Thermal Properties of Materials Specific Heat Capacity Specific








- Slides: 27

Thermal Properties of Materials Specific Heat Capacity Specific Latent Heat Internal Energy First Law of Thermodynamics

Quantifying Heat n n n Heat and Temperature Equation Specific Heat Capacity n n n Change of Phase Equation Latent Heat

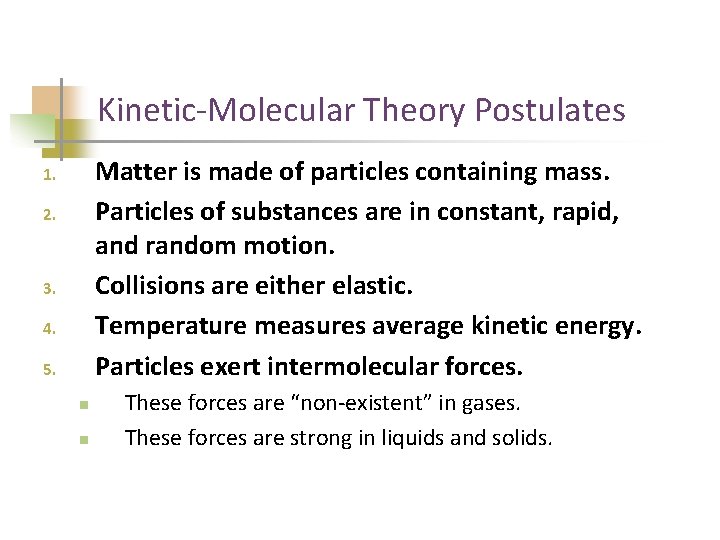
Kinetic-Molecular Theory Postulates Matter is made of particles containing mass. Particles of substances are in constant, rapid, and random motion. Collisions are either elastic. Temperature measures average kinetic energy. Particles exert intermolecular forces. 1. 2. 3. 4. 5. n n These forces are “non-existent” in gases. These forces are strong in liquids and solids.

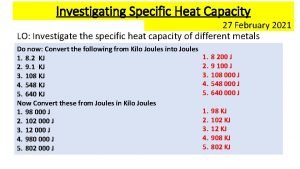
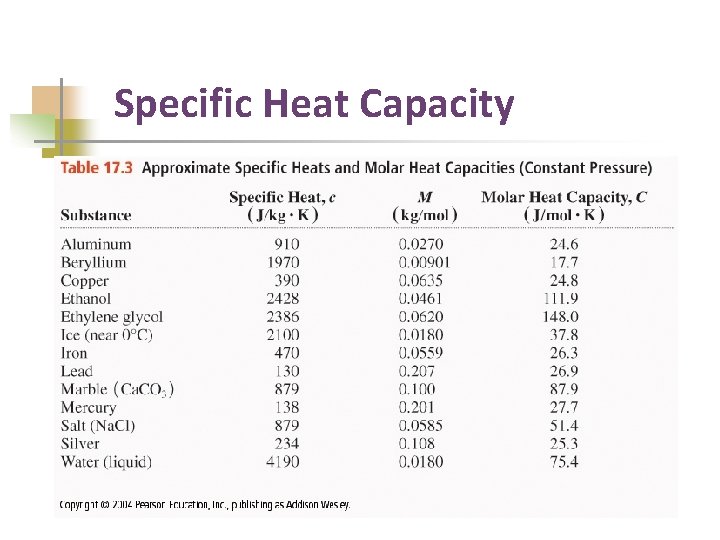
Specific Heat Capacity

