Douglas T DIETRICH 1 Treatment of HCV in















- Slides: 55

Douglas T. DIETRICH 1

Treatment of HCV in HIV Disease: New Challenges, New Promise Douglas T. Dieterich, M. D Professor of Medicine Division of Liver Diseases, Gastroenterology and Infectious Diseases Director of CME Department of Medicine Mt. Sinai School of Medicine New York, New York P-2

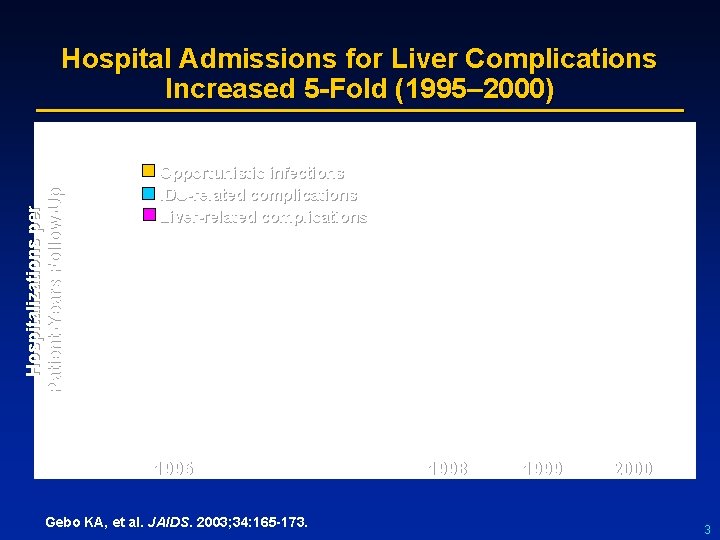
Hospitalizations per Patient-Years Follow-Up Hospital Admissions for Liver Complications Increased 5 -Fold (1995– 2000) Opportunistic infections IDU-related complications Liver-related complications 1995 1996 1997 1998 1999 2000 Gebo KA, et al. JAIDS. 2003; 34: 165 -173. 3

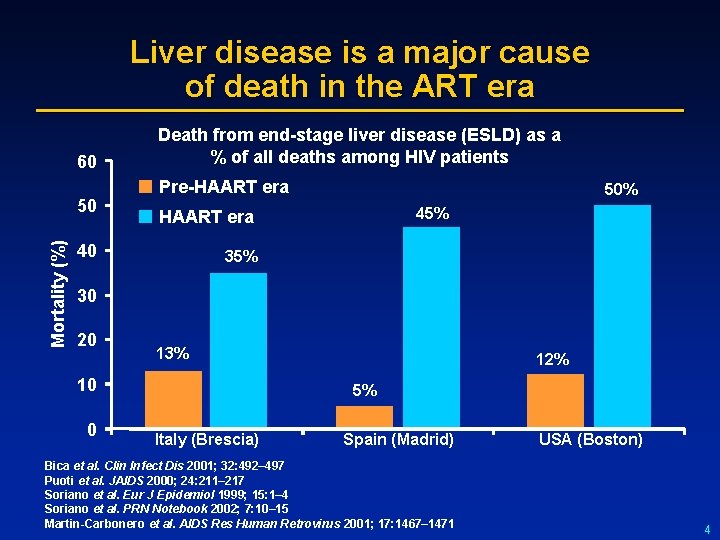
Liver disease is a major cause of death in the ART era 60 Mortality (%) 50 Death from end-stage liver disease (ESLD) as a % of all deaths among HIV patients Pre-HAART era 50% 45% HAART era 40 35% 30 20 13% 10 0 12% 5% Italy (Brescia) Spain (Madrid) Bica et al. Clin Infect Dis 2001; 32: 492– 497 Puoti et al. JAIDS 2000; 24: 211– 217 Soriano et al. Eur J Epidemiol 1999; 15: 1– 4 Soriano et al. PRN Notebook 2002; 7: 10– 15 Martin-Carbonero et al. AIDS Res Human Retrovirus 2001; 17: 1467– 1471 USA (Boston) 4

