Essential Strategies for Eliminating HCV Among Persons With














- Slides: 35

Essential Strategies for Eliminating HCV Among Persons With Current or Recent Injection Drug Use Cosimo Colletta Liver group 2 luglio 2019 This activity is supported by independent educational grants from Abb. Vie

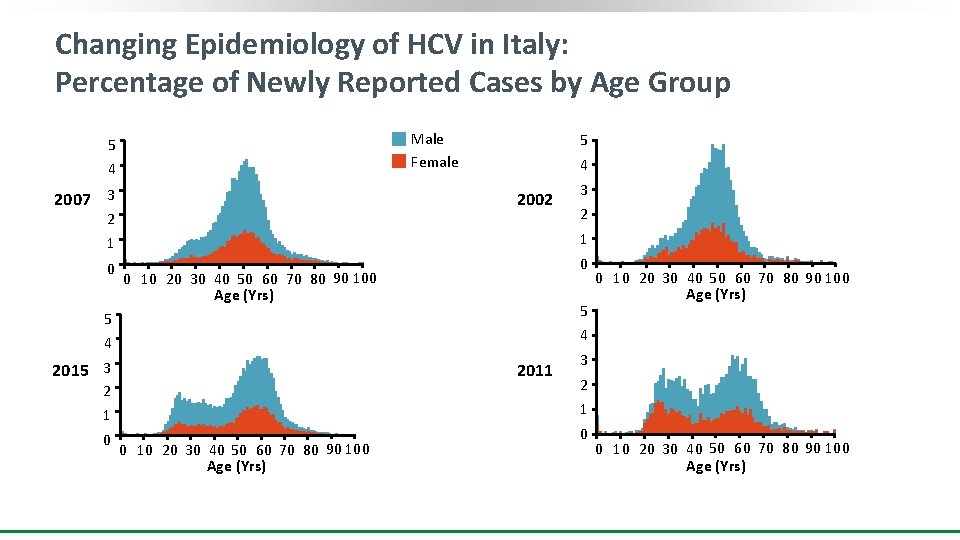
Changing Epidemiology of HCV in Italy: Percentage of Newly Reported Cases by Age Group Male Female 5 4 2007 3 5 4 2002 2 0 0 10 20 30 40 50 60 70 80 90 100 Age (Yrs) 5 5 4 0 10 20 30 40 50 60 70 80 90 100 Age (Yrs) 4 2015 3 2011 2 1 0 2 1 1 0 3 3 2 1 0 10 20 30 40 50 60 70 80 90 100 Age (Yrs) 0 0 10 20 30 40 50 60 70 80 90 100 Age (Yrs)

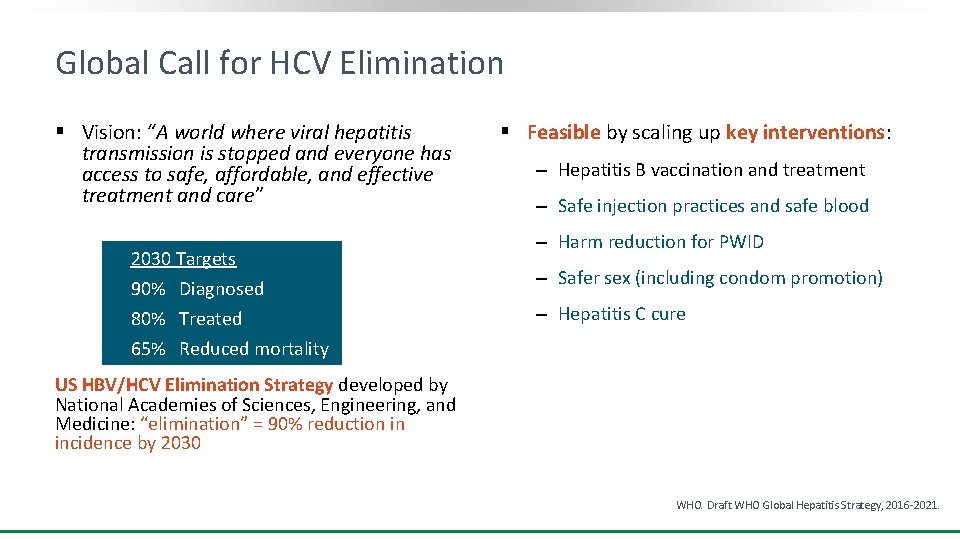
Global Call for HCV Elimination § Vision: “A world where viral hepatitis transmission is stopped and everyone has access to safe, affordable, and effective treatment and care” 2030 Targets 90% Diagnosed 80% Treated 65% Reduced mortality § Feasible by scaling up key interventions: ‒ Hepatitis B vaccination and treatment ‒ Safe injection practices and safe blood ‒ Harm reduction for PWID ‒ Safer sex (including condom promotion) ‒ Hepatitis C cure US HBV/HCV Elimination Strategy developed by National Academies of Sciences, Engineering, and Medicine: “elimination” = 90% reduction in incidence by 2030 WHO. Draft WHO Global Hepatitis Strategy, 2016 -2021.

Progress Toward HCV Elimination Goals § 12 countries are currently on track to achieve 2030 HCV elimination goals: ‒ Australia, Egypt, France, Georgia, Iceland, Italy, Japan, Mongolia, the Netherlands, Spain, Switzerland, and United Kingdom ‒ The United States is NOT among them ‒ We are missing in important population for elimination efforts by not expanding treatment further to PWID population http: //polarisobservatory. org/polaris_view/hep. C. htm

Patient Case: Young, Female PWID § A young woman presents at a drug treatment center and explains that she recently experienced a heroin overdose and is afraid of dying if it happens again § She lives in remote rural area

