Hepatitis C Virus HCV ss RNA virus Envelope




















- Slides: 55


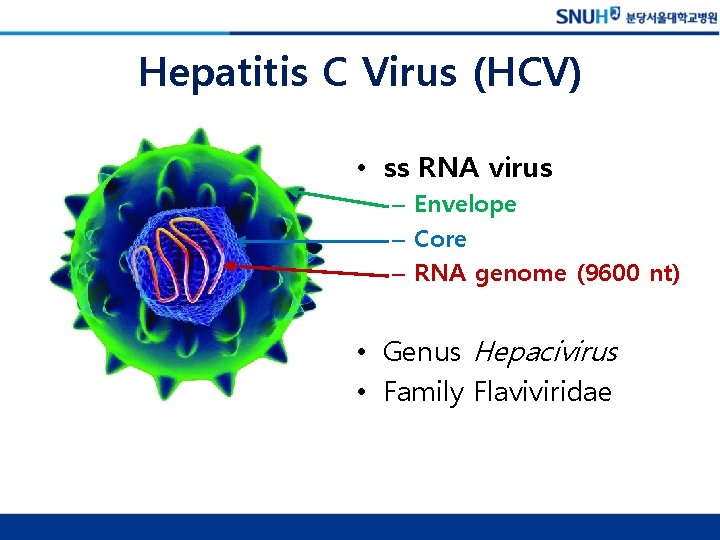
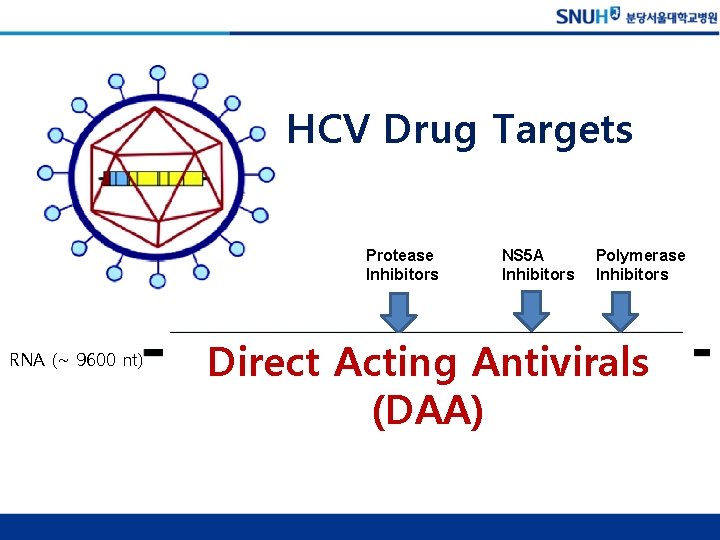
Hepatitis C Virus (HCV) • ss RNA virus – Envelope – Core – RNA genome (9600 nt) • Genus Hepacivirus • Family Flaviviridae

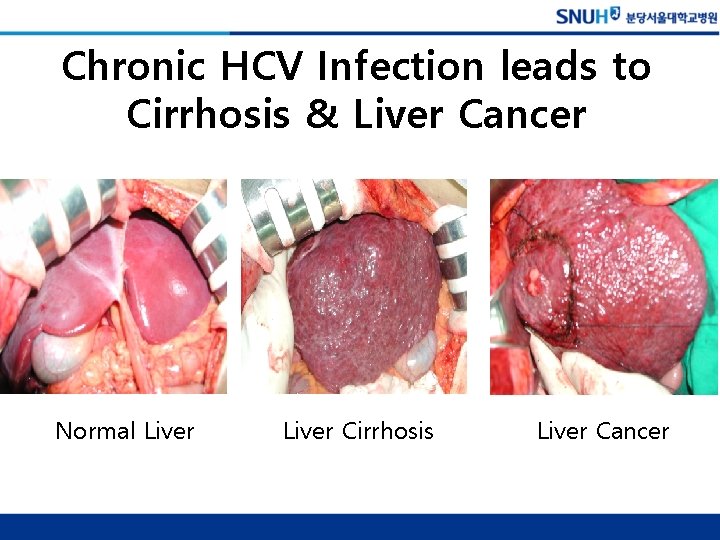
Chronic HCV Infection leads to Cirrhosis & Liver Cancer Normal Liver Cirrhosis Liver Cancer


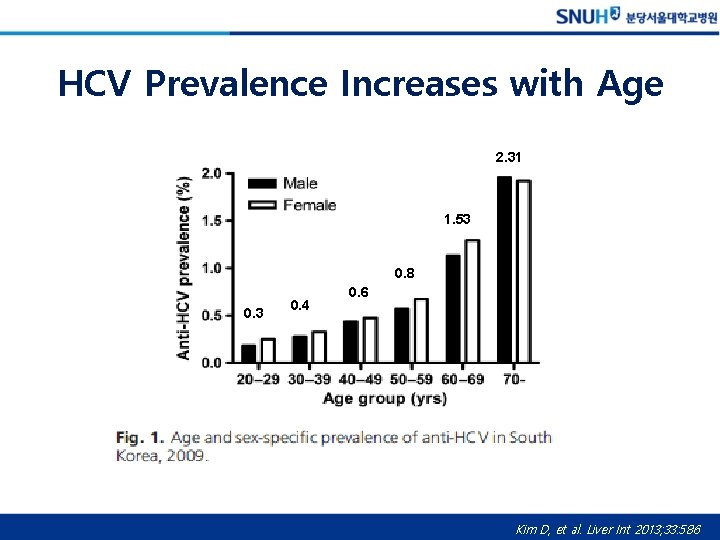
HCV Prevalence in Korea • Anti-HCV Prevalence (≥ 20 yr) : 0. 78% • Estimated number of adult people with • anti-HCV (+): ~327, 000 (96, 000 -868, 000) • Viremic rate: 56. 1% • HCV RNA(+): 183, 000 (54, 000 -487, 000) Gower E, et al. J Hepatol 2014; 61: S 45 -57, Kim D, et al. Liver Int 2013; 33: 586