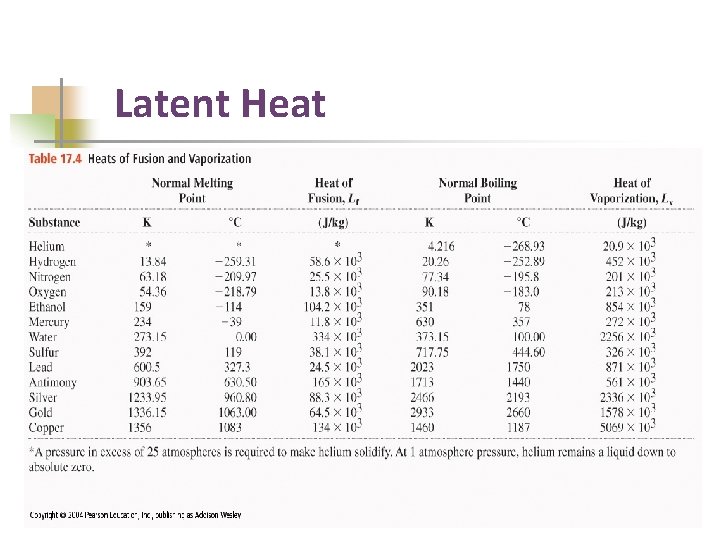
Latent Heat

Questions n n Which is greater, an increase in temperature of 1 C or an increase of 1 F ? If you drop a hot rock into a pail of water, the temperature of the rock and water will change until both are equal. The rock will cool and the water will warm. Does this hold true if the rock is dropped in the Pacific Ocean? Explain.

Questions n n n Adding the same amount of heat to two different objects does not necessarily produce the same increase in temperature. Why not? Why will watermelon stay cool for a longer time than sandwiches when both are removed from a cooler on a hot day? The desert sand is very hot in the day and very cool at night. What does this tell you about its specific heat?

Examples n 135 g of aluminum (initially at 400 o. C) are mixed with an unknown mass of water (initially at 25 o. C). When thermal equilibrium is reached, the system has a temperature of 80 o. C. Find the mass of the water.

Examples n In the lab, an experimenter mixes 75. 0 g of water (initially at 30 o. C) with 83. 8 g of a solid metal (initially at 600 o. C). At thermal equilibrium, he measures a final temperature of 50. 0 o. C. What metal did the experimenter probably use?

Examples n A 38 g sample of ice at – 11 o. C is placed into 214 g of water at 56 o. C. Find the system’s final temperature.

Examples n 25 g of 116 o. C steam are bubbled into 0. 2384 kg of water at 8 o. C. Find the final temperature of the system.

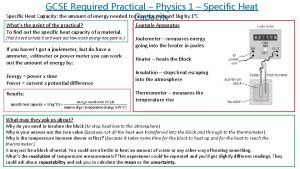
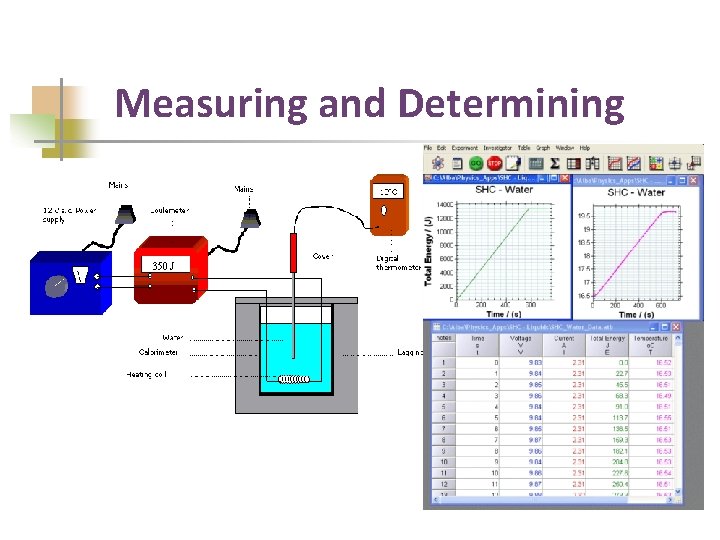
Measuring and Determining