Causes of Liver Disease in HIV Infection 5

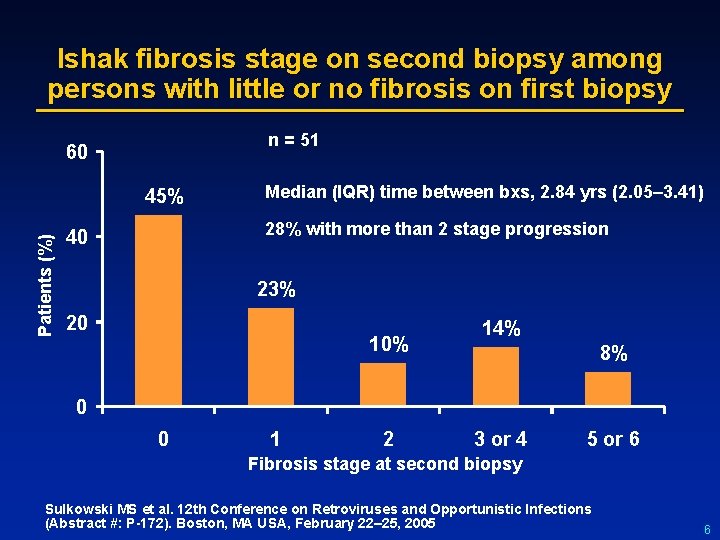
Ishak fibrosis stage on second biopsy among persons with little or no fibrosis on first biopsy n = 51 60 Patients (%) 45% Median (IQR) time between bxs, 2. 84 yrs (2. 05– 3. 41) 28% with more than 2 stage progression 40 23% 20 10% 14% 8% 0 0 1 2 3 or 4 5 or 6 Fibrosis stage at second biopsy Sulkowski MS et al. 12 th Conference on Retroviruses and Opportunistic Infections (Abstract #: P-172). Boston, MA USA, February 22– 25, 2005 6

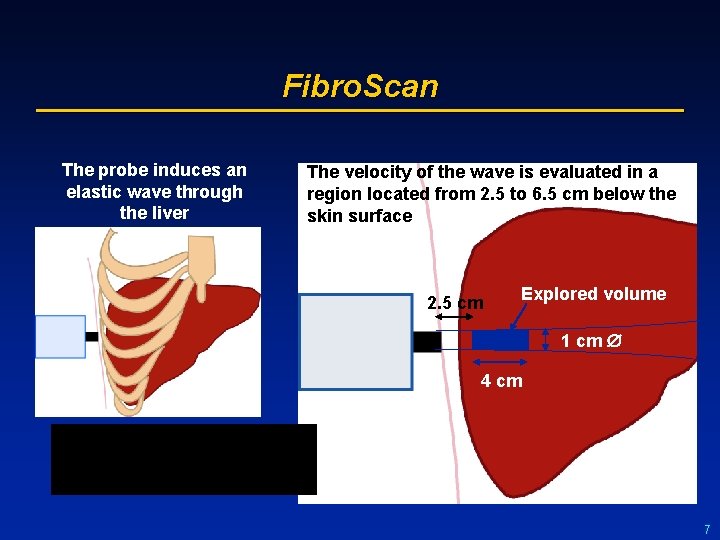
Fibro. Scan The probe induces an elastic wave through the liver The velocity of the wave is evaluated in a region located from 2. 5 to 6. 5 cm below the skin surface 2. 5 cm Explored volume 1 cm 4 cm LB: 1/50, 000 of the liver Fibro. Scan: 1/500 of the liver 7