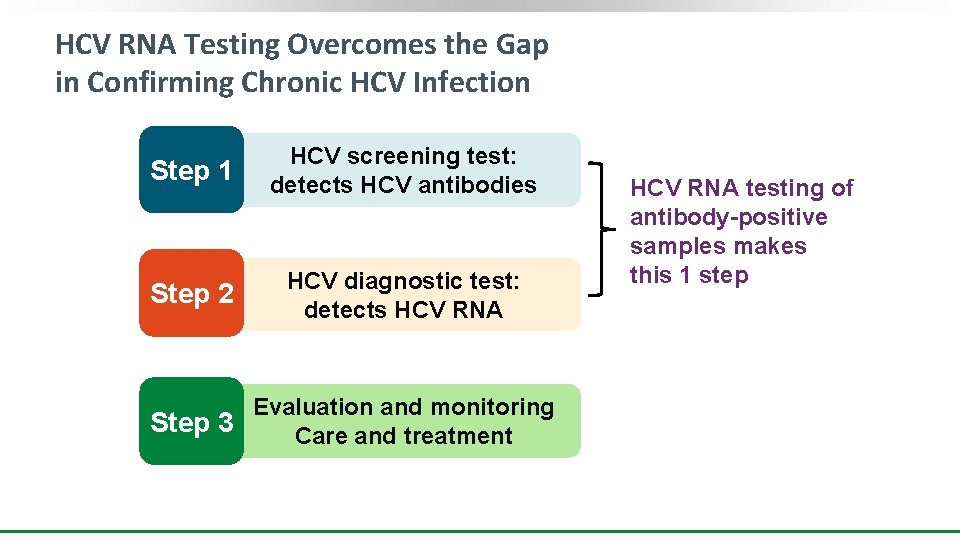
HCV RNA Testing Overcomes the Gap in Confirming Chronic HCV Infection Step 1 HCV screening test: detects HCV antibodies Step 2 HCV diagnostic test: detects HCV RNA Evaluation and monitoring Step 3 Care and treatment HCV RNA testing of antibody-positive samples makes this 1 step

Full Range of Care Services Needed in This Case § HCV screening § Screening/prevention of other infections, HBV, HAV, HIV, bacterial § Harm reduction access § OAT § Overdose prevention Case Details § § A young white woman PWID (heroin), recent overdose She lives in remote rural area

Care Linkage and Engagement—Enormous Benefits of Colocalized Addiction and HCV Treatment Services § Linkage does not always translate into treatment § Often HCV care and drug treatment exist in separate silos § If separate, need to establish strong communication avenues between the 2 § 2 -way street: addiction medicine needs to understand HCV epidemic and management and HCV treaters need to understand addiction medicine

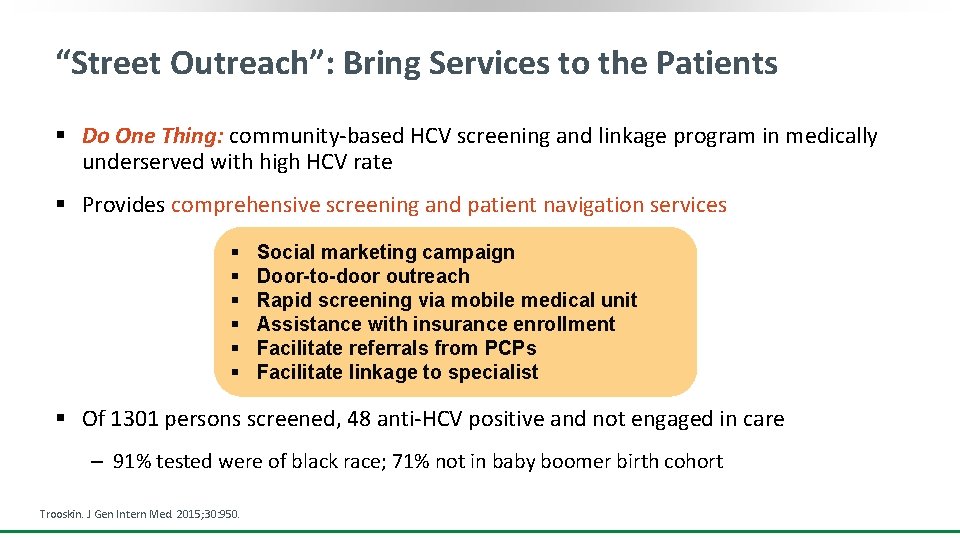
“Street Outreach”: Bring Services to the Patients § Do One Thing: community-based HCV screening and linkage program in medically underserved with high HCV rate § Provides comprehensive screening and patient navigation services § § § Social marketing campaign Door-to-door outreach Rapid screening via mobile medical unit Assistance with insurance enrollment Facilitate referrals from PCPs Facilitate linkage to specialist § Of 1301 persons screened, 48 anti-HCV positive and not engaged in care ‒ 91% tested were of black race; 71% not in baby boomer birth cohort Trooskin. J Gen Intern Med. 2015; 30: 950.

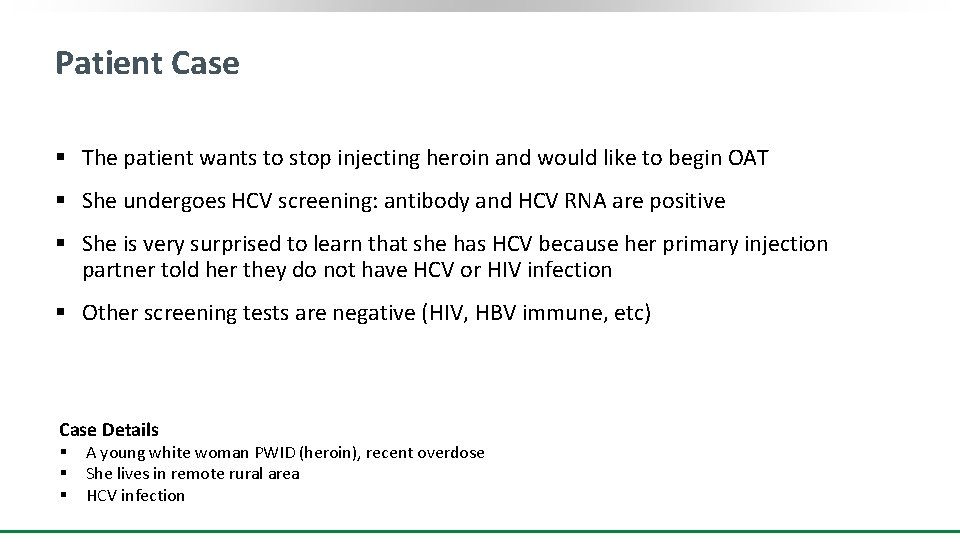
Patient Case § The patient wants to stop injecting heroin and would like to begin OAT § She undergoes HCV screening: antibody and HCV RNA are positive § She is very surprised to learn that she has HCV because her primary injection partner told her they do not have HCV or HIV infection § Other screening tests are negative (HIV, HBV immune, etc) Case Details § § § A young white woman PWID (heroin), recent overdose She lives in remote rural area HCV infection