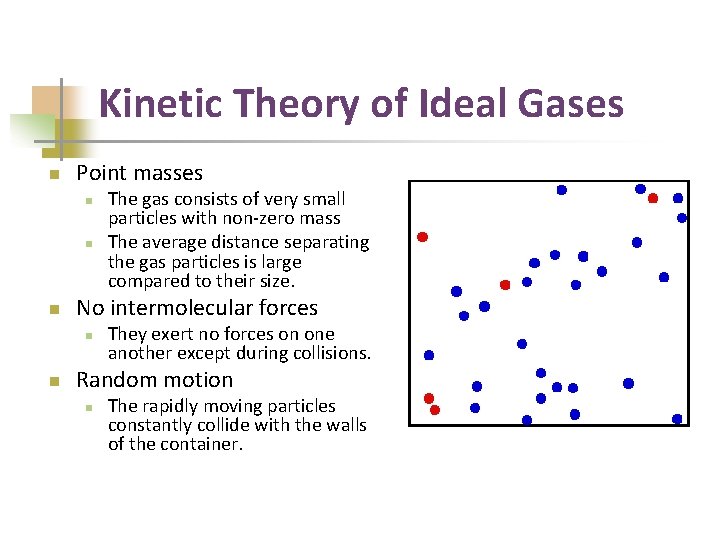
Kinetic Theory of Ideal Gases n Point masses n n n No intermolecular forces n n The gas consists of very small particles with non-zero mass The average distance separating the gas particles is large compared to their size. They exert no forces on one another except during collisions. Random motion n The rapidly moving particles constantly collide with the walls of the container.

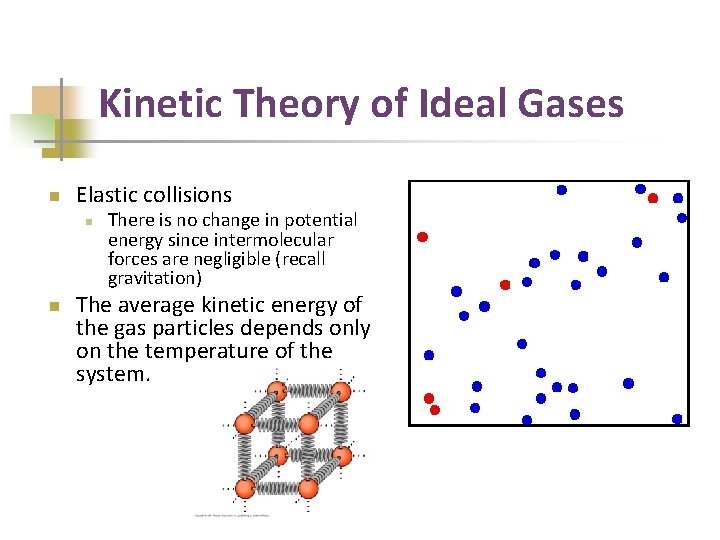
Kinetic Theory of Ideal Gases n Elastic collisions n n There is no change in potential energy since intermolecular forces are negligible (recall gravitation) The average kinetic energy of the gas particles depends only on the temperature of the system.

Energy n n n Mechanical Energy: KE, PE, SE Work is done by energy transfer. Heat is another form of energy. Need to expand the conservation of energy principle to accommodate thermal systems.

First Law of Thermodynamics n Energy cannot be created or destroyed but can only be transformed from one form to another n n The heat energy supplied to the system n n Conservation of energy increases the internal energy Or enables it to do work Or both Equation

Internal Energy n Sum of the kinetic and potential energies of a system or substance.

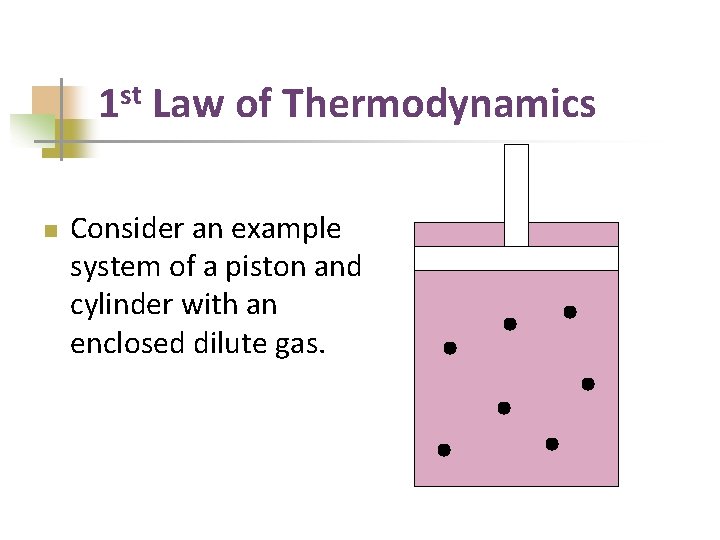
st 1 n Law of Thermodynamics Consider an example system of a piston and cylinder with an enclosed dilute gas.

