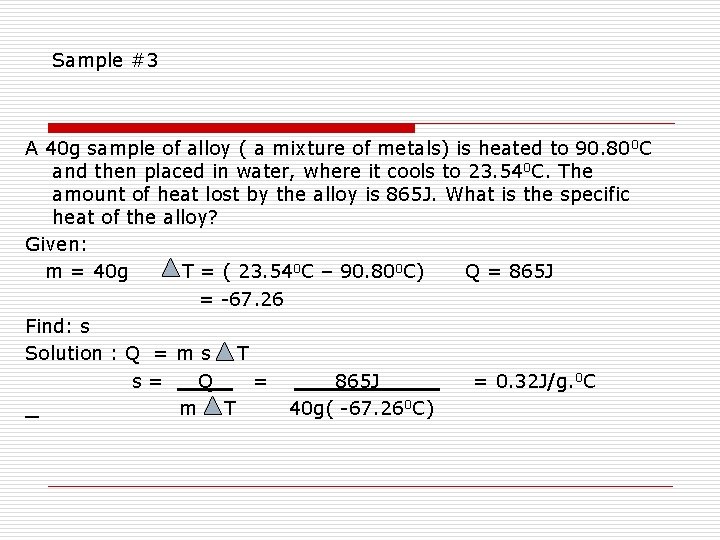
Sample #3 A 40 g sample of alloy ( a mixture of metals) is heated to 90. 80 0 C and then placed in water, where it cools to 23. 540 C. The amount of heat lost by the alloy is 865 J. What is the specific heat of the alloy? Given: m = 40 g T = ( 23. 540 C – 90. 800 C) Q = 865 J = -67. 26 Find: s Solution : Q = m s T s= Q = 865 J = 0. 32 J/g. 0 C m T 40 g( -67. 260 C)

Enthalpy

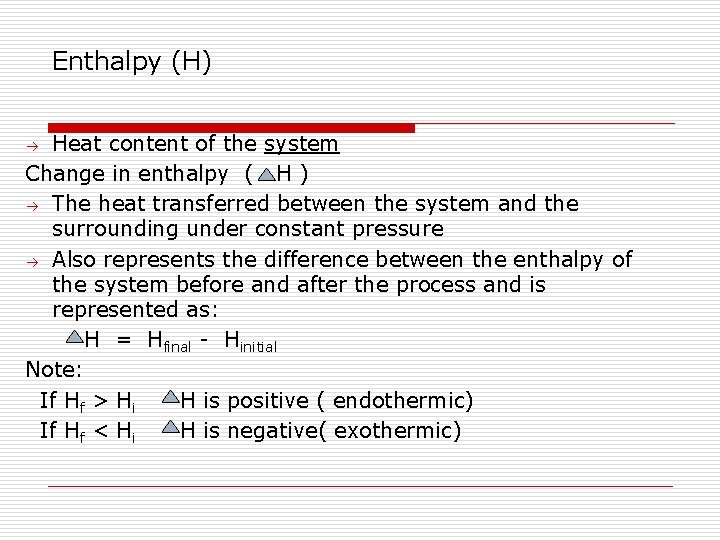
Enthalpy (H) Heat content of the system Change in enthalpy ( H ) The heat transferred between the system and the surrounding under constant pressure Also represents the difference between the enthalpy of the system before and after the process and is represented as: H = Hfinal - Hinitial Note: If Hf > Hi H is positive ( endothermic) If Hf < Hi H is negative( exothermic)