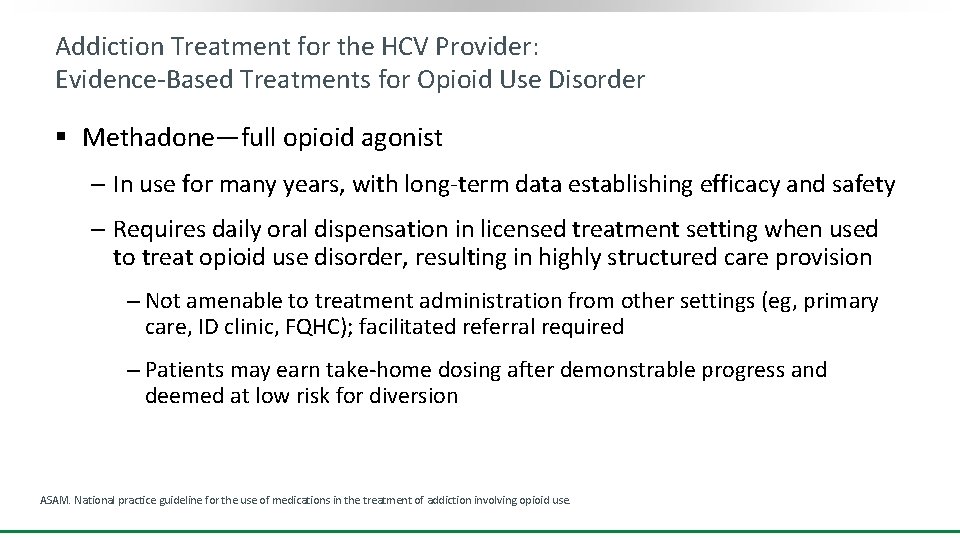
Addiction Treatment for the HCV Provider: Evidence-Based Treatments for Opioid Use Disorder § Methadone—full opioid agonist ‒ In use for many years, with long-term data establishing efficacy and safety ‒ Requires daily oral dispensation in licensed treatment setting when used to treat opioid use disorder, resulting in highly structured care provision ‒ Not amenable to treatment administration from other settings (eg, primary care, ID clinic, FQHC); facilitated referral required ‒ Patients may earn take-home dosing after demonstrable progress and deemed at low risk for diversion ASAM. National practice guideline for the use of medications in the treatment of addiction involving opioid use.

Addiction Treatment for the HCV Provider: Evidence-Based Treatments for Opioid Use Disorder § Buprenorphine (often combined with naloxone)—partial opioid agonist/antagonist ‒ Highly effective and established safety ‒ Daily SL administration as tablet or film; “bad taste” reported by some ‒ Monthly injectable: injection-site reactions, irreversibility of treatment ‒ Implant (6 mos): stable on 8 mg/day; site infection, must be removed ASAM. National practice guideline for the use of medications in the treatment of addiction involving opioid use.

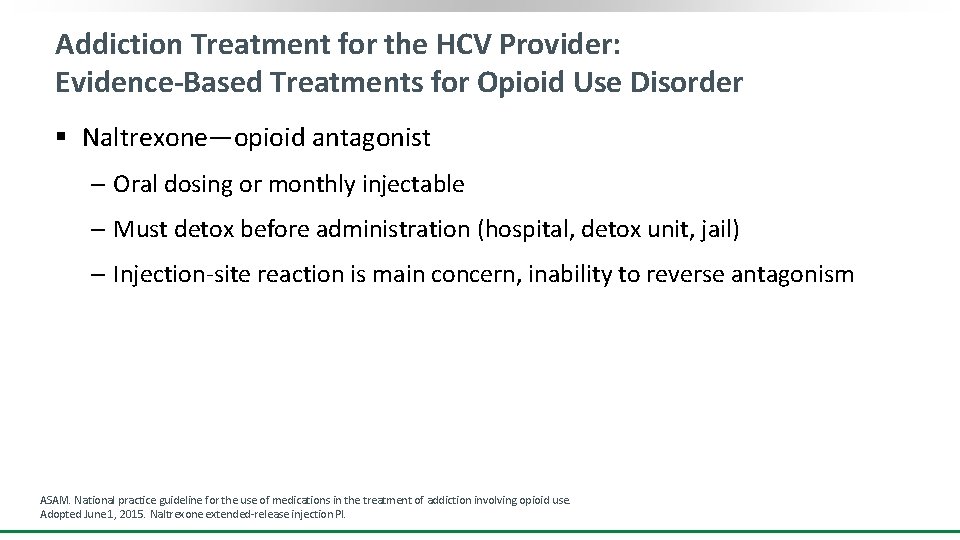
Addiction Treatment for the HCV Provider: Evidence-Based Treatments for Opioid Use Disorder § Naltrexone—opioid antagonist ‒ Oral dosing or monthly injectable ‒ Must detox before administration (hospital, detox unit, jail) ‒ Injection-site reaction is main concern, inability to reverse antagonism ASAM. National practice guideline for the use of medications in the treatment of addiction involving opioid use. Adopted June 1, 2015. Naltrexone extended-release injection PI.

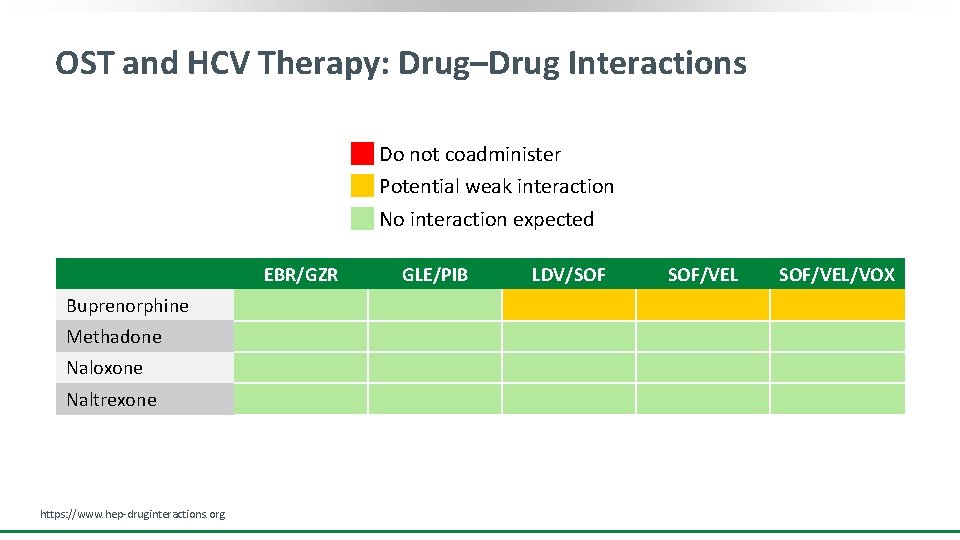
OST and HCV Therapy: Drug–Drug Interactions Do not coadminister Potential weak interaction No interaction expected EBR/GZR Buprenorphine Methadone Naloxone Naltrexone https: //www. hep-druginteractions. org GLE/PIB LDV/SOF SOF/VEL/VOX