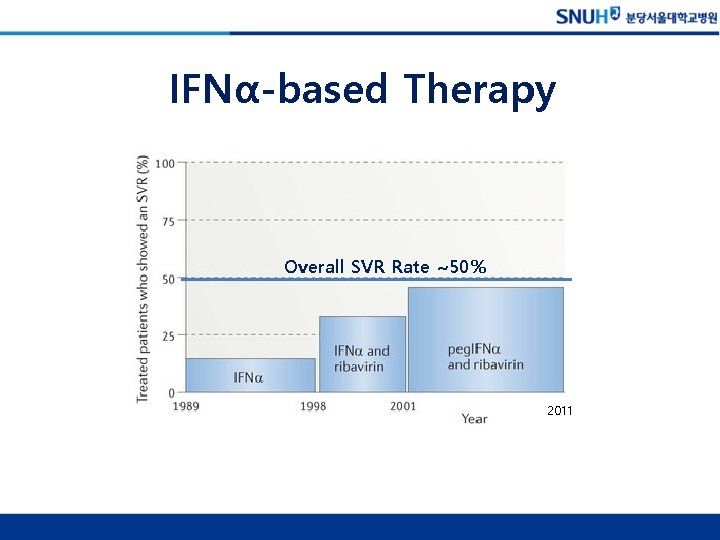
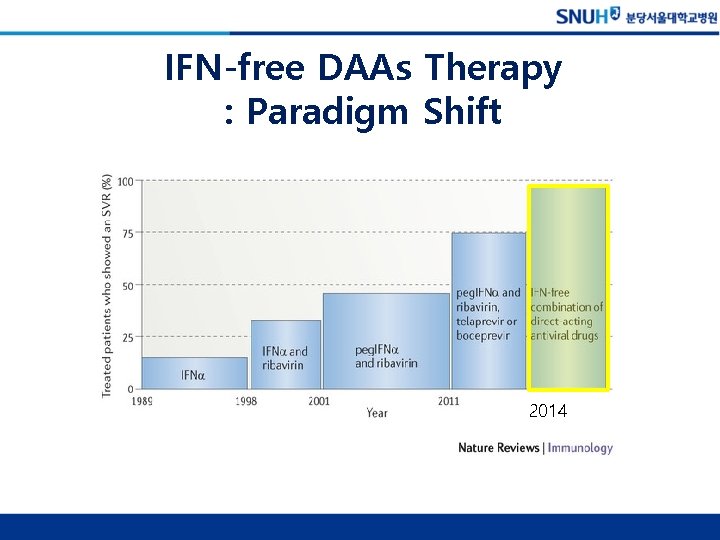
IFNα-based Therapy Overall SVR Rate ~50% 2011

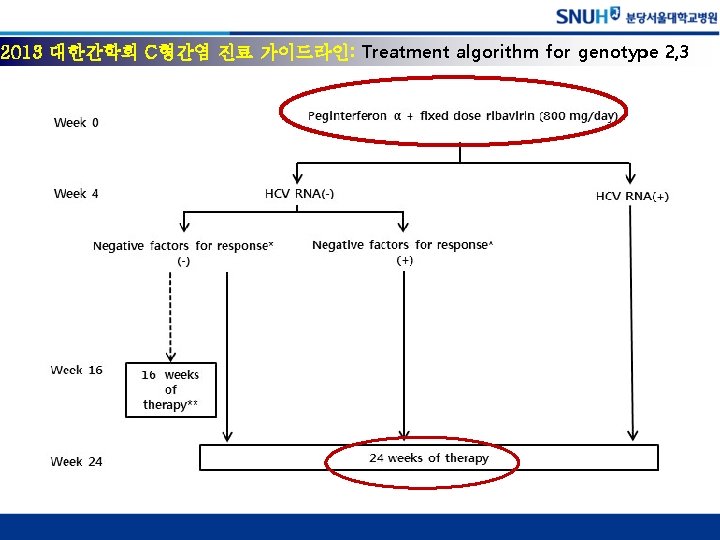
Response-guided Tx http: //www. kasl. org/html/sub 05_03. asp

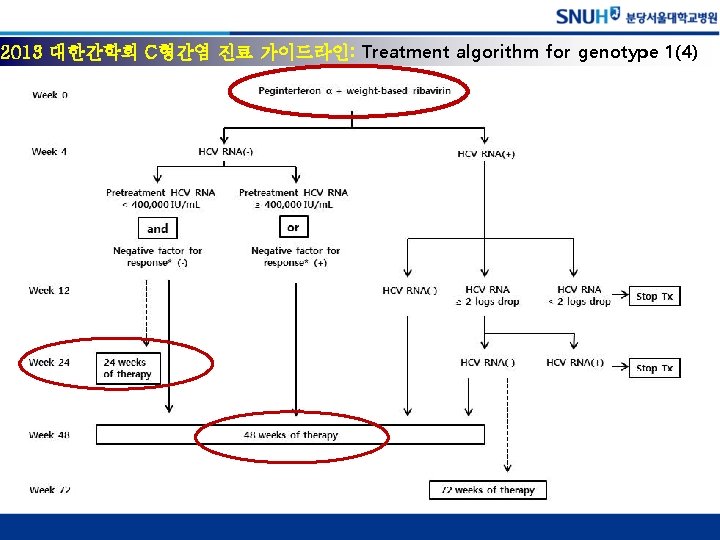
2013 대한간학회 C형간염 진료 가이드라인: Treatment algorithm for genotype 2, 3

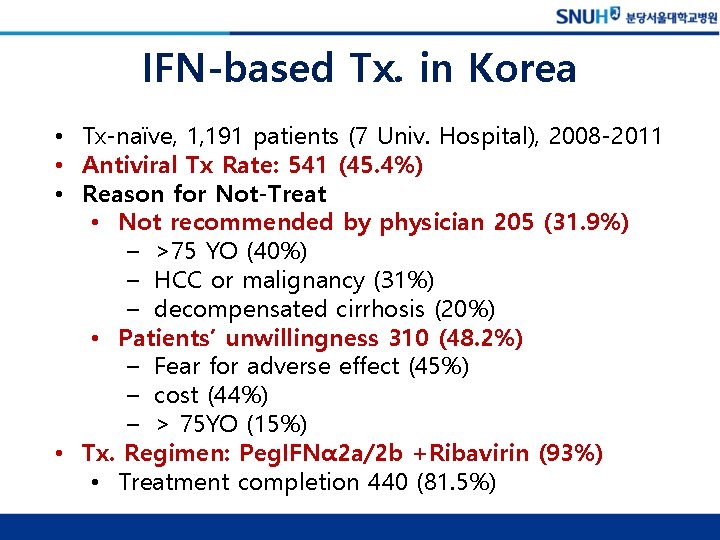
2013 대한간학회 C형간염 진료 가이드라인: Treatment algorithm for genotype 1(4)

IFN-based Tx. in Korea • Tx-naïve, 1, 191 patients (7 Univ. Hospital), 2008 -2011 • Antiviral Tx Rate: 541 (45. 4%) • Reason for Not-Treat • Not recommended by physician 205 (31. 9%) – >75 YO (40%) – HCC or malignancy (31%) – decompensated cirrhosis (20%) • Patients’ unwillingness 310 (48. 2%) – Fear for adverse effect (45%) – cost (44%) – > 75 YO (15%) • Tx. Regimen: Peg. IFNα 2 a/2 b +Ribavirin (93%) • Treatment completion 440 (81. 5%)

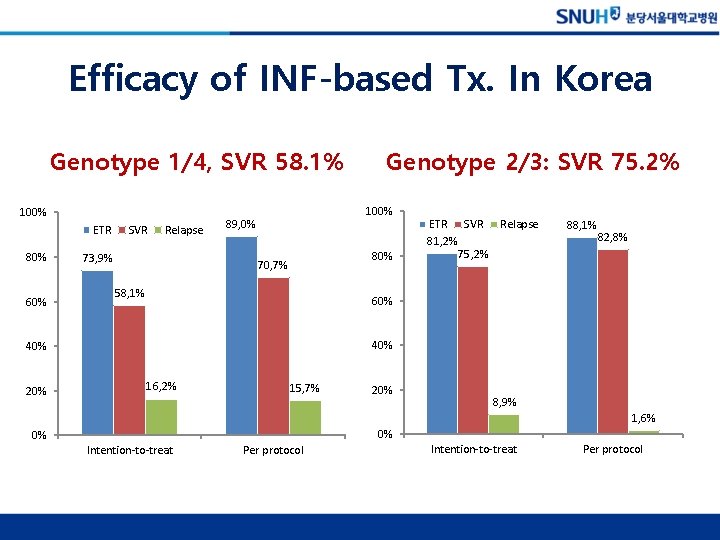
Efficacy of INF-based Tx. In Korea Genotype 1/4, SVR 58. 1% 100% ETR 80% 60% SVR Relapse 73, 9% 100% 89, 0% 80% 70, 7% 58, 1% ETR SVR 81, 2% 75, 2% Relapse 88, 1% 82, 8% 60% 40% 20% Genotype 2/3: SVR 75. 2% 16, 2% 15, 7% 20% 8, 9% 1, 6% 0% 0% Intention-to-treat Per protocol

