SPECIFIC HEAT OF A METAL PROCEDURE 1 Fill








- Slides: 16

SPECIFIC HEAT OF A METAL

PROCEDURE 1) Fill a 250 m. L beaker about 3/4 of the way full with tap water and place it on the hotplate to begin boiling.

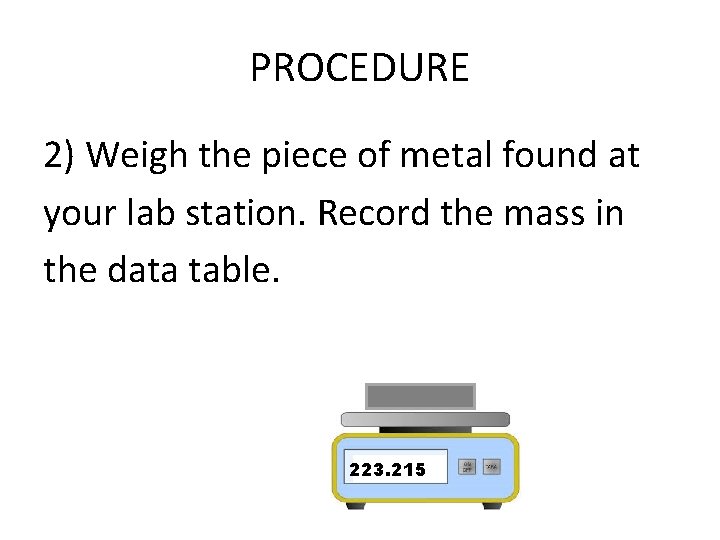
PROCEDURE 2) Weigh the piece of metal found at your lab station. Record the mass in the data table. 223. 215

PROCEDURE 3) Measure out 150 m. L of the cold water from the 600 m. L beaker. Do not use any ice cubes. Pour the water into the nested styrofoam cups. This is called a calorimeter.

PROCEDURE 4) Measure the temperature of the water in the calorimeter. Record the temperature in the data table. 2. 0 C

PROCEDURE 5) Carefully place the metal into the boiling water. Be careful not to drop the metal into the beaker use the tongs.

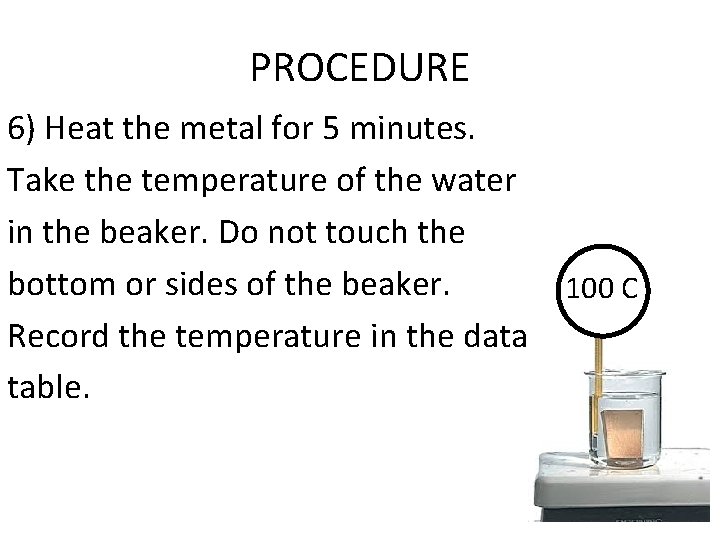
PROCEDURE 6) Heat the metal for 5 minutes. Take the temperature of the water in the beaker. Do not touch the bottom or sides of the beaker. 100 C Record the temperature in the data table.

PROCEDURE 7) Carefully remove the metal from the boiling water using the tongs and place the metal into the calorimeter. Place thermometer into the calorimeter. While determining the temperature of the metal and the water do not punch a hole into the calorimeter bottom or you will be starting again.

PROCEDURE 8) Record the temperature every 30 seconds for 5 minutes immediately after you add the metal to the water.

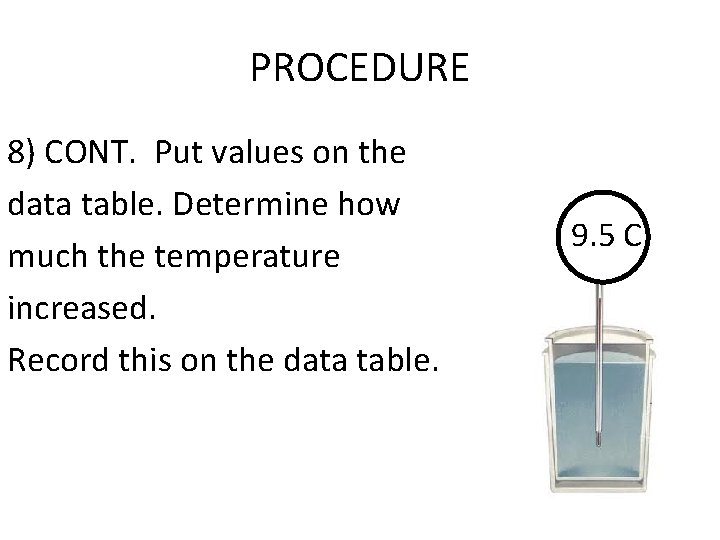
PROCEDURE 8) CONT. Put values on the data table. Determine how much the temperature increased. Record this on the data table. 9. 5 C

PROCEDURE 9) Pour the water into the sink and clean-up your lab station. Be sure to unplug the hotplate.