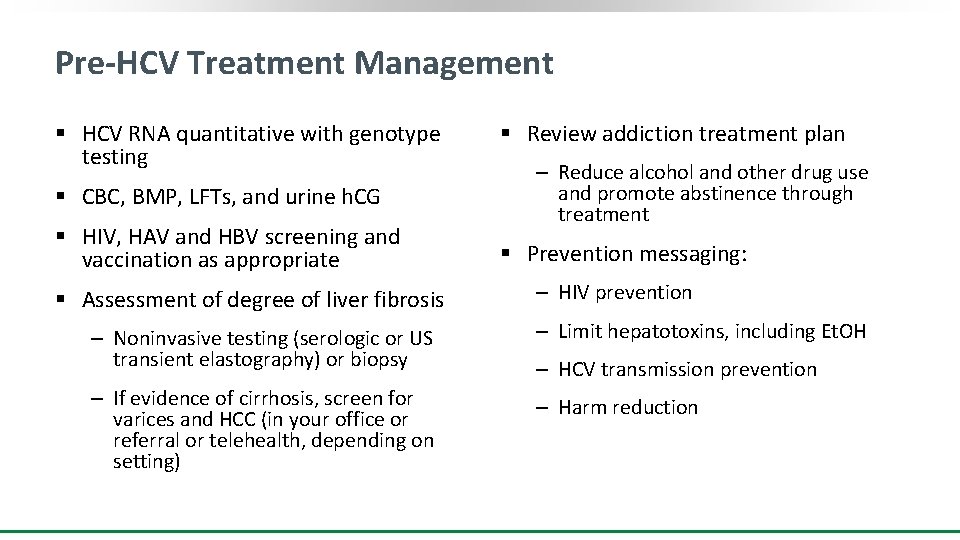
Pre-HCV Treatment Management § HCV RNA quantitative with genotype testing § CBC, BMP, LFTs, and urine h. CG § HIV, HAV and HBV screening and vaccination as appropriate § Assessment of degree of liver fibrosis § Review addiction treatment plan ‒ Reduce alcohol and other drug use and promote abstinence through treatment § Prevention messaging: ‒ HIV prevention ‒ Noninvasive testing (serologic or US transient elastography) or biopsy ‒ Limit hepatotoxins, including Et. OH ‒ If evidence of cirrhosis, screen for varices and HCC (in your office or referral or telehealth, depending on setting) ‒ Harm reduction ‒ HCV transmission prevention

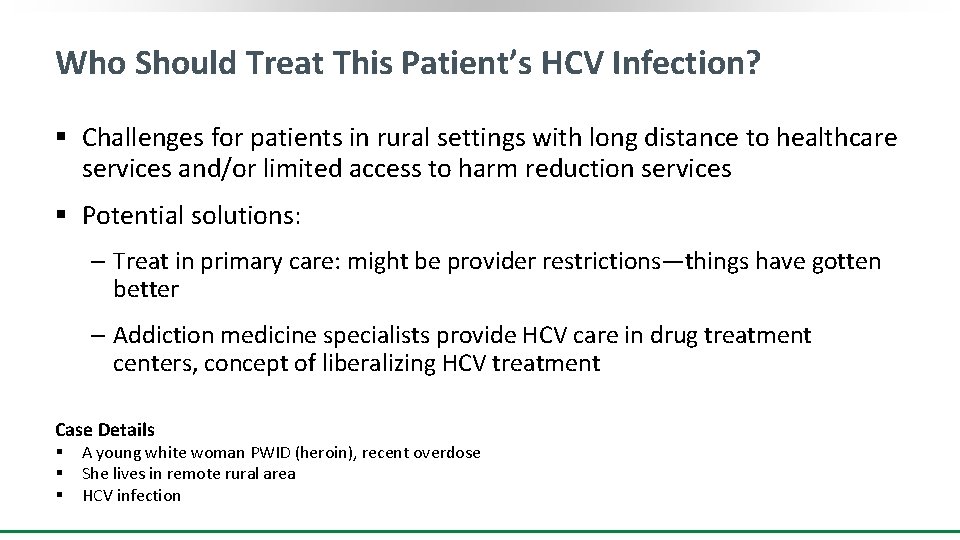
Who Should Treat This Patient’s HCV Infection? § Challenges for patients in rural settings with long distance to healthcare services and/or limited access to harm reduction services § Potential solutions: ‒ Treat in primary care: might be provider restrictions—things have gotten better ‒ Addiction medicine specialists provide HCV care in drug treatment centers, concept of liberalizing HCV treatment Case Details § § § A young white woman PWID (heroin), recent overdose She lives in remote rural area HCV infection

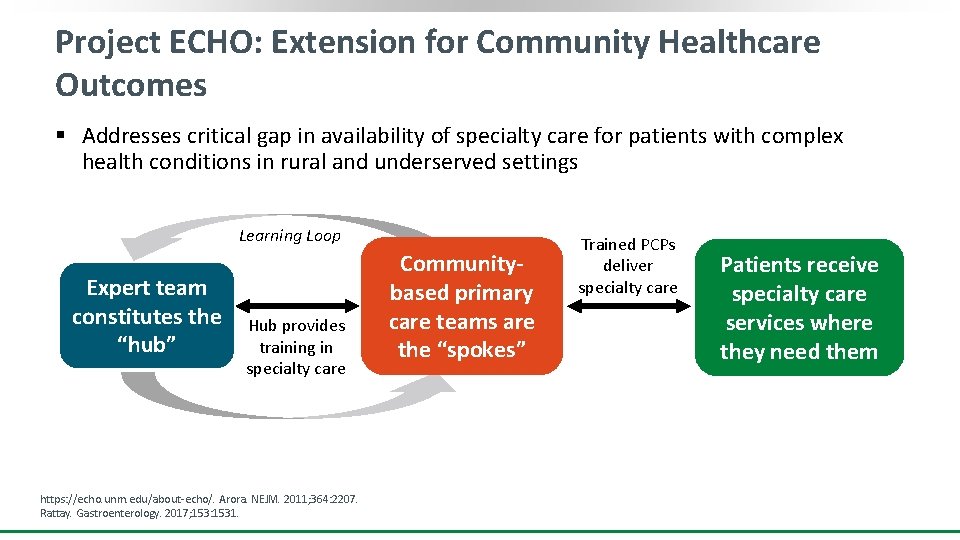
Project ECHO: Extension for Community Healthcare Outcomes § Addresses critical gap in availability of specialty care for patients with complex health conditions in rural and underserved settings Learning Loop Expert team constitutes the “hub” Hub provides training in specialty care https: //echo. unm. edu/about-echo/. Arora. NEJM. 2011; 364: 2207. Rattay. Gastroenterology. 2017; 153: 1531. Communitybased primary care teams are the “spokes” Trained PCPs deliver specialty care Patients receive specialty care services where they need them