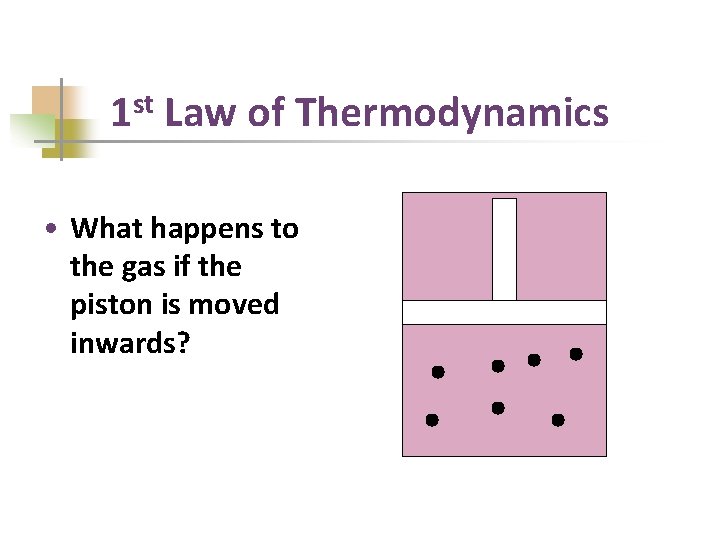
1 st Law of Thermodynamics • What happens to the gas if the piston is moved inwards?

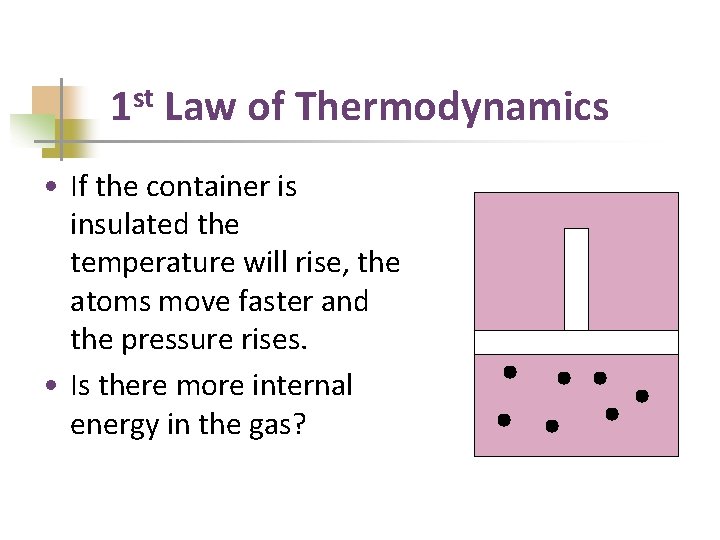
1 st Law of Thermodynamics • If the container is insulated the temperature will rise, the atoms move faster and the pressure rises. • Is there more internal energy in the gas?