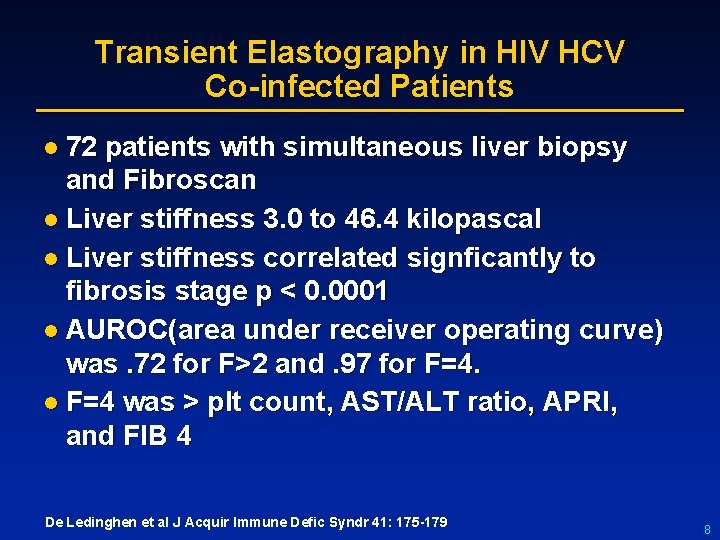
Transient Elastography in HIV HCV Co-infected Patients l 72 patients with simultaneous liver biopsy and Fibroscan l Liver stiffness 3. 0 to 46. 4 kilopascal l Liver stiffness correlated signficantly to fibrosis stage p < 0. 0001 l AUROC(area under receiver operating curve) was. 72 for F>2 and. 97 for F=4. l F=4 was > plt count, AST/ALT ratio, APRI, and FIB 4 De Ledinghen et al J Acquir Immune Defic Syndr 41: 175 -179 8

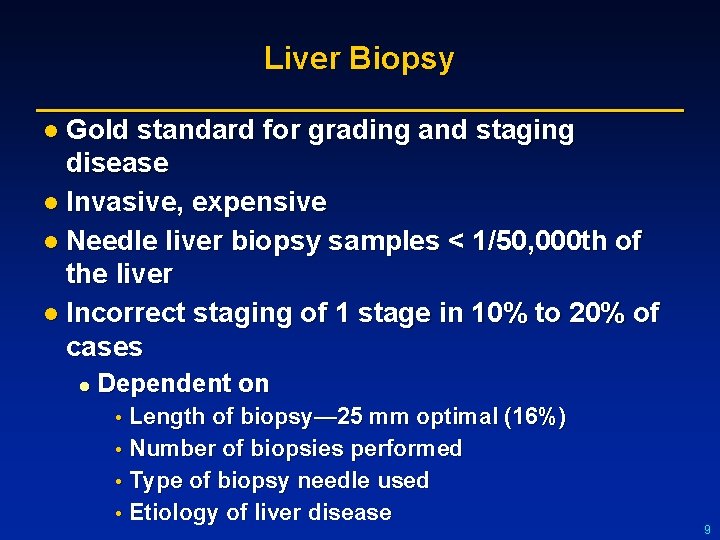
Liver Biopsy l Gold standard for grading and staging disease l Invasive, expensive l Needle liver biopsy samples < 1/50, 000 th of the liver l Incorrect staging of 1 stage in 10% to 20% of cases l Dependent on Length of biopsy— 25 mm optimal (16%) • Number of biopsies performed • Type of biopsy needle used • Etiology of liver disease • 9

The Importance of Liver Biopsy 10

Conflicting Data on the Effects of HCV Upon HIV Disease l Meta-analysis involving 6216 patients from 8 trials l l Mean increase in CD 4 cell count among HIV/HCV coinfected patients was 33. 4 cells/mm 3 less than that observed in HIV-monoinfected patients Euro. SIDA l After adjusting for baseline factors, investigators concluded that HIV/HCV-coinfected patients do not have a greater risk of progressing to AIDS Miller et al. Clin Inf Dis. 2005; 41: 713 -720. Rockstroh et al. J Inf Dis. 2005; 192: 992 -1002 11