Pregnancy Potential in Young Adult Women: Additional Urgency for HCV Diagnosis § 89% increase in HCV among women at time of delivery: 1. 8/1000 live births in 2009 to 3. 4/1000 live births in 2014 § Although mother-to-child HCV transmission rates low (~ 4% if viremic), risk exists and is not on the radar of many providers § HCV identification during pregnancy critical for appropriate follow-up of infants § Breastfeeding Patrick. MMWR Morb Mortal Wkly Rep 2017; 66: 470. Smith. MMWR Recomm Rep. 2012; 61(RR-4): 1. AASLD-IDSA. HCV guidance. 2018.

Patient Case § You discuss reproductive intentions with her and she does not want to become pregnant Case Details § § § A young white woman PWID (heroin), recent overdose She lives in remote rural area HCV infection

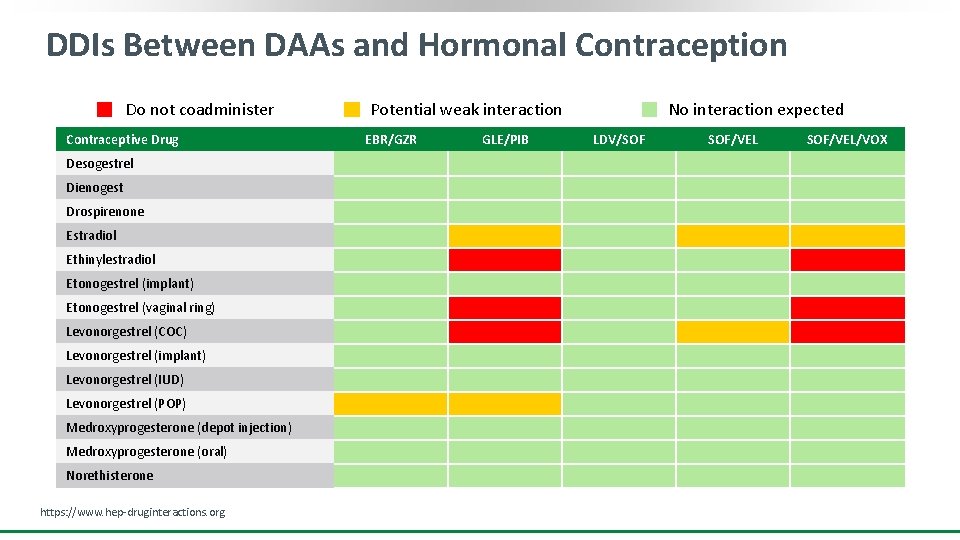
DDIs Between DAAs and Hormonal Contraception Do not coadminister Contraceptive Drug Desogestrel Dienogest Drospirenone Estradiol Ethinylestradiol Etonogestrel (implant) Etonogestrel (vaginal ring) Levonorgestrel (COC) Levonorgestrel (implant) Levonorgestrel (IUD) Levonorgestrel (POP) Medroxyprogesterone (depot injection) Medroxyprogesterone (oral) Norethisterone https: //www. hep-druginteractions. org Potential weak interaction EBR/GZR GLE/PIB No interaction expected LDV/SOF SOF/VEL/VOX

Patient Case § Results of HCV workup: GT 3 a (second most common transmitted GT), HCV RNA 300, 000 IU/m. L § It turns out that she can be treated for HCV, and a primary care provider will manage her care through the Project ECHO § The patient is doing well after 2 wks of buprenorphine but she is still having heroin cravings and some withdrawal symptoms. She is worried about being able to stay abstinent from heroin Case Details § § § A young white woman PWID (heroin), recent overdose She lives in remote rural area HCV infection

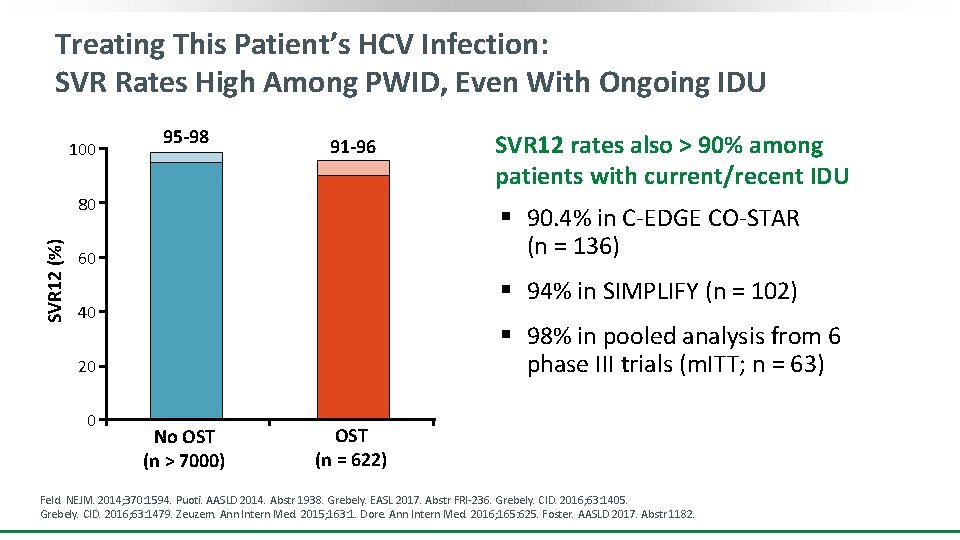
Treating This Patient’s HCV Infection: SVR Rates High Among PWID, Even With Ongoing IDU 100 95 -98 91 -96 SVR 12 (%) 80 § 90. 4% in C-EDGE CO-STAR (n = 136) 60 § 94% in SIMPLIFY (n = 102) 40 § 98% in pooled analysis from 6 phase III trials (m. ITT; n = 63) 20 0 SVR 12 rates also > 90% among patients with current/recent IDU No OST (n > 7000) OST (n = 622) Feld. NEJM. 2014; 370: 1594. Puoti. AASLD 2014. Abstr 1938. Grebely. EASL 2017. Abstr FRI-236. Grebely. CID. 2016; 63: 1405. Grebely. CID. 2016; 63: 1479. Zeuzem. Ann Intern Med. 2015; 163: 1. Dore. Ann Intern Med. 2016; 165: 625. Foster. AASLD 2017. Abstr 1182.