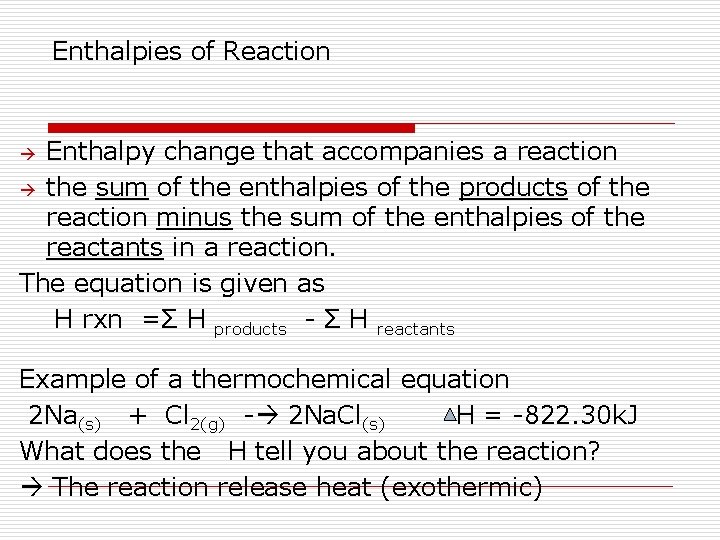
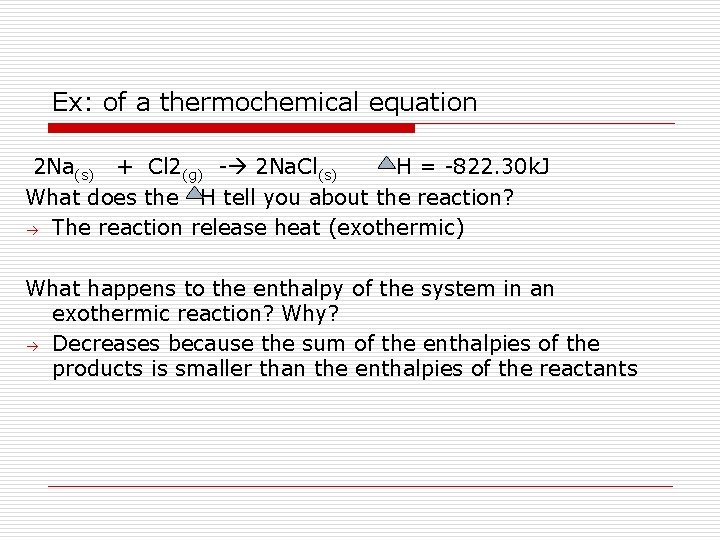
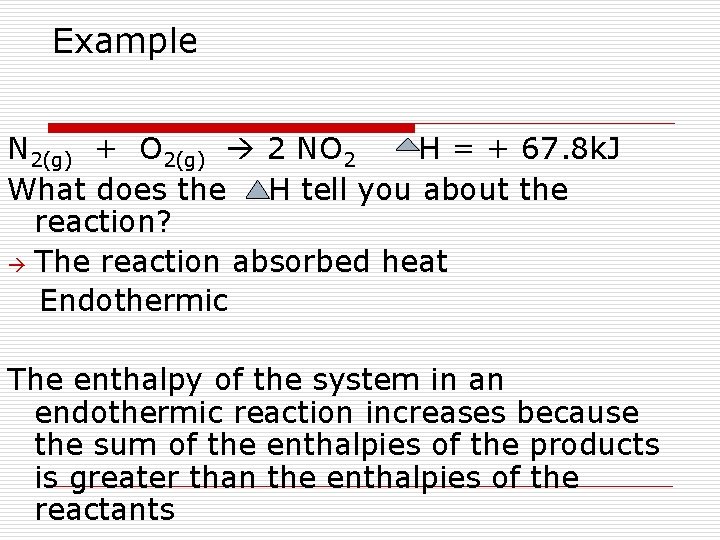
Enthalpies of Reaction Enthalpy change that accompanies a reaction the sum of the enthalpies of the products of the reaction minus the sum of the enthalpies of the reactants in a reaction. The equation is given as H rxn =Σ H products - Σ H reactants Example of a thermochemical equation 2 Na(s) + Cl 2(g) - 2 Na. Cl(s) H = -822. 30 k. J What does the H tell you about the reaction? The reaction release heat (exothermic)

Ex: of a thermochemical equation 2 Na(s) + Cl 2(g) - 2 Na. Cl(s) H = -822. 30 k. J What does the H tell you about the reaction? The reaction release heat (exothermic) What happens to the enthalpy of the system in an exothermic reaction? Why? Decreases because the sum of the enthalpies of the products is smaller than the enthalpies of the reactants