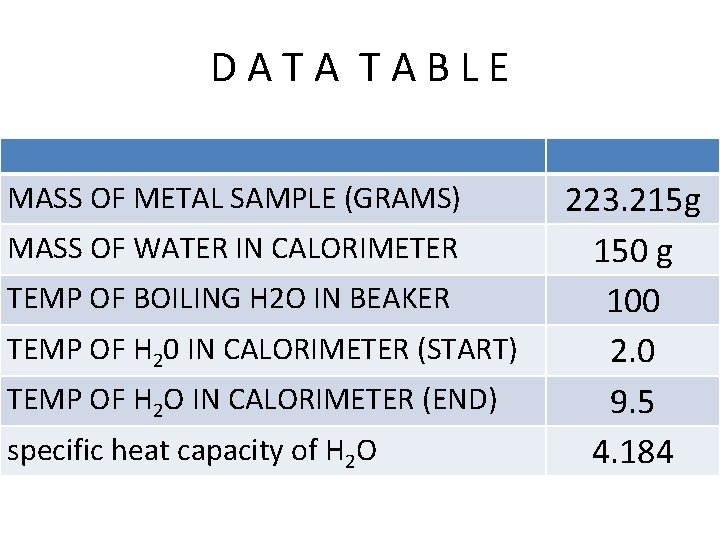
D A T A B L E MASS OF METAL SAMPLE (GRAMS) MASS OF WATER IN CALORIMETER TEMP OF BOILING H 2 O IN BEAKER TEMP OF H 20 IN CALORIMETER (START) TEMP OF H 2 O IN CALORIMETER (END) specific heat capacity of H 2 O 223. 215 g 150 g 100 2. 0 9. 5 4. 184

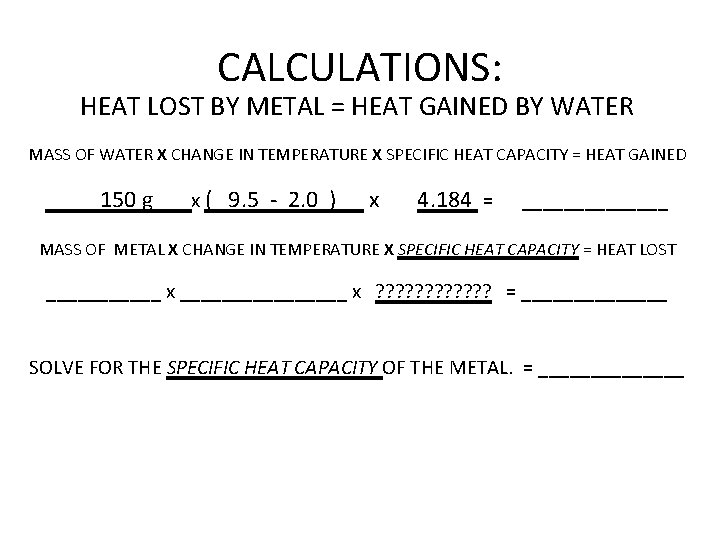
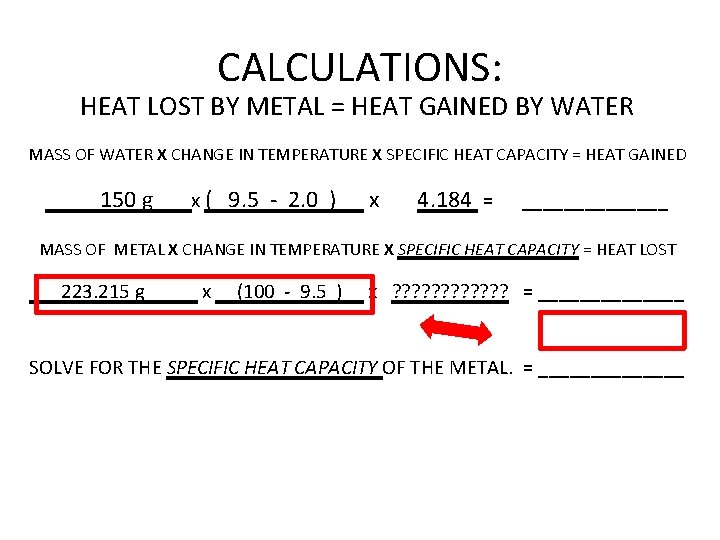
CALCULATIONS: HEAT LOST BY METAL = HEAT GAINED BY WATER MASS OF WATER X CHANGE IN TEMPERATURE X SPECIFIC HEAT CAPACITY = HEAT GAINED 150 g x ( 9. 5 - 2. 0 ) x 4. 184 = _______ MASS OF METAL X CHANGE IN TEMPERATURE X SPECIFIC HEAT CAPACITY = HEAT LOST ______ x ________ x ? ? ? = _______ SOLVE FOR THE SPECIFIC HEAT CAPACITY OF THE METAL. = _______