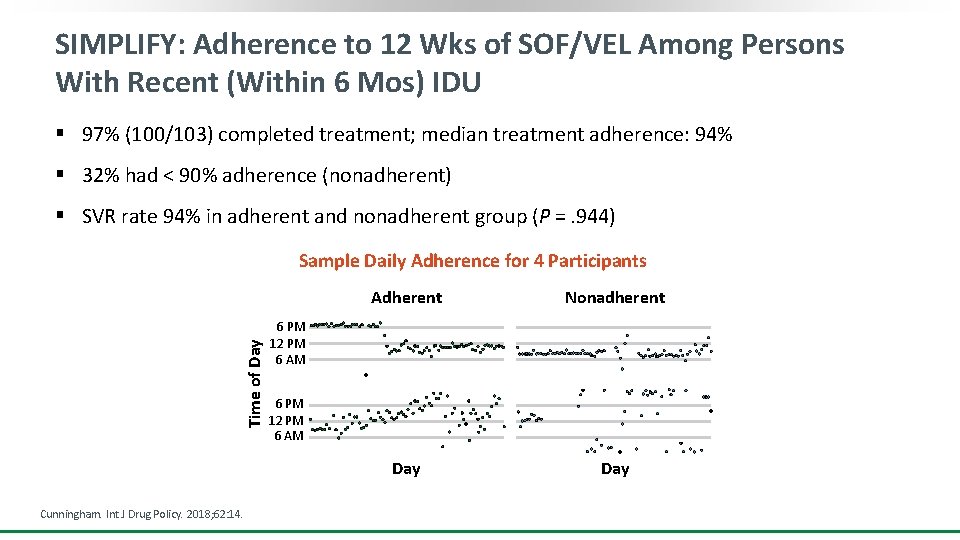
SIMPLIFY: Adherence to 12 Wks of SOF/VEL Among Persons With Recent (Within 6 Mos) IDU § 97% (100/103) completed treatment; median treatment adherence: 94% § 32% had < 90% adherence (nonadherent) § SVR rate 94% in adherent and nonadherent group (P = . 944) Time of Day Sample Daily Adherence for 4 Participants Cunningham. Int J Drug Policy. 2018; 62: 14. Adherent Nonadherent Day 6 PM 12 PM 6 AM

Strategies to Promote Engagement and Completion of HCV Therapy § Concurrent medications for addiction treatment helps § Colocalization of addiction treatment and HCV care § Leaving appointment spots open for drop-ins or to get someone in quickly when they have missed an appointment § Peer navigation services § After hrs/weekend services to facilitate appointment attendance

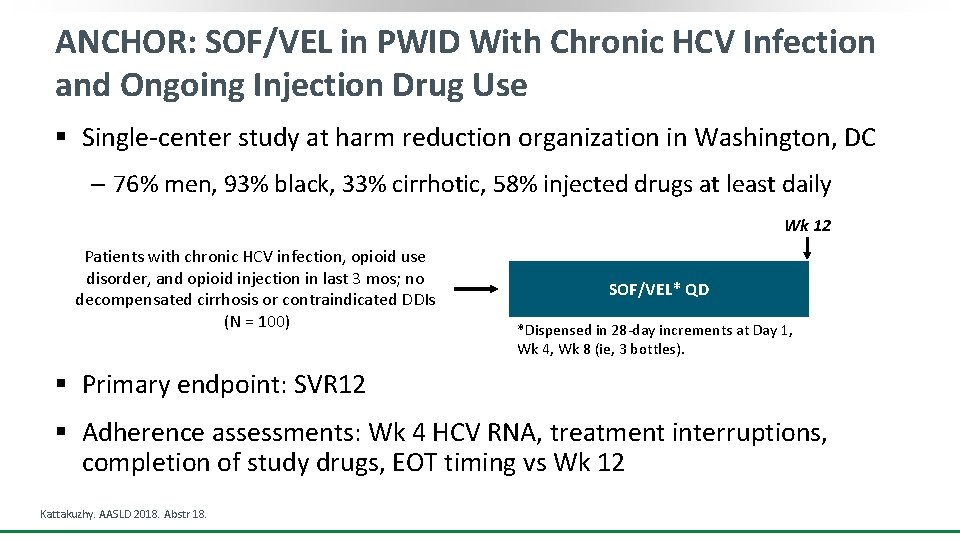
ANCHOR: SOF/VEL in PWID With Chronic HCV Infection and Ongoing Injection Drug Use § Single-center study at harm reduction organization in Washington, DC ‒ 76% men, 93% black, 33% cirrhotic, 58% injected drugs at least daily Wk 12 Patients with chronic HCV infection, opioid use disorder, and opioid injection in last 3 mos; no decompensated cirrhosis or contraindicated DDIs (N = 100) SOF/VEL* QD *Dispensed in 28 -day increments at Day 1, Wk 4, Wk 8 (ie, 3 bottles). § Primary endpoint: SVR 12 § Adherence assessments: Wk 4 HCV RNA, treatment interruptions, completion of study drugs, EOT timing vs Wk 12 Kattakuzhy. AASLD 2018. Abstr 18.

ANCHOR: Efficacy and Adherence § SVR 12 in ITT population: 78% (73/93) (m. ITT 90%; 52/58) ‒ Virologic success unaffected by BL demographics such as frequency of drug use, housing stability, MAT § Through Wk 12 in full study population (N = 100) Adherence Measure in ITT Population Kattakuzhy. AASLD 2018. Abstr 18. P Value Wk 4 HCV RNA < 200 IU/m. L § Yes (n = 80) § No (n = 8) 86 25 . 0005 No treatment interruptions § Yes (n = 76) § No (n = 12) 86 67 . 22 Completed 2 or 3 of 3 SOF/VEL bottles § Yes (n = 87) § No (n = 6) 84 0 . 0001 Finished SOF/VEL on time (vs late) § Yes (n = 20) § No (n = 43) 95 88 . 65 ‒ SOF/VEL prescriptions dispensed: 92% to 97% ‒ Visit attendance: 70% to 88% SVR 12, %