Example N 2(g) + O 2(g) 2 NO 2 H = + 67. 8 k. J What does the H tell you about the reaction? The reaction absorbed heat Endothermic The enthalpy of the system in an endothermic reaction increases because the sum of the enthalpies of the products is greater than the enthalpies of the reactants

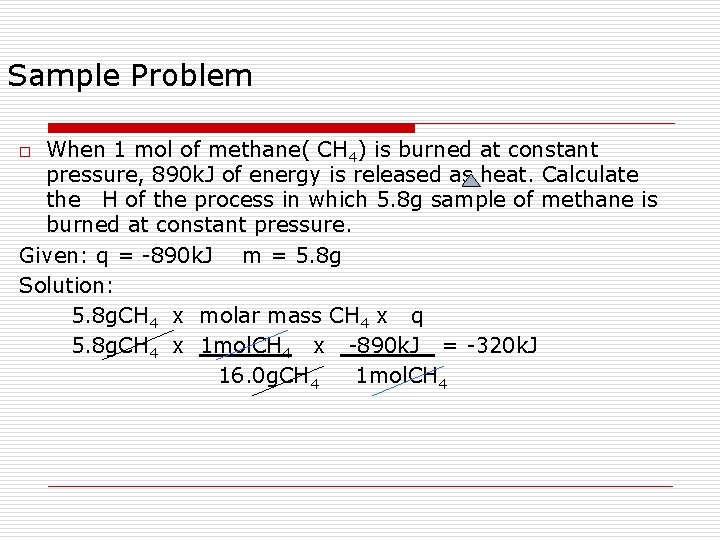
Sample Problem When 1 mol of methane( CH 4) is burned at constant pressure, 890 k. J of energy is released as heat. Calculate the H of the process in which 5. 8 g sample of methane is burned at constant pressure. Given: q = -890 k. J m = 5. 8 g Solution: 5. 8 g. CH 4 x molar mass CH 4 x q 5. 8 g. CH 4 x 1 mol. CH 4 x -890 k. J = -320 k. J 16. 0 g. CH 4 1 mol. CH 4 o

Self-Check 10. 5 p. 302 The reaction that occurs in the heat packs to treat sports injuries is 4 Fe(s) + 3 O 2(g) ---> 2 Fe 2 O 3(s) H= -1652 k. J How much heat is released when 1. 00 g Fe(s) is reacted with excess O 2? Solution: molar mass Fe = 55. 85 g/mol 1. 00 g Fe x 1 mol Fe = 1. 79 x 10 -2 mol Fe 55. 85 g. Fe 1. 79 x 10 -2 mol Fe x -1652 k. J = -7. 39 k. J 4 mol. Fe


Objective: Explain the energy involve during the phase change Change of phase Occurs when a substance change from one state to another Note: Change of phase always occur with a change in the amount of heat of a substance Heat travels spontaneously from a warmer body to a colder body

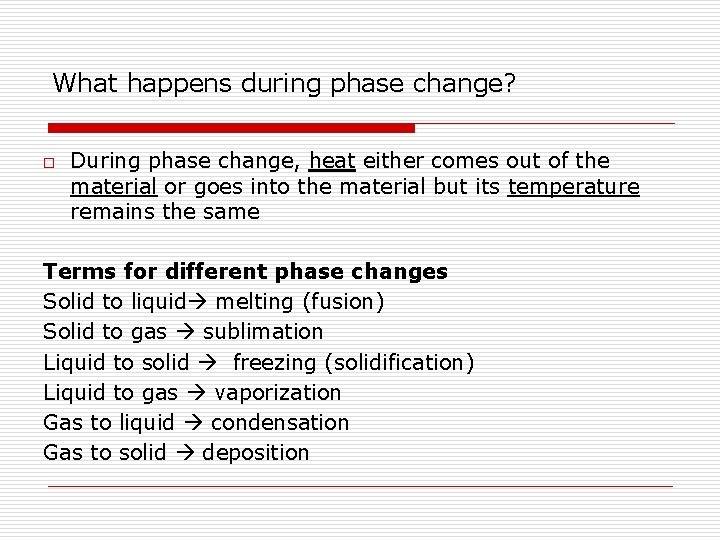
What happens during phase change? o During phase change, heat either comes out of the material or goes into the material but its temperature remains the same Terms for different phase changes Solid to liquid melting (fusion) Solid to gas sublimation Liquid to solid freezing (solidification) Liquid to gas vaporization Gas to liquid condensation Gas to solid deposition