IFN-free DAAs Therapy : Paradigm Shift 2014

HCV Drug Targets Protease Inhibitors RNA (~ 9600 nt) NS 5 A Inhibitors Polymerase Inhibitors Direct Acting Antivirals (DAA)

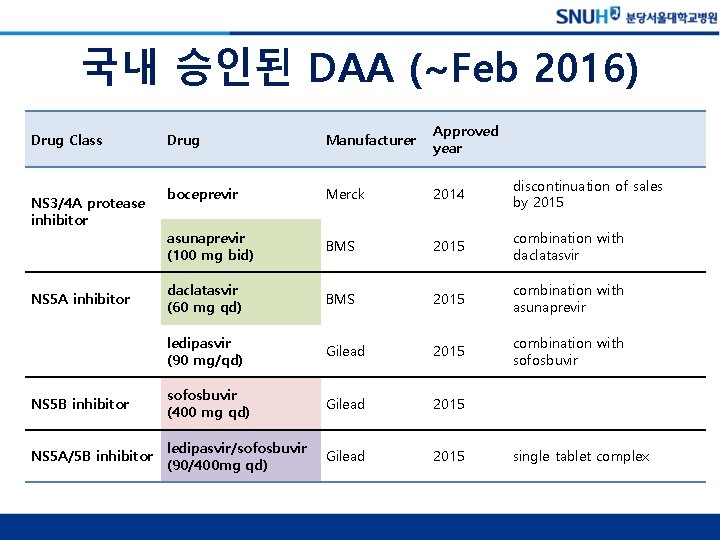
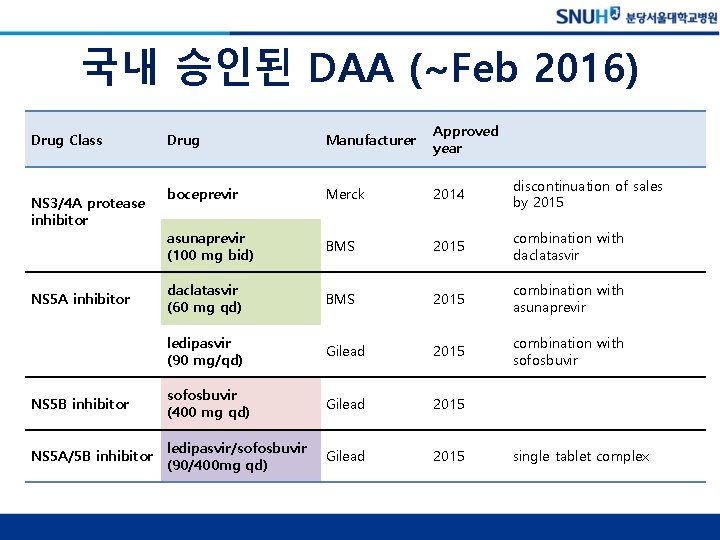
국내 승인된 DAA (~Feb 2016) Drug Manufacturer Approved year boceprevir Merck 2014 discontinuation of sales by 2015 asunaprevir (100 mg bid) BMS 2015 combination with daclatasvir (60 mg qd) BMS 2015 combination with asunaprevir ledipasvir (90 mg/qd) Gilead 2015 combination with sofosbuvir NS 5 B inhibitor sofosbuvir (400 mg qd) Gilead 2015 NS 5 A/5 B inhibitor ledipasvir/sofosbuvir (90/400 mg qd) Gilead 2015 Drug Class NS 3/4 A protease inhibitor NS 5 A inhibitor single tablet complex

http: //www. kasl. org/bbs/? code=guide



HCV Prevalence Increases with Age 2. 31 1. 53 0. 8 0. 3 0. 4 0. 6 Kim D, et al. Liver Int 2013; 33: 586


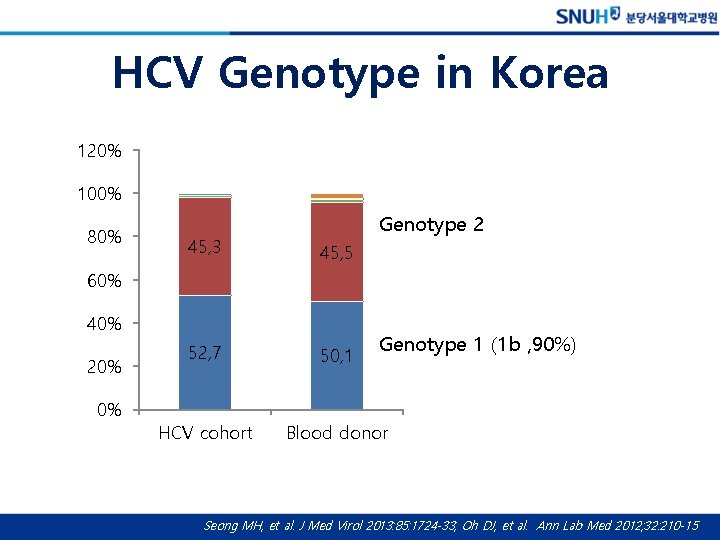
HCV Genotype in Korea 120% 100% 80% Genotype 2 45, 3 45, 5 52, 7 50, 1 HCV cohort Blood donor 60% 40% 20% 0% Genotype 1 (1 b , 90%) Seong MH, et al. J Med Virol 2013: 85: 1724 -33, Oh DJ, et al. Ann Lab Med 2012; 32: 210 -15




국내 승인된 DAA (~Feb 2016) Drug Manufacturer Approved year boceprevir Merck 2014 discontinuation of sales by 2015 asunaprevir (100 mg bid) BMS 2015 combination with daclatasvir (60 mg qd) BMS 2015 combination with asunaprevir ledipasvir (90 mg/qd) Gilead 2015 combination with sofosbuvir NS 5 B inhibitor sofosbuvir (400 mg qd) Gilead 2015 NS 5 A/5 B inhibitor ledipasvir/sofosbuvir (90/400 mg qd) Gilead 2015 Drug Class NS 3/4 A protease inhibitor NS 5 A inhibitor single tablet complex