What if. . . the Patient Returned to Injecting Opioids § The patient is having a hard time with her recovery from opioid use disorder. When it is time to start HCV therapy she is still injecting daily, really struggling with basic survival at this point § What do you do? Do you still start HCV therapy? Case Details § § § A young white woman PWID (heroin), recent overdose She lives in remote rural area HCV infection

Real-World DAA Outcomes Among PWID in the United States: Hepatitis C Real Options (HERO) § All patients treated with 12 wks of SOF/VEL with monthly follow-up and followed for SVR 12 with quarterly follow-up after SVR 12; up to 12 wks permitted for HCV treatment initiation after enrollment Patients with any GT HCV infection, IDU in previous 3 mos, ± current OAT, DAA naive, ± HIV coinfection (N = 600) Modified Directly Observed Therapy (methadone maintenance program: n = 150; community health center: n = 150)* Standardized Intervention: Patient Navigation (methadone maintenance program: n = 150; community health center: n = 150) *Patients in modified directly observed therapy arm treated at methadone maintenance programs receive SOF/VEL daily with daily methadone and those treated in community health centers record themselves taking SOF/VEL each day using emocha app. Taylor. INHSU 2018. Abstr.

Back to Original Case: HCV Treatment and OAT § The patient receives 8 wks of HCV therapy and achieves SVR § She continued to do fairly well with buprenorphine during HCV therapy ‒ She had 1 injection event during the 8 wks, but she is still determined to stay in treatment § Counseling on reducing her risk of reinfection; importance of harm reduction Case Details § § A young white woman PWID (heroin), recent overdose She lives in remote rural area HCV infection Initiated HCV therapy and achieved SVR

Unique Elements of On-Site Addiction Center Treatment § Provide HCV education to patients ‒ Review modes of transmission ‒ Discuss chronology/evolution of HCV ‒ Involve patient’s partner and family, and encourage screening for themselves § Arrange frequent follow-up ‒ Distribution of medication at clinic in short duration and individualized packs ‒ Utilize specialty pharmacy with medication delivery directly to clinic § Coordinate care across services ‒ OTP clinician, social worker, psychiatrist

Patient Case: After HCV Cure § Unfortunately, the patient returned to heroin use after achieving SVR to HCV therapy § There is no access to clean needles in her area § On periodic HCV RNA test, HCV reinfection is identified ‒ This time GT 1 a Case Details § § § A young white woman PWID (heroin), recent overdose She lives in remote rural area HCV infection Initiated HCV therapy and achieved SVR Relapsed IDU, HCV reinfection

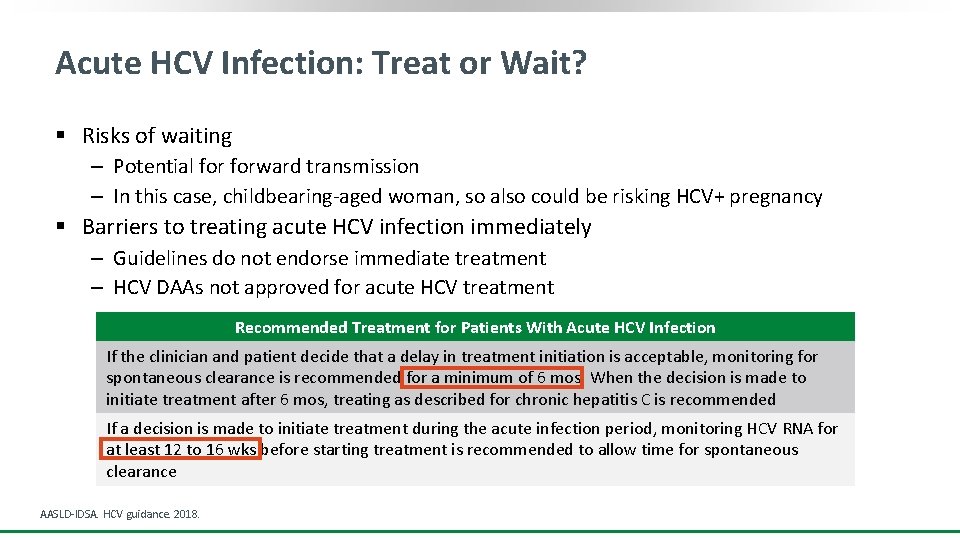
Acute HCV Infection: Treat or Wait? § Risks of waiting ‒ Potential forward transmission ‒ In this case, childbearing-aged woman, so also could be risking HCV+ pregnancy § Barriers to treating acute HCV infection immediately ‒ Guidelines do not endorse immediate treatment ‒ HCV DAAs not approved for acute HCV treatment Recommended Treatment for Patients With Acute HCV Infection If the clinician and patient decide that a delay in treatment initiation is acceptable, monitoring for spontaneous clearance is recommended for a minimum of 6 mos. When the decision is made to initiate treatment after 6 mos, treating as described for chronic hepatitis C is recommended If a decision is made to initiate treatment during the acute infection period, monitoring HCV RNA for at least 12 to 16 wks before starting treatment is recommended to allow time for spontaneous clearance AASLD-IDSA. HCV guidance. 2018.

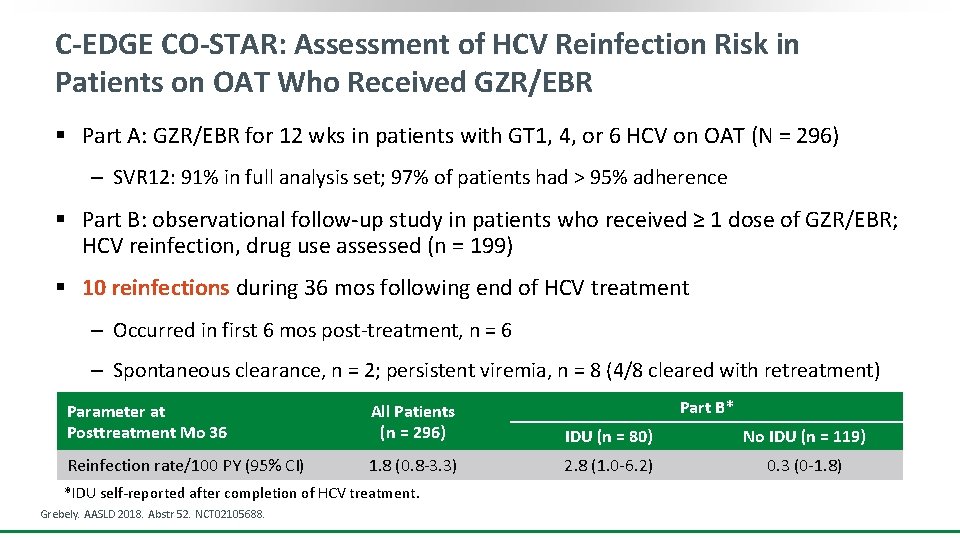
C-EDGE CO-STAR: Assessment of HCV Reinfection Risk in Patients on OAT Who Received GZR/EBR § Part A: GZR/EBR for 12 wks in patients with GT 1, 4, or 6 HCV on OAT (N = 296) ‒ SVR 12: 91% in full analysis set; 97% of patients had > 95% adherence § Part B: observational follow-up study in patients who received ≥ 1 dose of GZR/EBR; HCV reinfection, drug use assessed (n = 199) § 10 reinfections during 36 mos following end of HCV treatment ‒ Occurred in first 6 mos post-treatment, n = 6 ‒ Spontaneous clearance, n = 2; persistent viremia, n = 8 (4/8 cleared with retreatment) Part B* Parameter at Posttreatment Mo 36 All Patients (n = 296) IDU (n = 80) No IDU (n = 119) Reinfection rate/100 PY (95% CI) 1. 8 (0. 8 -3. 3) 2. 8 (1. 0 -6. 2) 0. 3 (0 -1. 8) *IDU self-reported after completion of HCV treatment. Grebely. AASLD 2018. Abstr 52. NCT 02105688.