GAS vaporization condensation sublimation LIQUID melting (fusion) freezing (solidification) SOLID deposition

o o The amount of heat absorbed by one mole of a substance when it changes from solid to liquid The quantity of heat absorbed during fusion is equal to the heat released during freezing

The amount of heat released by one mole of a substance to change from liquid to solid.

Molar heat of vaporization Amount of heat required by one mole of a substance to change from liquid to gas

Molar heat of condensation Amount of heat released by one mole of a substance to change from gas to liquid

Explain the kinetic energy of the water molecule in the heating curve o When all the ice has turned to liquid and heat continues to flow into the water, the heat goes into increasing the kinetic energy of the molecules resulting in an increase in temperature



a process that occurs by itself. Which means no external force is needed to continue the process Ex: Sugar dissolving in hot coffee rusting of iron

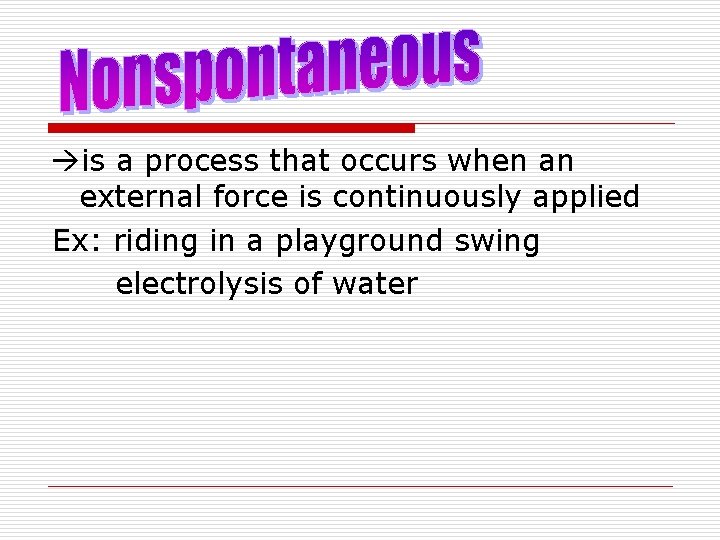
is a process that occurs when an external force is continuously applied Ex: riding in a playground swing electrolysis of water

Note: Most exothermic reactions are spontaneous Most endothermic reactions are non spontaneous However there are some endothermic reactions that are nonspontaneous Ex: the melting of ice at room temperature or above 00 C

Consider the melting of ice cube Melting of an ice cube is spontaneous if the temperature is above the melting point Nonspontaneous is below the melting point

Degree of freedom that particles have Degree of disorder in a system The greater the degree of randomness or disorder, in a system, the greater is its entropy Which has a greater entropy liquid or gas? Gas has greater entropy than pure liquids Entropy is measured by J/K

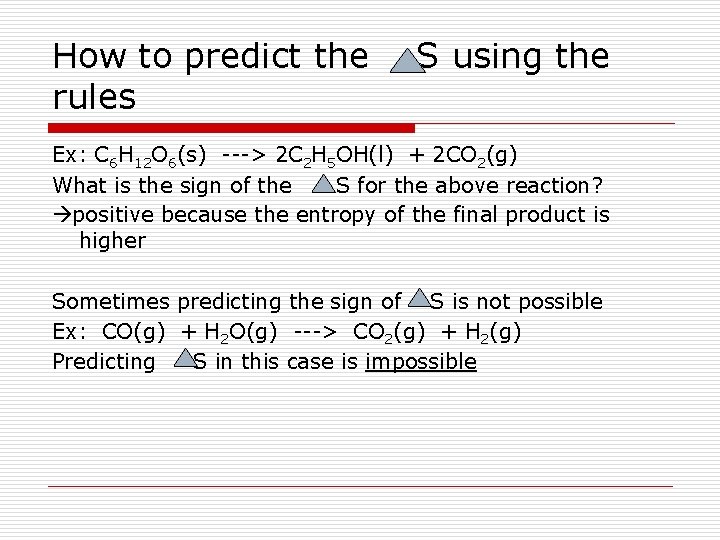
How to predict the rules S using the Ex: C 6 H 12 O 6(s) ---> 2 C 2 H 5 OH(l) + 2 CO 2(g) What is the sign of the S for the above reaction? positive because the entropy of the final product is higher Sometimes predicting the sign of S is not possible Ex: CO(g) + H 2 O(g) ---> CO 2(g) + H 2(g) Predicting S in this case is impossible