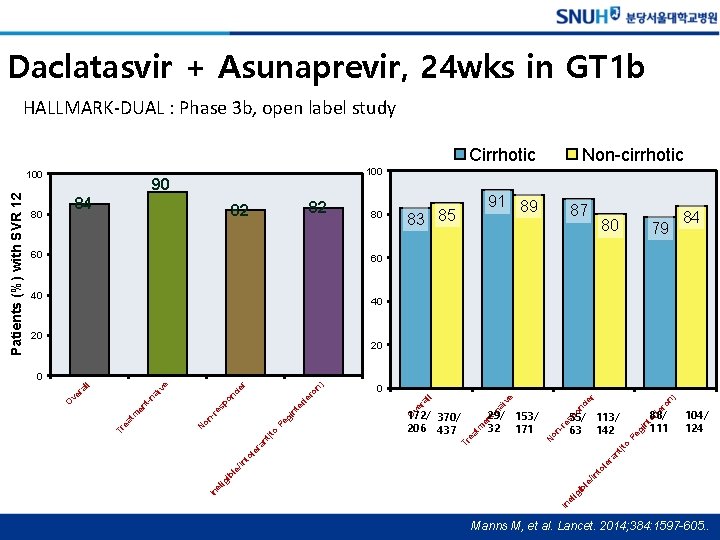
Daclatasvir + Asunaprevir, 24 wks in GT 1 b HALLMARK-DUAL : Phase 3 b, open label study Cirrhotic 80 90 84 82 82 60 80 91 89 83 85 87 80 79 55/ 113/ 63 142 88/ 111 84 60 40 40 20 20 104/ 124 (to Pe gi nt er sp o N on -r e en t tm ea Tr fe ro nd aï -n 29/ 153/ 32 171 n) er ve ll ra fe ro er nt Pe gi (to nt ra 172/ 370/ 206 437 In el ig ib el le ig ib /In le to le /In ra to nt le 192/ 235 0 O ve n) er nd -r e on 168/ 205 N In 182/ 203 sp o t-n tm en ea Tr 542/ 643 aï ra ve ll 0 O ve Patients (%) with SVR 12 100 Non-cirrhotic 100 Manns M, et al. Lancet. 2014; 384: 1597 -605. .

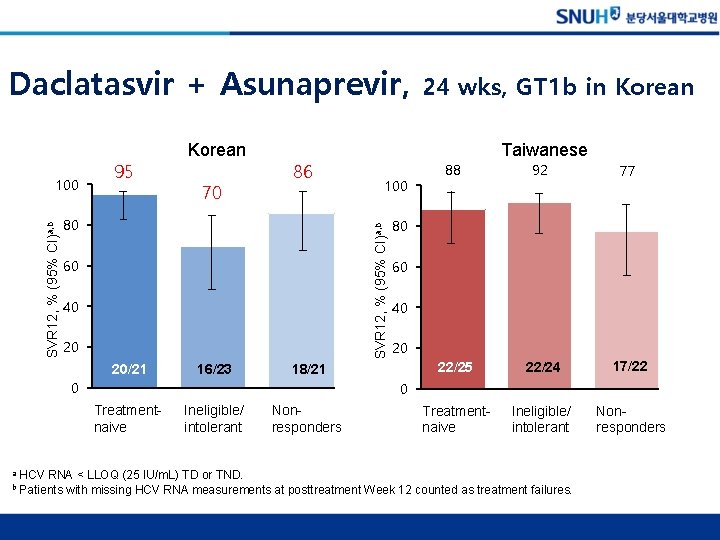
Daclatasvir + Asunaprevir, 70 86 80 60 40 20 20/21 16/23 88 92 77 22/25 22/24 17/22 Treatmentnaive Ineligible/ intolerant Nonresponders 80 60 40 20 0 Treatmentnaive b 100 18/21 0 a Taiwanese SVR 12, % (95% CI)a, b 100 95 Korean 24 wks, GT 1 b in Korean Ineligible/ intolerant Nonresponders HCV RNA < LLOQ (25 IU/m. L) TD or TND. Patients with missing HCV RNA measurements at posttreatment Week 12 counted as treatment failures.

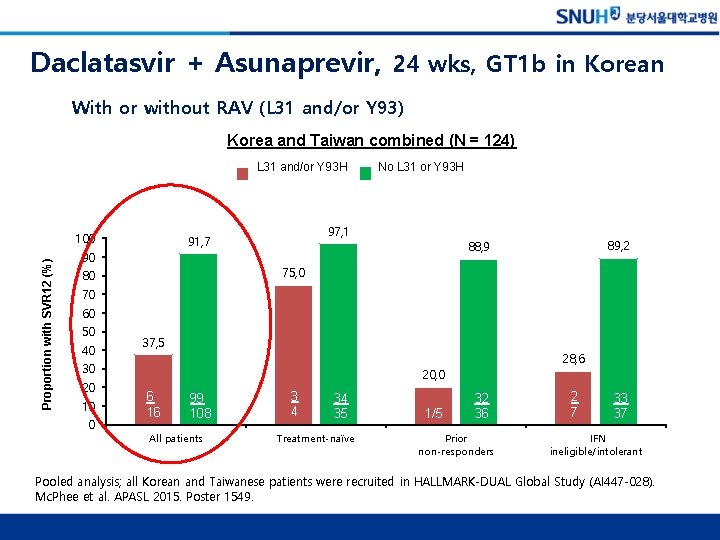
Daclatasvir + Asunaprevir, 24 wks, GT 1 b in Korean With or without RAV (L 31 and/or Y 93) Korea and Taiwan combined (N = 124) L 31 and/or Y 93 H Proportion with SVR 12 (%) 100 97, 1 91, 7 90 No L 31 or Y 93 H 89, 2 88, 9 75, 0 80 70 60 50 40 37, 5 28, 6 30 20 10 0 20, 0 6 16 99 108 All patients 3 4 34 35 Treatment-naïve 1/5 32 36 Prior non-responders 2 7 33 37 IFN ineligible/intolerant Pooled analysis; all Korean and Taiwanese patients were recruited in HALLMARK-DUAL Global Study (AI 447 -028). Mc. Phee et al. APASL 2015. Poster 1549.

Sofosbuvir • NS 5 B polymerase inhibitor • 400 mg 정, 실온 보관 – 식사와 함께 또는 관계없이 1회 투여 – 18시간 이내는 바로 복용, 이후에는 다음날 1정 복용 • 금기: <GFR 30 m. L/min • 병용투여 금기: amiodarone – flecainaide, propafenone, thioridazine – Rifampin, cyclosporine, sirolimus, gemfibrozil • 부작용 – 2% 미만, 두통, 피로, 설사, 구역 • 비급여, 12주 가격 3800만원

Ledipasvir/Sofosbuvir • NS 5 A inhibitor/NS 5 B polymerase inhibitor • 90 mg/400 mg 단일 정제, 실온 보관 – 식사와 함께 또는 관계없이 1회 투여 – 18시간 이내는 바로 복용, 이후에는 다음날 1정 복용 • 금기: <GFR 30 m. L/min • 병용투여 금기: amiodarone – flecainaide, propafenone, thioridazine – Rifampin, cyclosporine, sirolimus, gemfibrozil • 부작용 – 2% 미만, 두통, 피로, 설사, 구역 • 비급여, 12주 가격 4600만원