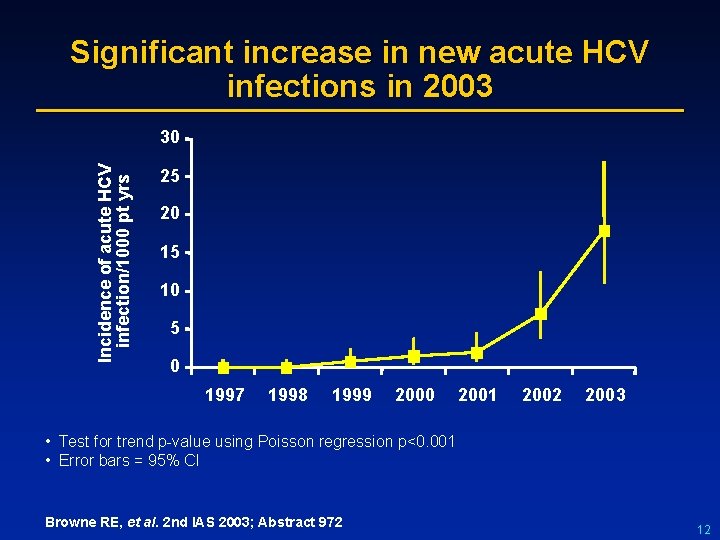
Significant increase in new acute HCV infections in 2003 Incidence of acute HCV infection/1000 pt yrs 30 25 20 15 10 5 0 1997 1998 1999 2000 2001 2002 2003 • Test for trend p-value using Poisson regression p<0. 001 • Error bars = 95% CI Browne RE, et al. 2 nd IAS 2003; Abstract 972 12

HCV: A new epidemic among MSM? l Newly infected male, MSM, HCV+ patients at a London clinic, January 1997 – May 2003; n=44 l Reasons for testing: ALT (35), sexual contact with HCV+ person (3), jaundice (3), HIV screening (2), H/O IVDU (1) l Risk factors identified: IVDU (1), sexual contact (39), none (4) Browne RE, et al. 2 nd IAS 2003; Abstract 972 13

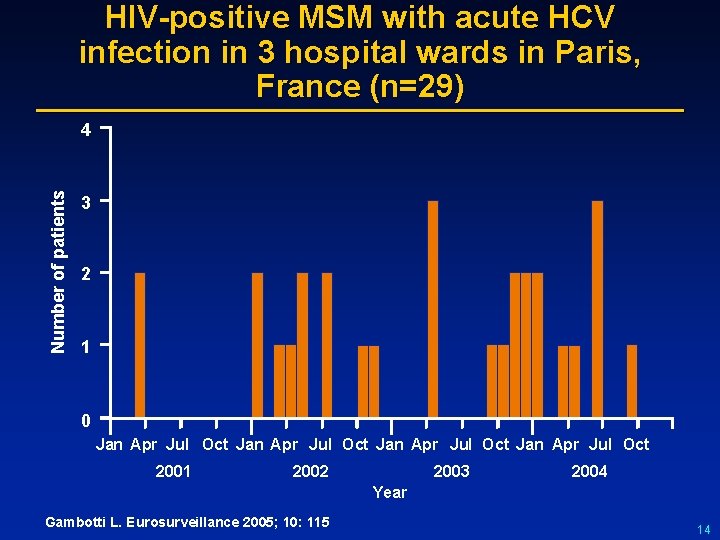
HIV-positive MSM with acute HCV infection in 3 hospital wards in Paris, France (n=29) Number of patients 4 3 2 1 0 Jan Apr Jul Oct 2001 2002 2003 2004 Year Gambotti L. Eurosurveillance 2005; 10: 115 14