Symbol ( S) Is the difference between the final entropy and the initial entropy of a system. S = Sfinal - Sinitial S is positive if there is an entropy increase Ex: solid ---> liquid or gas S is negative if there is an entropy decrease Ex: when a precipitate for Ag+(aq) + Cl-(aq) Ag. Cl(s) The entropy of the solid is lower than the original aqueous ions

Rules to predict the sign of S Generally entropy increases when A reaction breaks up a larger molecule into smaller molecular fragments A reaction occurs in which there is an increase in the mole of gas in the product A process where a solid changes to a liquid or gas or a liquid to a gas o

Exercises o o 2 NH 4 NO 3(s) --->2 N 2(g) + 4 H 2 O(g) + O 2(g) 2 SO 2(g) + O 2(g) --> 2 SO 3(g) C 12 H 22 O 11(aq) C 12 H 22 O 11(s) CO 2(g) + H 20(g) ---> CO 2(g) + H 2(g)

Hess Law States that if the reaction occurs in two or more steps, the enthalpy for the reaction is the sum of the enthalpies of the individual steps Why is it useful? because it allows us to calculate heats of reaction that are difficult or inconvenient to measure in a calorimeter



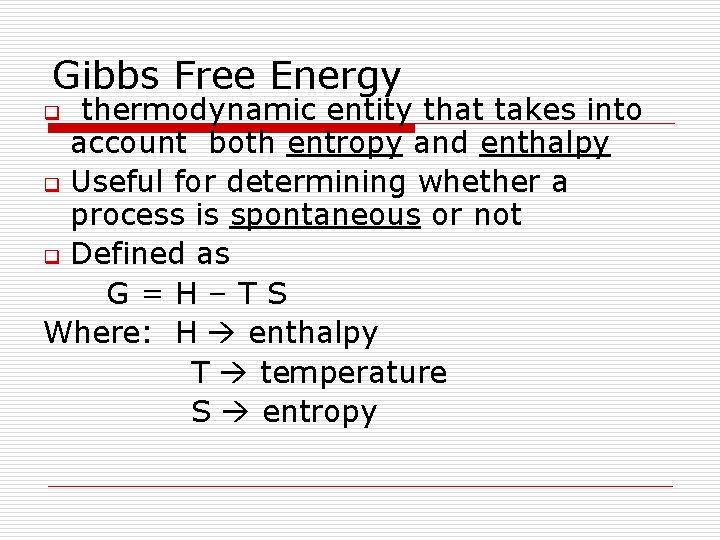
Gibbs Free Energy thermodynamic entity that takes into account both entropy and enthalpy q Useful for determining whether a process is spontaneous or not q Defined as G=H–TS Where: H enthalpy T temperature S entropy q