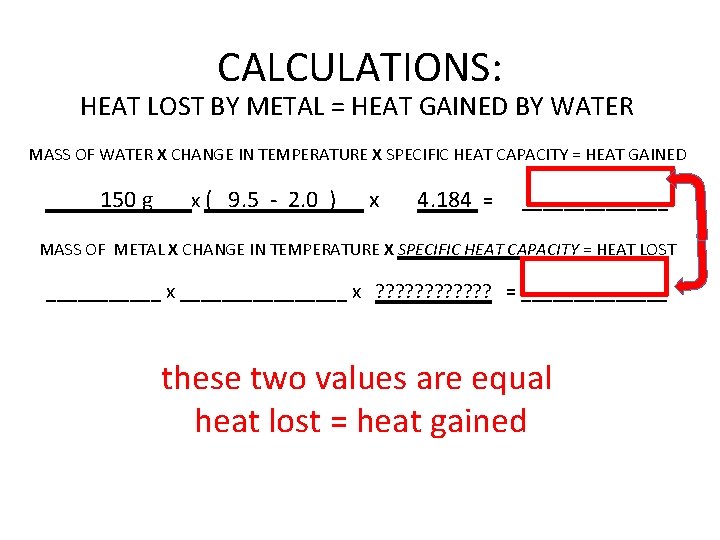
CALCULATIONS: HEAT LOST BY METAL = HEAT GAINED BY WATER MASS OF WATER X CHANGE IN TEMPERATURE X SPECIFIC HEAT CAPACITY = HEAT GAINED 150 g x ( 9. 5 - 2. 0 ) x 4. 184 = _______ MASS OF METAL X CHANGE IN TEMPERATURE X SPECIFIC HEAT CAPACITY = HEAT LOST ______ x ________ x ? ? ? = _______ these two values are equal heat lost = heat gained

CALCULATIONS: HEAT LOST BY METAL = HEAT GAINED BY WATER MASS OF WATER X CHANGE IN TEMPERATURE X SPECIFIC HEAT CAPACITY = HEAT GAINED 150 g x ( 9. 5 - 2. 0 ) x 4. 184 = _______ MASS OF METAL X CHANGE IN TEMPERATURE X SPECIFIC HEAT CAPACITY = HEAT LOST ___223. 215 g_____ x __(100 - 9. 5 )__ x ? ? ? = _______ SOLVE FOR THE SPECIFIC HEAT CAPACITY OF THE METAL. = _______

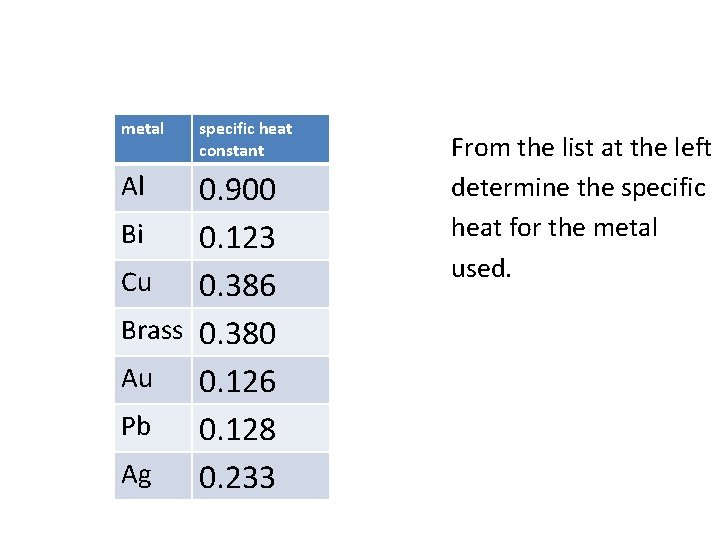
metal specific heat constant 0. 900 Bi 0. 123 Cu 0. 386 Brass 0. 380 Au 0. 126 Pb 0. 128 Ag 0. 233 Al From the list at the left determine the specific heat for the metal used.
 Specific heat of water
Specific heat of water Latent heat problems
Latent heat problems Boundary fill 4(x-1,y, fillcolor,________)
Boundary fill 4(x-1,y, fillcolor,________) Perbedaan boundary fill dan flood fill
Perbedaan boundary fill dan flood fill Area filling
Area filling Chemical bond def
Chemical bond def Acidity trends periodic table
Acidity trends periodic table Pure substances on the periodic table
Pure substances on the periodic table Nonmetals examples
Nonmetals examples Non metals melting and boiling points
Non metals melting and boiling points Metals react with nonmetals to form ionic compounds by
Metals react with nonmetals to form ionic compounds by Venn diagram gas liquid solid
Venn diagram gas liquid solid El sodio es metal o no metal
El sodio es metal o no metal 0 25
0 25 P
P Metal vs non metal
Metal vs non metal Uses of non metals
Uses of non metals