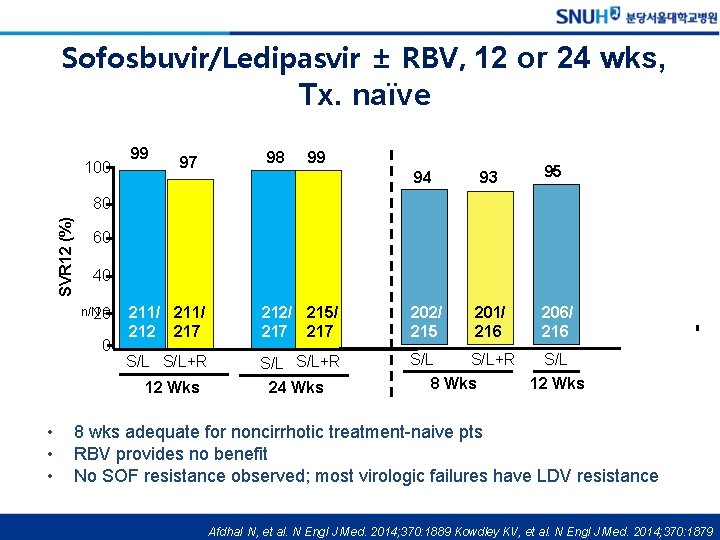
Sofosbuvir/Ledipasvir ± RBV, 12 or 24 wks, Tx. naïve 100 99 97 98 99 94 93 95 SVR 12 (%) 80 60 40 n/N 20= 0 211/ 212 217 212/ 215/ 217 202/ 215 201/ 216 206/ 216 S/L S/L+R S/L 12 Wks • • • 24 Wks 8 Wks 12 Wks 8 wks adequate for noncirrhotic treatment-naive pts RBV provides no benefit No SOF resistance observed; most virologic failures have LDV resistance Afdhal N, et al. N Engl J Med. 2014; 370: 1889 Kowdley KV, et al. N Engl J Med. 2014; 370: 1879

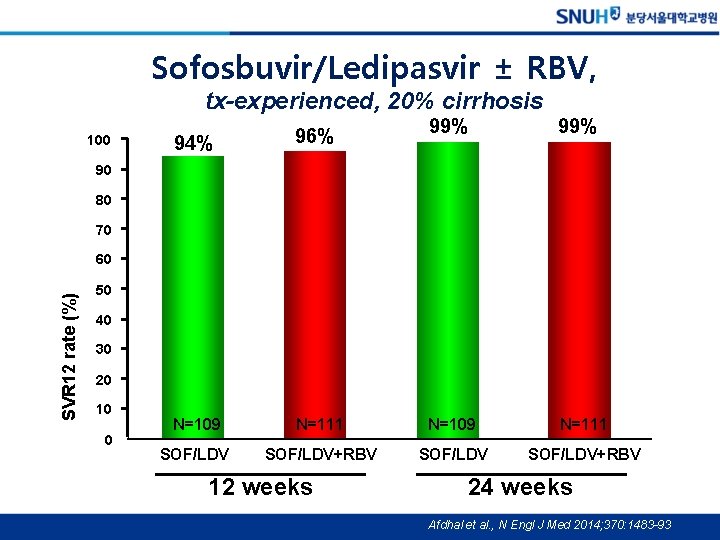
Sofosbuvir/Ledipasvir ± RBV, tx-experienced, 20% cirrhosis 100 99% 94% 96% N=109 N=111 SOF/LDV+RBV 90 80 70 SVR 12 rate (%) 60 50 40 30 20 10 0 12 weeks 24 weeks Afdhal et al. , N Engl J Med 2014; 370: 1483 -93

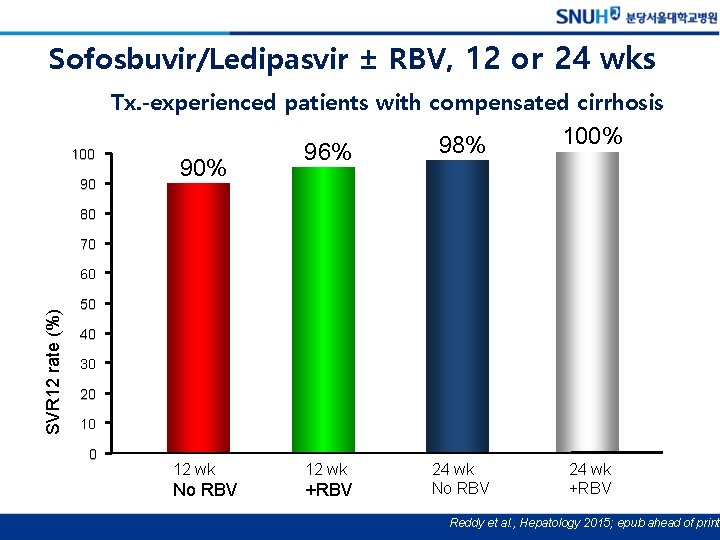
Sofosbuvir/Ledipasvir ± RBV, 12 or 24 wks Tx. -experienced patients with compensated cirrhosis 100 90 96% 98% 12 wk No RBV +RBV 24 wk No RBV 90% 100% 80 70 SVR 12 rate (%) 60 50 40 30 20 10 0 24 wk +RBV Reddy et al. , Hepatology 2015; epub ahead of print

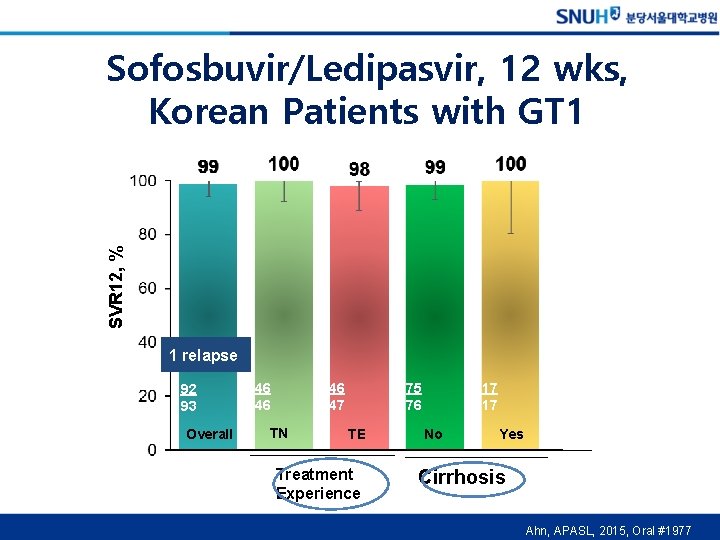
SVR 12, % Sofosbuvir/Ledipasvir, 12 wks, Korean Patients with GT 1 1 relapse 92 93 Overall 46 47 46 46 TN 75 76 TE Treatment Experience 17 17 No Yes Cirrhosis Ahn, APASL, 2015, Oral #1977

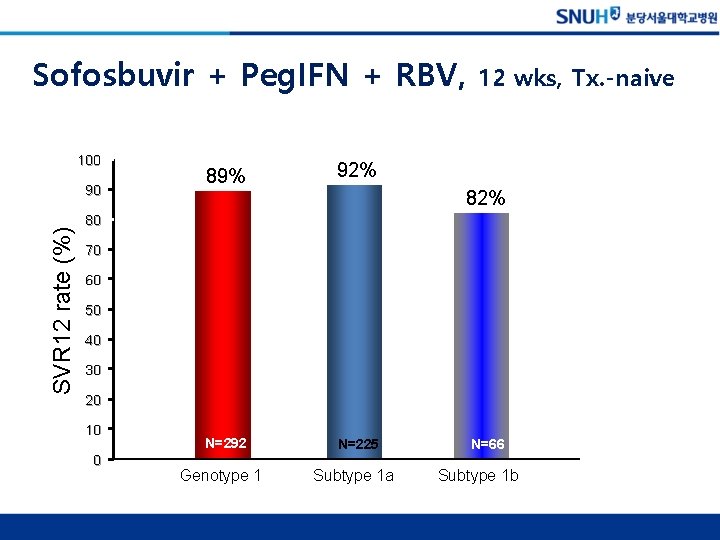
Sofosbuvir + Peg. IFN + RBV, 100 SVR 12 rate (%) 90 89% 92% N=292 N=225 12 wks, Tx. -naive 82% 80 70 60 50 40 30 20 10 0 Genotype 1 Subtype 1 a N=66 Subtype 1 b