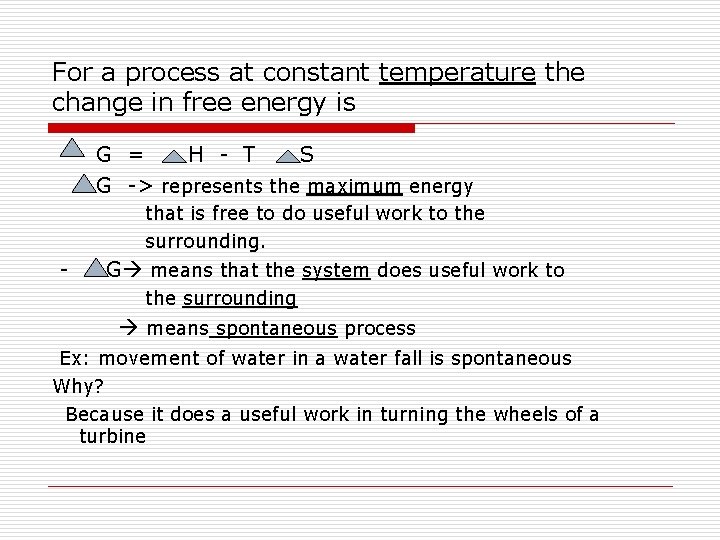
For a process at constant temperature the change in free energy is G = H - T S G -> represents the maximum energy that is free to do useful work to the surrounding. - G means that the system does useful work to the surrounding means spontaneous process Ex: movement of water in a water fall is spontaneous Why? Because it does a useful work in turning the wheels of a turbine

+ G indicates that work has to be done on the system for the process to take place means that energy will have to be absorbed from the surrounding means non spontaneous process Ex: maintenance of life It requires sustained input of work or energy that we get from food If G=0 The reaction is at equilibrium

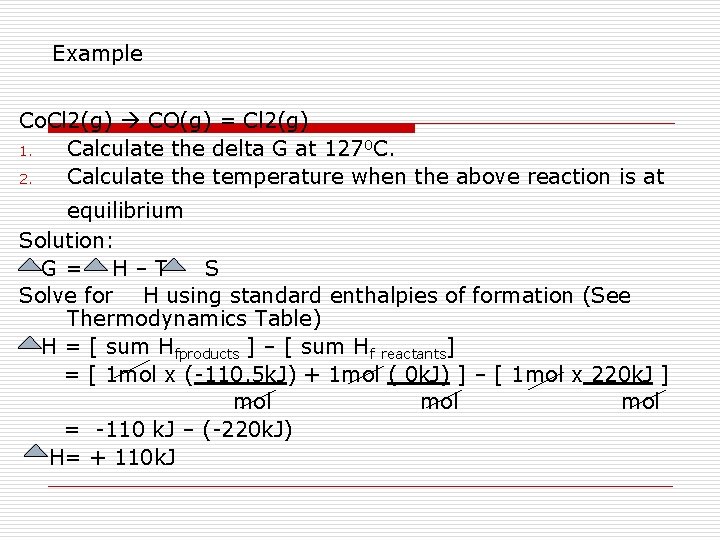
Example Co. Cl 2(g) CO(g) = Cl 2(g) 1. Calculate the delta G at 1270 C. 2. Calculate the temperature when the above reaction is at equilibrium Solution: G= H–T S Solve for H using standard enthalpies of formation (See Thermodynamics Table) H = [ sum Hfproducts ] – [ sum Hf reactants] = [ 1 mol x (-110. 5 k. J) + 1 mol ( 0 k. J) ] – [ 1 mol x 220 k. J ] mol mol = -110 k. J – (-220 k. J) H= + 110 k. J

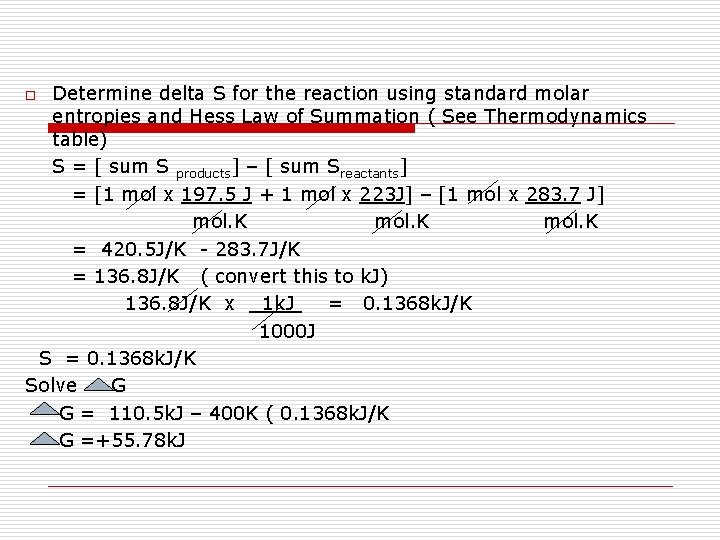
Determine delta S for the reaction using standard molar entropies and Hess Law of Summation ( See Thermodynamics table) S = [ sum S products] – [ sum Sreactants] = [1 mol x 197. 5 J + 1 mol x 223 J] – [1 mol x 283. 7 J] mol. K = 420. 5 J/K - 283. 7 J/K = 136. 8 J/K ( convert this to k. J) 136. 8 J/K x 1 k. J = 0. 1368 k. J/K 1000 J S = 0. 1368 k. J/K Solve G G = 110. 5 k. J – 400 K ( 0. 1368 k. J/K G =+55. 78 k. J o