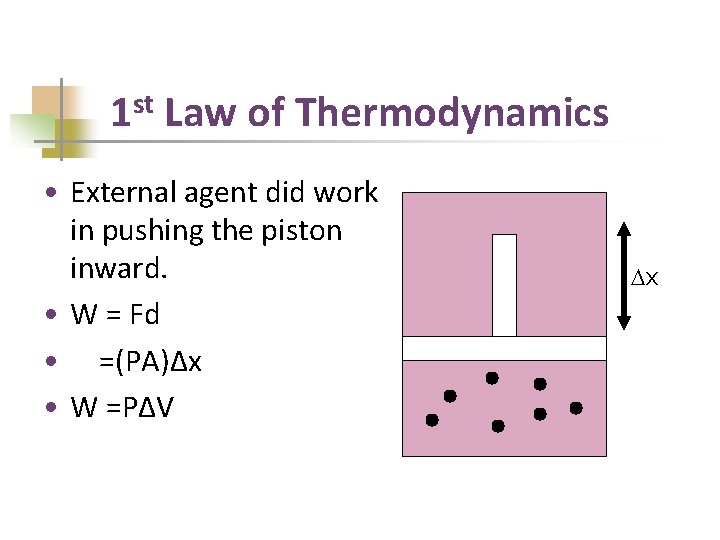
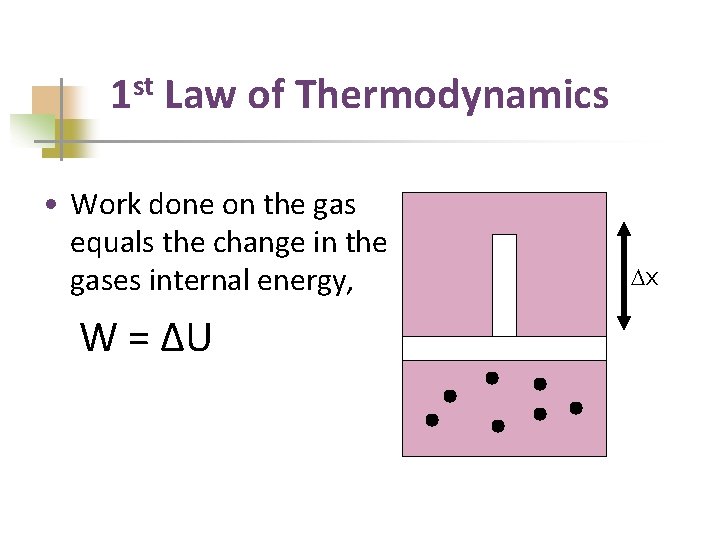
1 st Law of Thermodynamics • External agent did work in pushing the piston inward. • W = Fd • =(PA)Δx • W =PΔV Dx

1 st Law of Thermodynamics • Work done on the gas equals the change in the gases internal energy, W = ΔU Dx

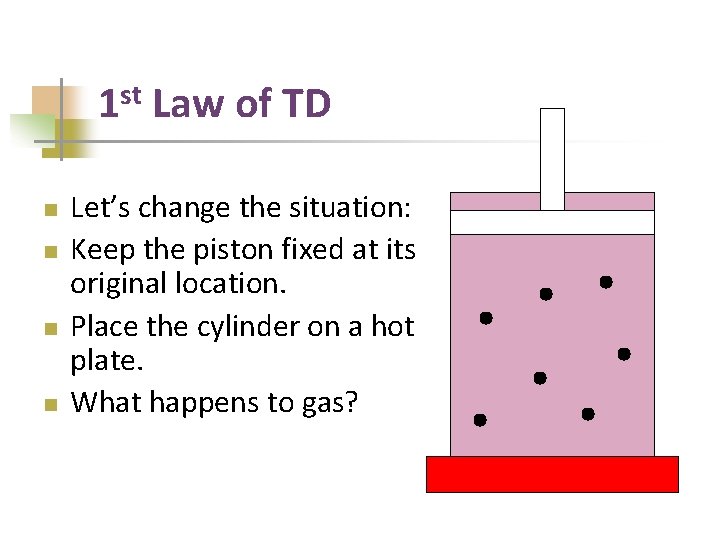
st 1 n n Law of TD Let’s change the situation: Keep the piston fixed at its original location. Place the cylinder on a hot plate. What happens to gas?