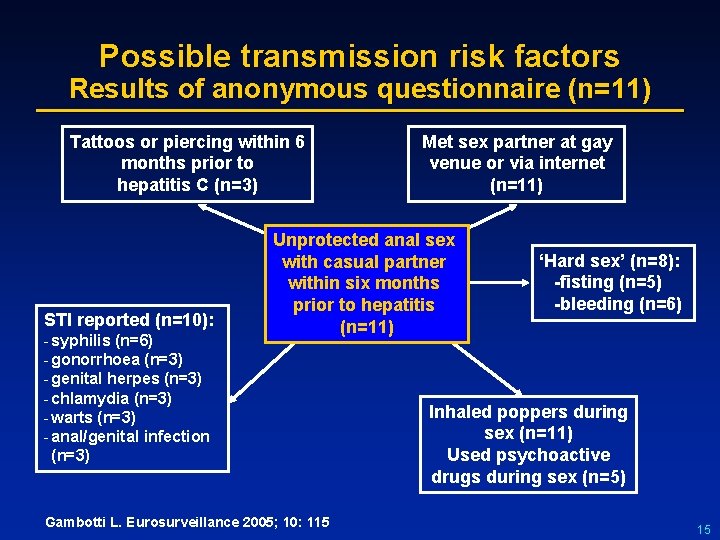
Possible transmission risk factors Results of anonymous questionnaire (n=11) Tattoos or piercing within 6 months prior to hepatitis C (n=3) STI reported (n=10): - syphilis (n=6) - gonorrhoea (n=3) - genital herpes (n=3) - chlamydia (n=3) - warts (n=3) - anal/genital infection (n=3) Met sex partner at gay venue or via internet (n=11) Unprotected anal sex with casual partner within six months prior to hepatitis (n=11) Gambotti L. Eurosurveillance 2005; 10: 115 ‘Hard sex’ (n=8): -fisting (n=5) -bleeding (n=6) Inhaled poppers during sex (n=11) Used psychoactive drugs during sex (n=5) 15

German acute Hepatitis C Trial – I (1998– 2000) interferon alfa-2 b monotherapy for 6 months HCV RNA negative 100% 98% 80% 60% n=44 40% 20% 0% 0 4 8 12 Weeks Jaeckel E, et al. N Engl J Med 2001; 345: 1452 16 20 24 48 (F/up Wk 24) 16

Acute HCV in IVDU: Swiss Association for the Study of the Liver Study (SASL 18) 27 patients 5 spontaneous clearance 22 patients treatment indicated 6 refused therapy 2 patients lost to observation 14 patients Peg-IFN treatment started 6 premature stop due to side-effects (1 SVR) 8 patients adherent to therapy 7 patients with SVR Broers, B et al. J Hepatol 2005; 42: 323 17

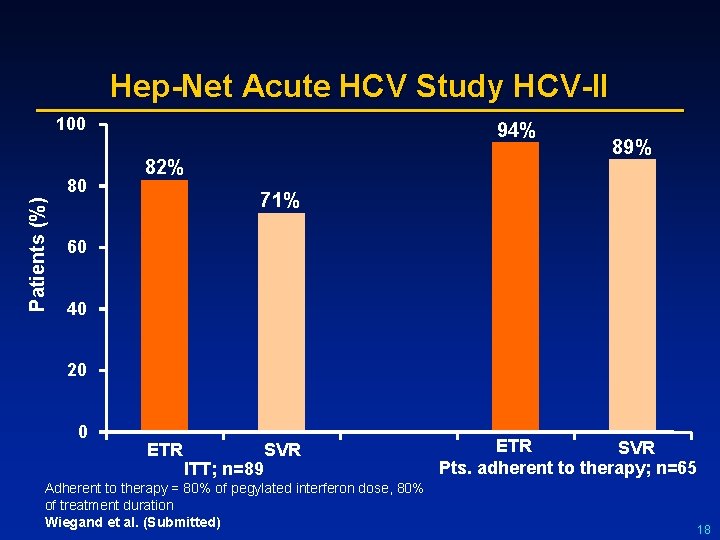
Hep-Net Acute HCV Study HCV-II 100 Patients (%) 80 94% 82% 89% 71% 60 40 20 0 ETR ITT; n=89 SVR Adherent to therapy = 80% of pegylated interferon dose, 80% of treatment duration Wiegand et al. (Submitted) ETR SVR Pts. adherent to therapy; n=65 18