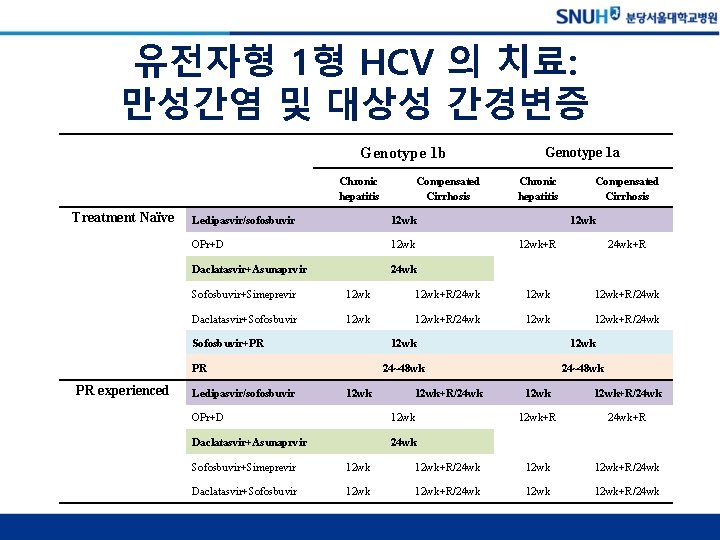
유전자형 1형 HCV 의 치료: 만성간염 및 대상성 간경변증 Genotype 1 b Chronic hepatitis Treatment Naïve Ledipasvir/sofosbuvir 12 wk OPr+D 12 wk Daclatasvir+Asunaprvir 24 wk Chronic hepatitis Compensated Cirrhosis 12 wk+R 24 wk+R Sofosbuvir+Simeprevir 12 wk+R/24 wk Daclatasvir+Sofosbuvir 12 wk+R/24 wk Sofosbuvir+PR PR PR experienced Compensated Cirrhosis Genotype 1 a Ledipasvir/sofosbuvir 12 wk 24~48 wk 12 wk+R/24 wk OPr+D 12 wk Daclatasvir+Asunaprvir 24 wk 12 wk+R/24 wk 12 wk+R 24 wk+R Sofosbuvir+Simeprevir 12 wk+R/24 wk Daclatasvir+Sofosbuvir 12 wk+R/24 wk


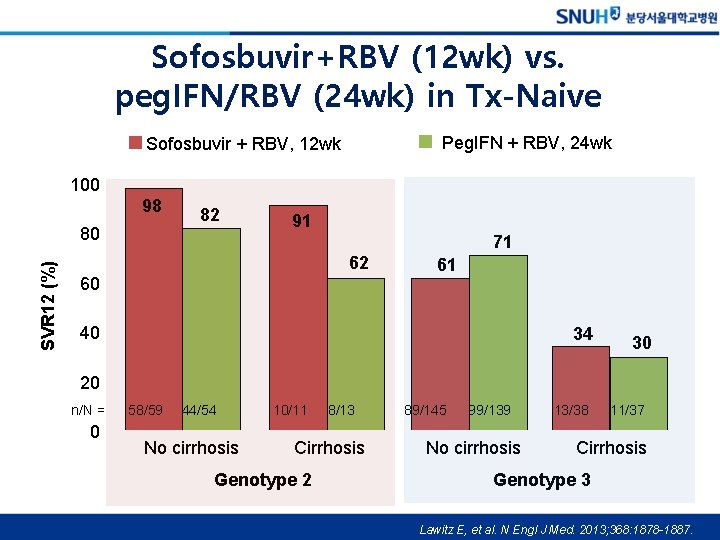
Sofosbuvir+RBV (12 wk) vs. peg. IFN/RBV (24 wk) in Tx-Naive Peg. IFN + RBV, 24 wk Sofosbuvir + RBV, 12 wk 100 98 SVR 12 (%) 80 82 91 71 62 60 61 40 34 30 20 n/N = 0 58/59 44/54 No cirrhosis 10/11 8/13 Cirrhosis Genotype 2 89/145 99/139 No cirrhosis 13/38 11/37 Cirrhosis Genotype 3 Lawitz E, et al. N Engl J Med. 2013; 368: 1878 -1887.

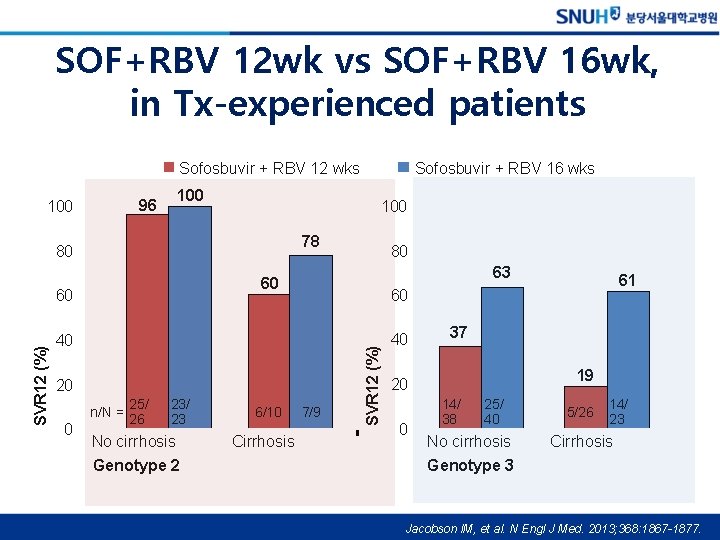
SOF+RBV 12 wk vs SOF+RBV 16 wk, in Tx-experienced patients Sofosbuvir + RBV 12 wks 96 100 100 78 80 63 40 20 n/N = 25/ 26 23/ 23 No cirrhosis Genotype 2 6/10 Cirrhosis 61 60 7/9 SVR 12 (%) 80 60 60 0 Sofosbuvir + RBV 16 wks 40 37 19 20 0 14/ 38 25/ 40 No cirrhosis Genotype 3 5/26 14/ 23 Cirrhosis Jacobson IM, et al. N Engl J Med. 2013; 368: 1867 -1877.