HCV Treatment Scale-up in High-Risk Populations Can Prevent Onward Transmission § Observed and modeled HCV chronic prevalence among PWID in Melbourne, Australia HCV Chronic Prevalence Among PWID (%) 100 Data No scale-up from baseline (3/1000 PWID annually) Scale-up to 10/1000 PWID annually Scale-up to 20/1000 PWID annually Scale-up to 40/1000 PWID annually Scale-up to 80/1000 PWID annually 80 60 40 2002 2007 2012 2017 Yr Martin. Hepatology. 2013; 58: 1598. 2022 2027

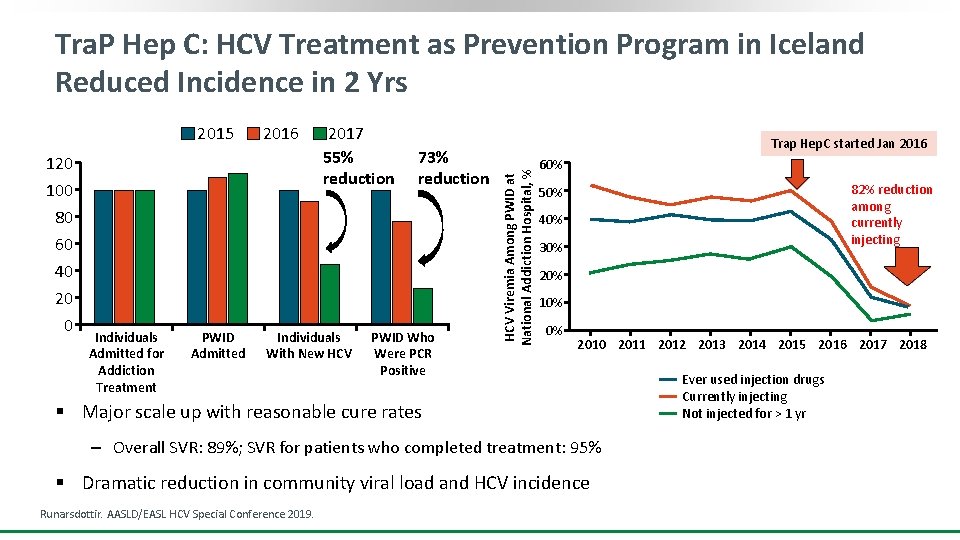
Tra. P Hep C: HCV Treatment as Prevention Program in Iceland Reduced Incidence in 2 Yrs 120 100 80 60 40 20 0 Individuals Admitted for Addiction Treatment PWID Admitted 2016 2017 55% reduction Individuals With New HCV 73% reduction PWID Who Were PCR Positive Trap Hep. C started Jan 2016 HCV Viremia Among PWID at National Addiction Hospital, % 2015 60% 82% reduction among currently injecting 50% 40% 30% 20% 10% 0% 2010 2011 2012 2013 2014 2015 2016 2017 2018 § Major scale up with reasonable cure rates ‒ Overall SVR: 89%; SVR for patients who completed treatment: 95% § Dramatic reduction in community viral load and HCV incidence Runarsdottir. AASLD/EASL HCV Special Conference 2019. Ever used injection drugs Currently injecting Not injected for > 1 yr
 One persons trash is another persons treasure
One persons trash is another persons treasure Persons essential being
Persons essential being Hcv treatment
Hcv treatment Hiv test window period
Hiv test window period Hcv treatment
Hcv treatment Hcv symptoms female
Hcv symptoms female Dulong's formula
Dulong's formula Douglas t dietrich
Douglas t dietrich Characteristics of lipids
Characteristics of lipids For top-down parsing left recursion removal is
For top-down parsing left recursion removal is Useless symbols
Useless symbols Eliminating the parameter trig
Eliminating the parameter trig The correct mixture of mirepoix is
The correct mixture of mirepoix is Involves eliminating columns in a table
Involves eliminating columns in a table Innovation and invention
Innovation and invention Conjuctive normal form concentrates on eliminating
Conjuctive normal form concentrates on eliminating Marzano's 9 high yield strategies
Marzano's 9 high yield strategies Livelihood opportunities for persons with disabilities
Livelihood opportunities for persons with disabilities Escala de sad persons
Escala de sad persons Married persons equality act 1 of 1996
Married persons equality act 1 of 1996 Structure of twelfth night
Structure of twelfth night Livelihood programs for persons with disabilities
Livelihood programs for persons with disabilities High close doji
High close doji Holy trinity explained
Holy trinity explained Postmodernism sociology examples
Postmodernism sociology examples It is name of persons places or things
It is name of persons places or things What is trafficking in persons
What is trafficking in persons Persons in lpscs careers must effectively communicate with
Persons in lpscs careers must effectively communicate with Personbunden fortæller
Personbunden fortæller Ctip for acquisition and contracting professionals
Ctip for acquisition and contracting professionals The principle of respect for persons
The principle of respect for persons Pronoun reference
Pronoun reference Sad persons scale nursing interventions
Sad persons scale nursing interventions Places, things, ideas.
Places, things, ideas. The principle of respect for persons
The principle of respect for persons Liable to vat
Liable to vat