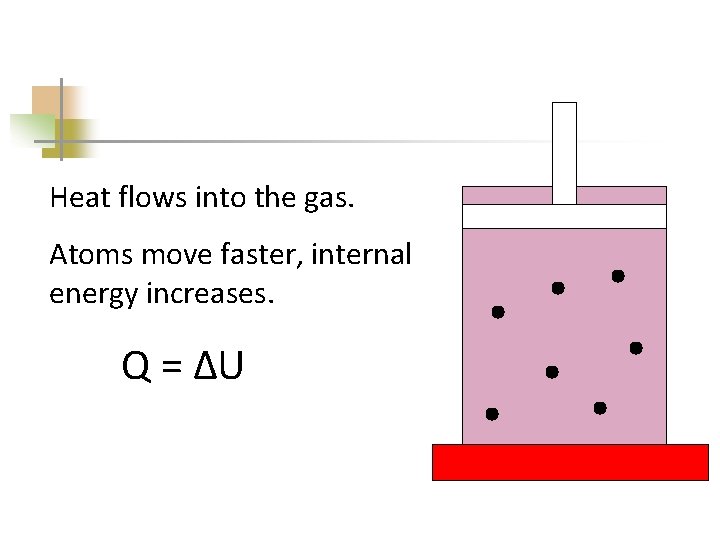
Heat flows into the gas. Atoms move faster, internal energy increases. Q = ΔU

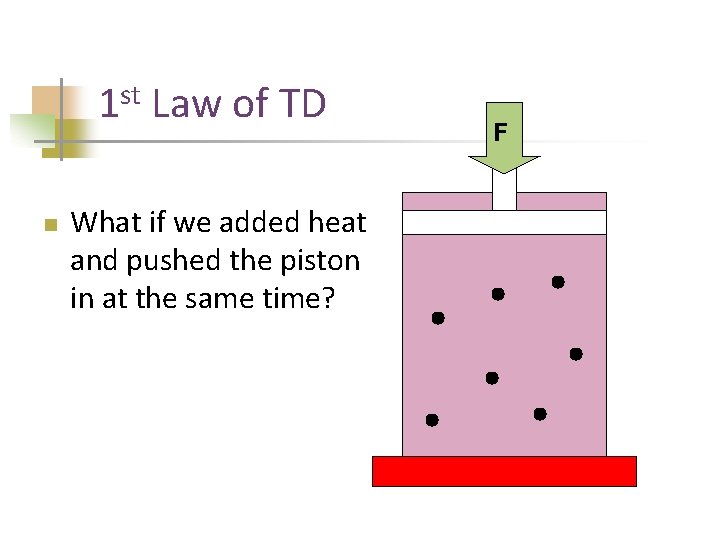
st 1 n Law of TD What if we added heat and pushed the piston in at the same time? F

Examples
 Specific heat of water
Specific heat of water Marginal percolation
Marginal percolation Unit for specific heat
Unit for specific heat What happens when you heat sugar
What happens when you heat sugar Specific heat capacity of ice cream
Specific heat capacity of ice cream Heat capacity at constant volume
Heat capacity at constant volume Specific latent heat formula
Specific latent heat formula Final temperature formula
Final temperature formula Specific heat capacity equation with power
Specific heat capacity equation with power How do i calculate specific heat
How do i calculate specific heat What is enthalpy of fusion
What is enthalpy of fusion How to find the specific heat capacity
How to find the specific heat capacity Symbol of specific heat capacity
Symbol of specific heat capacity Required practical specific heat capacity
Required practical specific heat capacity Latent heat of lead
Latent heat of lead Qlost = qgained
Qlost = qgained Specific heat chart
Specific heat chart Specific heat capacity unit
Specific heat capacity unit Specific heat capacity of lead j/kg c
Specific heat capacity of lead j/kg c Specific heat capacity question
Specific heat capacity question Enthalpy and heat equation
Enthalpy and heat equation Specific heat capacity of lead j/kg c
Specific heat capacity of lead j/kg c Specific heat capacity of cheese
Specific heat capacity of cheese Specific heat capacity calculator
Specific heat capacity calculator Specifc heat of water
Specifc heat of water Heat change unit
Heat change unit Heat capacity unit
Heat capacity unit Thermal energy
Thermal energy