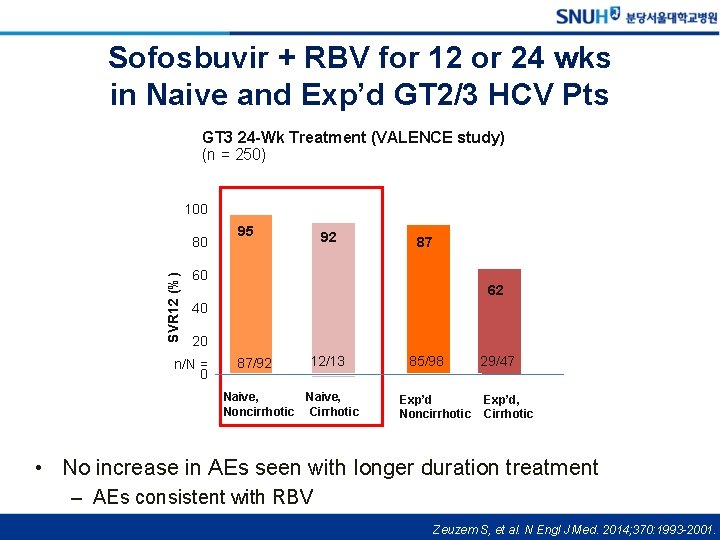
Sofosbuvir + RBV for 12 or 24 wks in Naive and Exp’d GT 2/3 HCV Pts GT 3 24 -Wk Treatment (VALENCE study) (n = 250) 100 SVR 12 (%) 80 95 92 87 60 62 40 20 n/N = 0 87/92 12/13 Naive, Noncirrhotic Cirrhotic 85/98 Exp’d Noncirrhotic 29/47 Exp’d, Cirrhotic • No increase in AEs seen with longer duration treatment – AEs consistent with RBV Zeuzem S, et al. N Engl J Med. 2014; 370: 1993 -2001.

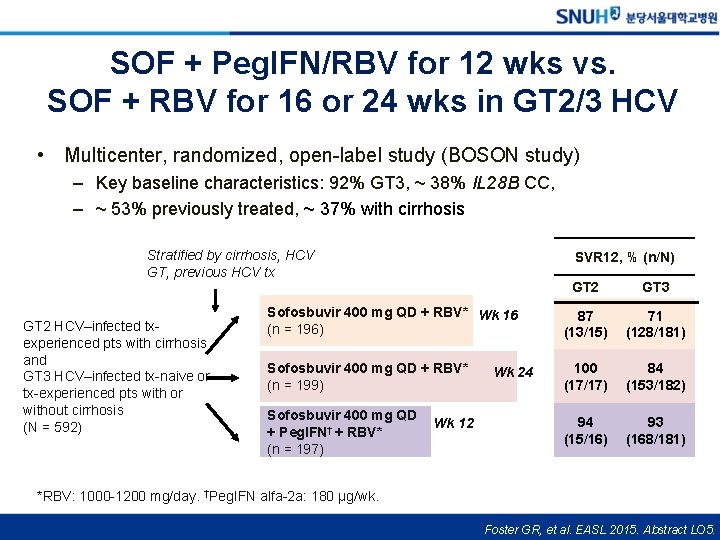
SOF + Peg. IFN/RBV for 12 wks vs. SOF + RBV for 16 or 24 wks in GT 2/3 HCV • Multicenter, randomized, open-label study (BOSON study) – Key baseline characteristics: 92% GT 3, ~ 38% IL 28 B CC, – ~ 53% previously treated, ~ 37% with cirrhosis Stratified by cirrhosis, HCV GT, previous HCV tx GT 2 HCV–infected txexperienced pts with cirrhosis and GT 3 HCV–infected tx-naive or tx-experienced pts with or without cirrhosis (N = 592) SVR 12, % (n/N) GT 2 GT 3 Sofosbuvir 400 mg QD + RBV* Wk 16 (n = 196) 87 (13/15) 71 (128/181) Sofosbuvir 400 mg QD + RBV* (n = 199) 100 (17/17) 84 (153/182) 94 (15/16) 93 (168/181) Sofosbuvir 400 mg QD + Peg. IFN† + RBV* (n = 197) Wk 12 Wk 24 *RBV: 1000 -1200 mg/day. †Peg. IFN alfa-2 a: 180 μg/wk. Foster GR, et al. EASL 2015. Abstract LO 5.

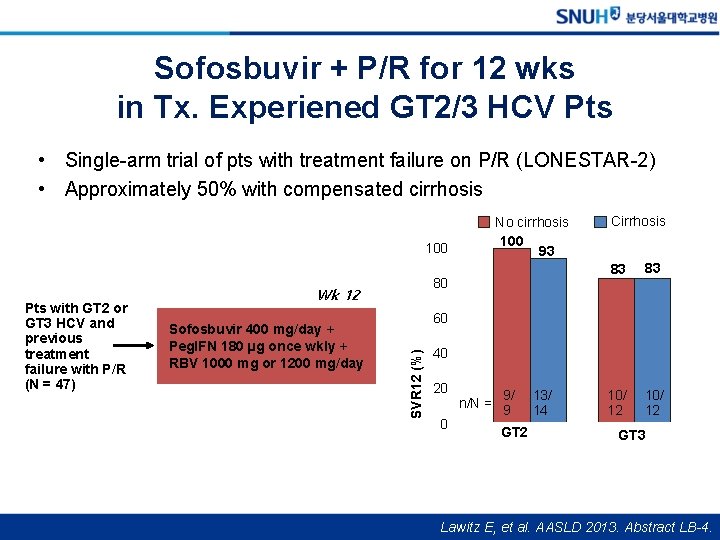
Sofosbuvir + P/R for 12 wks in Tx. Experiened GT 2/3 HCV Pts • Single-arm trial of pts with treatment failure on P/R (LONESTAR-2) • Approximately 50% with compensated cirrhosis No cirrhosis 100 93 100 Sofosbuvir 400 mg/day + Peg. IFN 180 µg once wkly + RBV 1000 mg or 1200 mg/day 83 83 10/ 12 60 SVR 12 (%) Pts with GT 2 or GT 3 HCV and previous treatment failure with P/R (N = 47) 80 Wk 12 Cirrhosis 40 20 0 n/N = 9/ 9 GT 2 13/ 14 GT 3 Lawitz E, et al. AASLD 2013. Abstract LB-4.

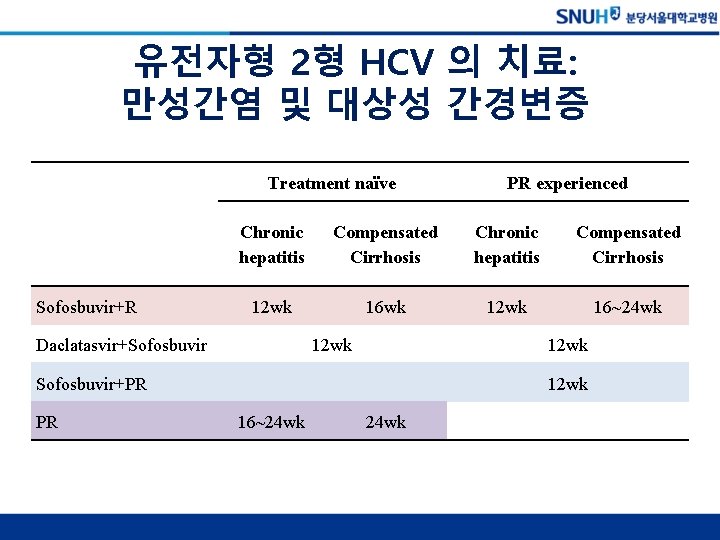
유전자형 2형 HCV 의 치료: 만성간염 및 대상성 간경변증 Treatment naïve Sofosbuvir+R Chronic hepatitis Compensated Cirrhosis 12 wk 16 wk 12 wk 16~24 wk Daclatasvir+Sofosbuvir 12 wk Sofosbuvir+PR PR PR experienced 12 wk 16~24 wk