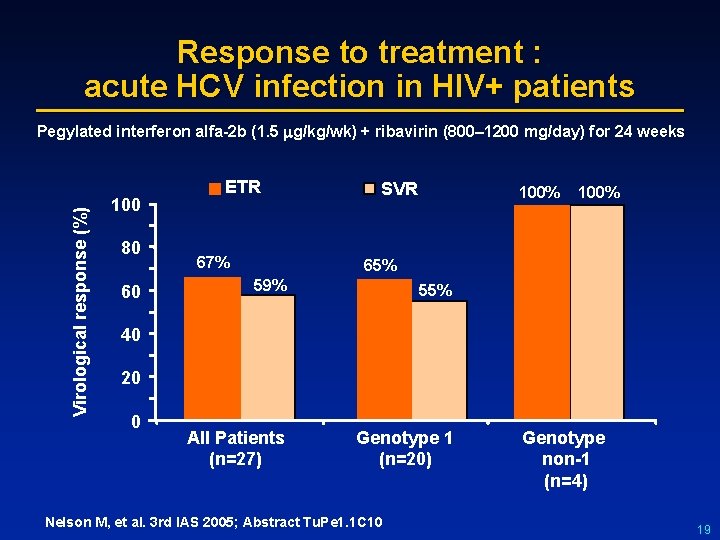
Response to treatment : acute HCV infection in HIV+ patients Virological response (%) Pegylated interferon alfa-2 b (1. 5 g/kg/wk) + ribavirin (800– 1200 mg/day) for 24 weeks 100 80 60 ETR 67% SVR 100% 65% 59% 55% All Patients (n=27) Genotype 1 (n=20) 40 20 0 Nelson M, et al. 3 rd IAS 2005; Abstract Tu. Pe 1. 1 C 10 Genotype non-1 (n=4) 19

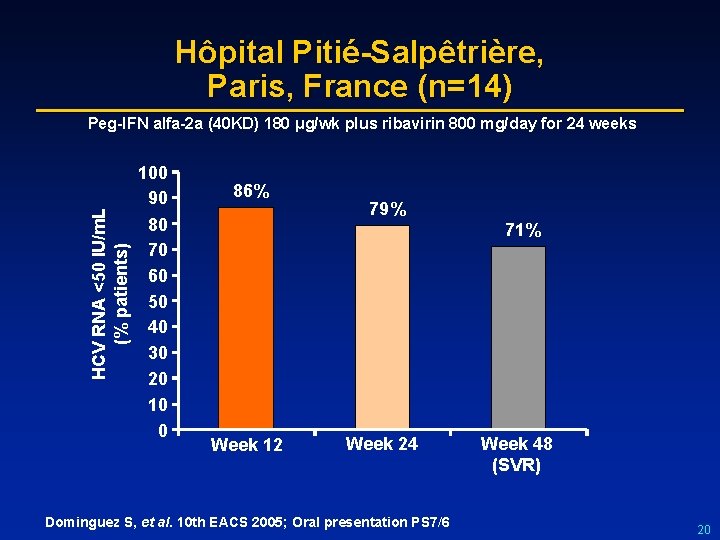
Hôpital Pitié-Salpêtrière, Paris, France (n=14) HCV RNA <50 IU/m. L (% patients) Peg-IFN alfa-2 a (40 KD) 180 μg/wk plus ribavirin 800 mg/day for 24 weeks 100 90 80 70 60 50 40 30 20 10 0 86% 79% 71% Week 12 Week 24 Dominguez S, et al. 10 th EACS 2005; Oral presentation PS 7/6 Week 48 (SVR) 20