2) When delta G = 0, solve T Solution G= H - T S 0 = 110. 5 k. J – T ( 0. 1368 k. J/K) (0. 1368 k. J/K)T = 110. 5 k. J 0. 1368 k. J/K T = 807. 7 K

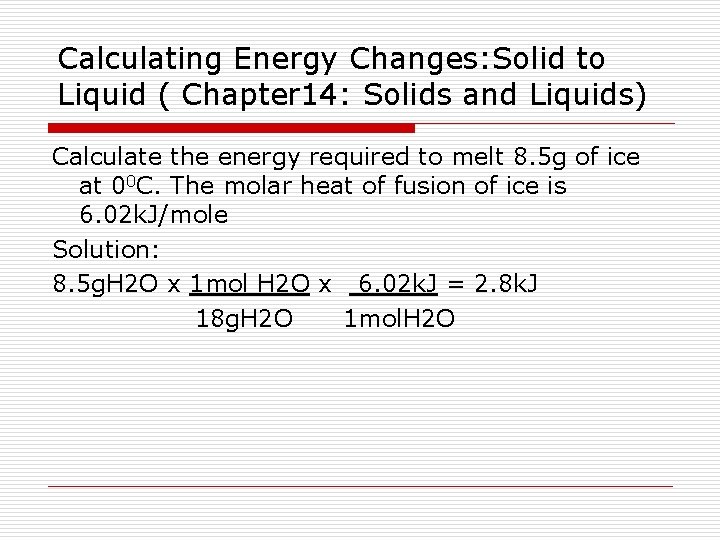
Calculating Energy Changes: Solid to Liquid ( Chapter 14: Solids and Liquids) Calculate the energy required to melt 8. 5 g of ice at 00 C. The molar heat of fusion of ice is 6. 02 k. J/mole Solution: 8. 5 g. H 2 O x 1 mol H 2 O x 6. 02 k. J = 2. 8 k. J 18 g. H 2 O 1 mol. H 2 O

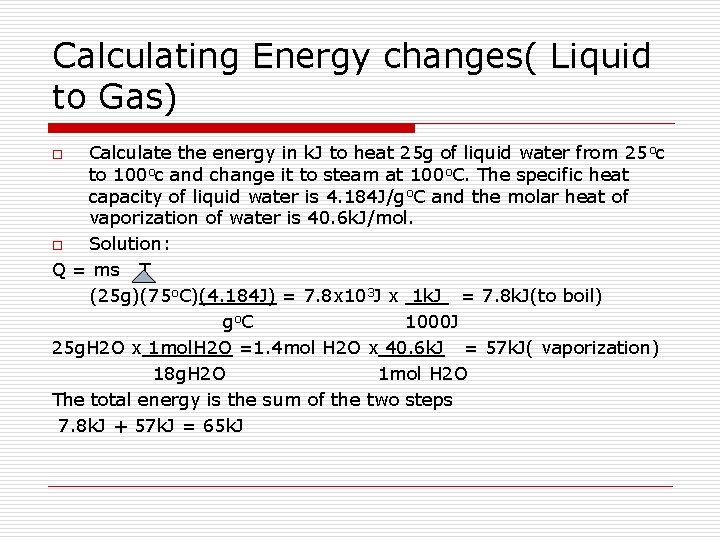
Calculating Energy changes( Liquid to Gas) Calculate the energy in k. J to heat 25 g of liquid water from 25 oc to 100 oc and change it to steam at 100 o. C. The specific heat capacity of liquid water is 4. 184 J/go. C and the molar heat of vaporization of water is 40. 6 k. J/mol. o Solution: Q = ms T (25 g)(75 o. C)(4. 184 J) = 7. 8 x 103 J x 1 k. J = 7. 8 k. J(to boil) go C 1000 J 25 g. H 2 O x 1 mol. H 2 O =1. 4 mol H 2 O x 40. 6 k. J = 57 k. J( vaporization) 18 g. H 2 O 1 mol H 2 O The total energy is the sum of the two steps 7. 8 k. J + 57 k. J = 65 k. J o