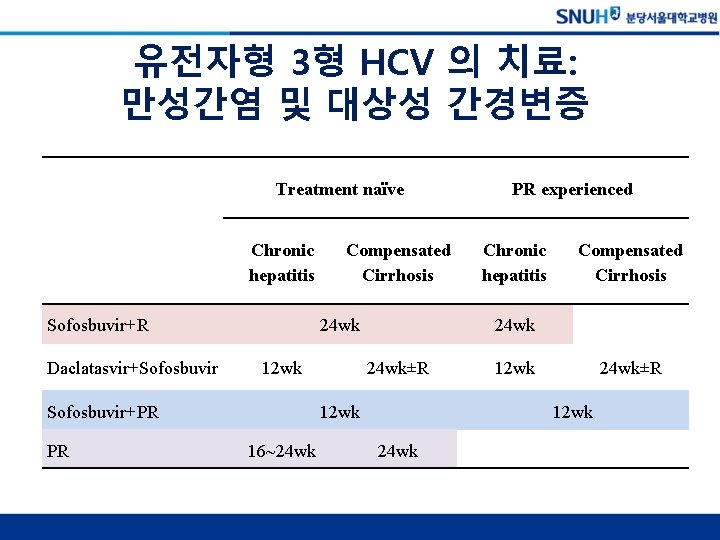
유전자형 3형 HCV 의 치료: 만성간염 및 대상성 간경변증 Treatment naïve Chronic hepatitis Sofosbuvir+R Daclatasvir+Sofosbuvir 24 wk 12 wk Sofosbuvir+PR PR Compensated Cirrhosis Chronic hepatitis Compensated Cirrhosis 24 wk±R 12 wk 16~24 wk PR experienced 12 wk 24 wk±R 12 wk 24 wk

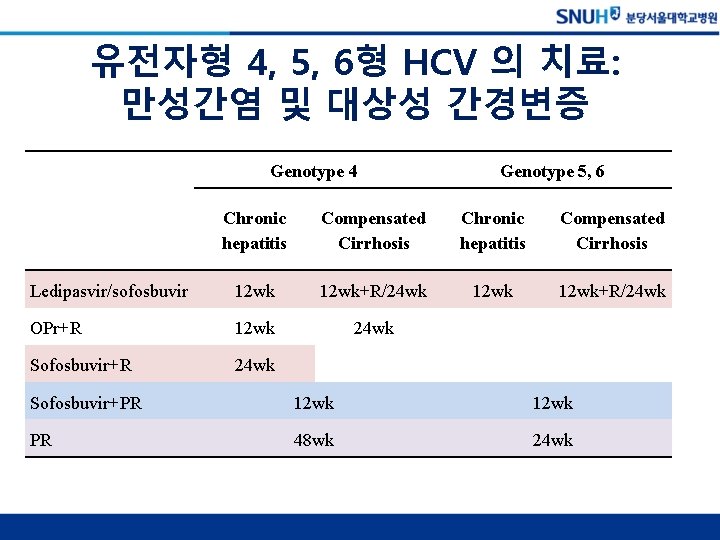
유전자형 4, 5, 6형 HCV 의 치료: 만성간염 및 대상성 간경변증 Genotype 4 Genotype 5, 6 Chronic hepatitis Compensated Cirrhosis Ledipasvir/sofosbuvir 12 wk+R/24 wk OPr+R 12 wk 24 wk Sofosbuvir+R 24 wk Sofosbuvir+PR 12 wk PR 48 wk 24 wk

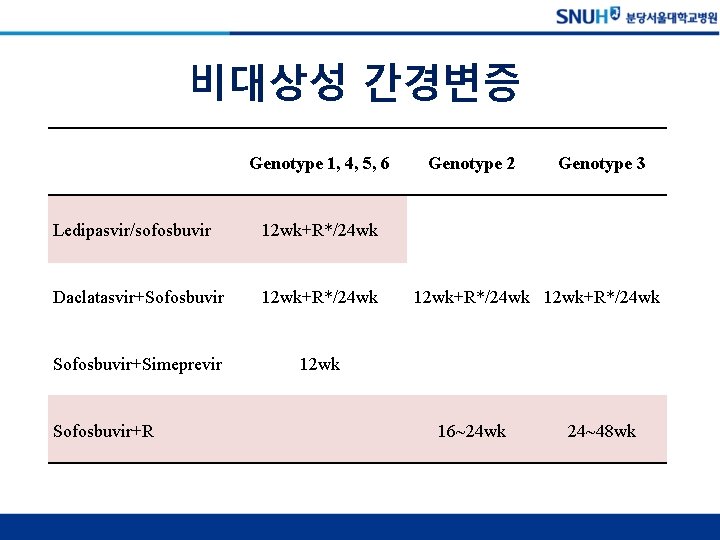
비대상성 간경변증 Genotype 1, 4, 5, 6 Ledipasvir/sofosbuvir 12 wk+R*/24 wk Daclatasvir+Sofosbuvir 12 wk+R*/24 wk Sofosbuvir+Simeprevir 12 wk Sofosbuvir+R Genotype 2 Genotype 3 12 wk+R*/24 wk 16~24 wk 24~48 wk

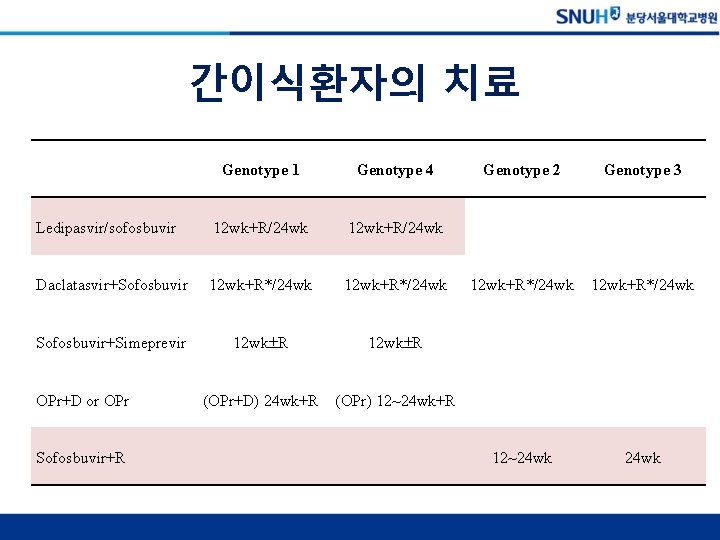
간이식환자의 치료 Genotype 1 Genotype 4 Ledipasvir/sofosbuvir 12 wk+R/24 wk Daclatasvir+Sofosbuvir 12 wk+R*/24 wk Sofosbuvir+Simeprevir 12 wk R (OPr+D) 24 wk+R (OPr) 12~24 wk+R OPr+D or OPr Sofosbuvir+R Genotype 2 Genotype 3 12 wk+R*/24 wk 12~24 wk





Thank You for Your Attention jsh@snubh. org