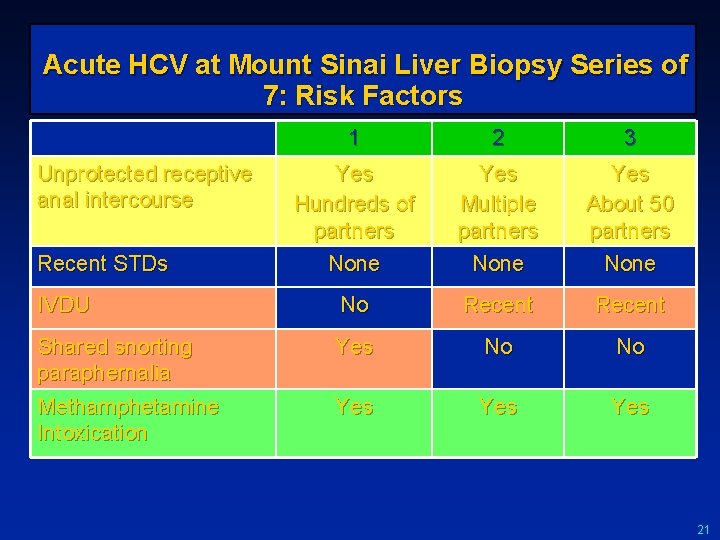
Acute HCV at Mount Sinai Liver Biopsy Series of 7: Risk Factors 1 2 3 Yes Hundreds of partners None Yes Multiple partners None Yes About 50 partners None IVDU No Recent Shared snorting paraphernalia Methamphetamine Intoxication Yes No No Yes Yes Unprotected receptive anal intercourse Recent STDs 21

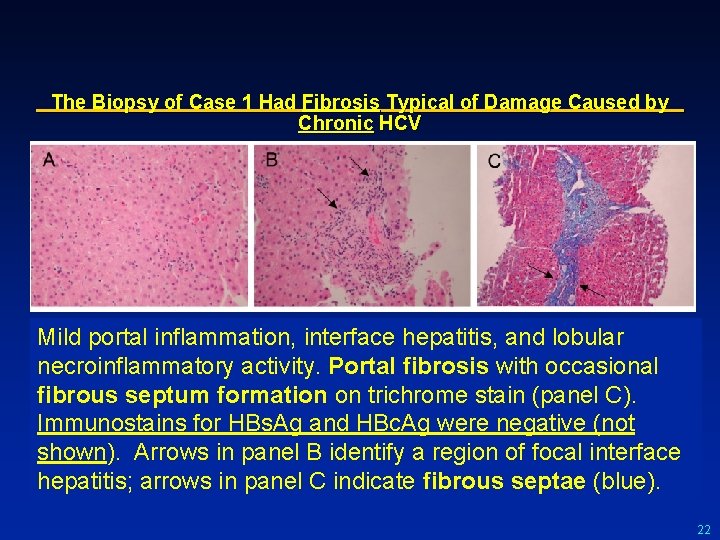
The Biopsy of Case 1 Had Fibrosis Typical of Damage Caused by Chronic HCV Mild portal inflammation, interface hepatitis, and lobular necroinflammatory activity. Portal fibrosis with occasional fibrous septum formation on trichrome stain (panel C). Immunostains for HBs. Ag and HBc. Ag were negative (not shown). Arrows in panel B identify a region of focal interface hepatitis; arrows in panel C indicate fibrous septae (blue). 22

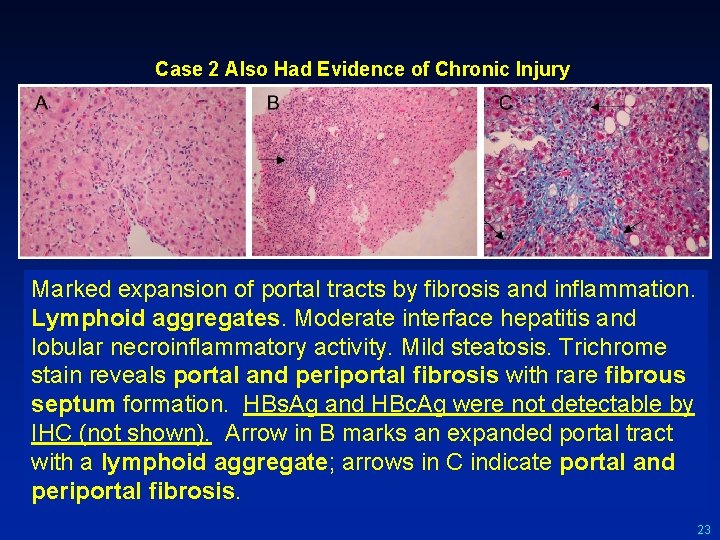
Case 2 Also Had Evidence of Chronic Injury Marked expansion of portal tracts by fibrosis and inflammation. Lymphoid aggregates. Moderate interface hepatitis and lobular necroinflammatory activity. Mild steatosis. Trichrome stain reveals portal and periportal fibrosis with rare fibrous septum formation. HBs. Ag and HBc. Ag were not detectable by IHC (not shown). Arrow in B marks an expanded portal tract with a lymphoid aggregate; arrows in C indicate portal and periportal fibrosis. 23