Heating and Cooling Curve

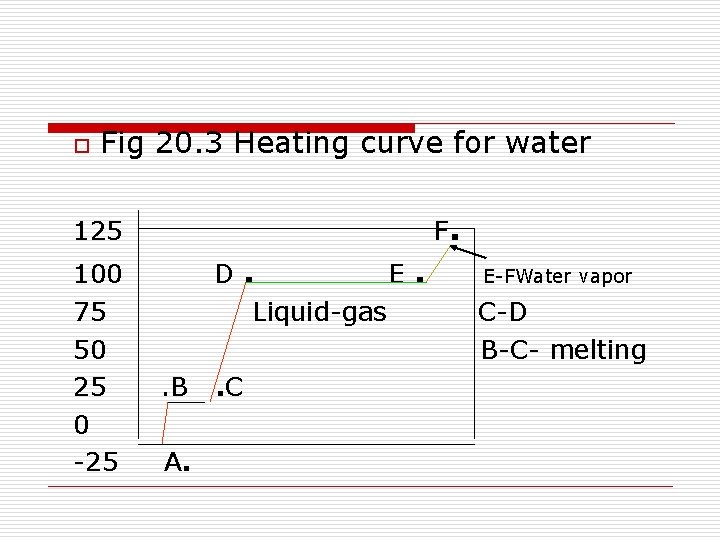
o Fig 20. 3 Heating curve for water F. 125 100 75 50 25 0 -25 D. E. Liquid-gas. B A. . C E-FWater vapor C-D B-C- melting

Cooling Curve o o Describes the change in temperature and the amount of heat during the cooling process The slope of the curve is decreasing when heat is removed from the material

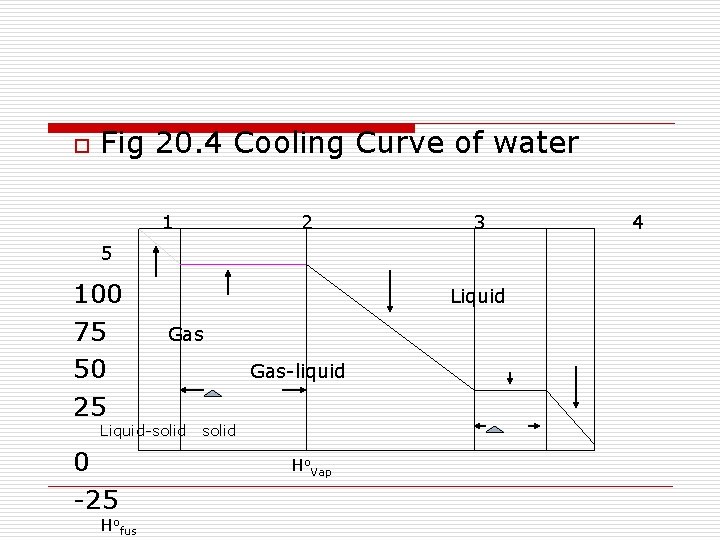
o Fig 20. 4 Cooling Curve of water 1 2 3 5 100 75 50 25 Liquid Gas Liquid-solid 0 -25 Hofus Gas-liquid solid Ho. Vap 4