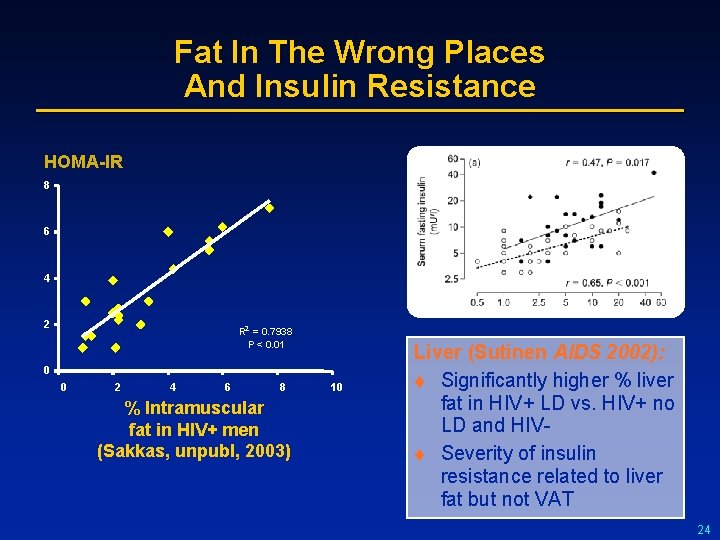
Fat In The Wrong Places And Insulin Resistance HOMA-IR 8 6 4 2 R 2 = 0. 7938 P < 0. 01 0 0 2 4 6 8 % Intramuscular fat in HIV+ men (Sakkas, unpubl, 2003) 10 Liver (Sutinen AIDS 2002): t Significantly higher % liver fat in HIV+ LD vs. HIV+ no LD and HIVt Severity of insulin resistance related to liver fat but not VAT 24

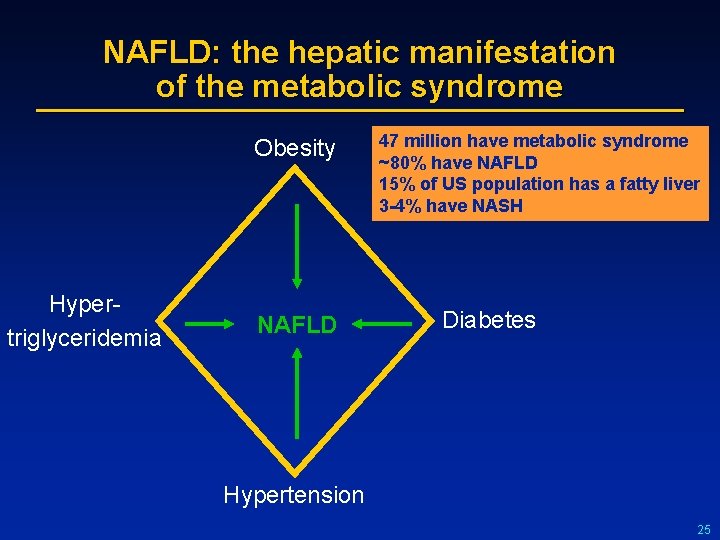
NAFLD: the hepatic manifestation of the metabolic syndrome Obesity Hypertriglyceridemia NAFLD 47 million have metabolic syndrome ~80% have NAFLD 15% of US population has a fatty liver 3 -4% have NASH Diabetes Hypertension 25

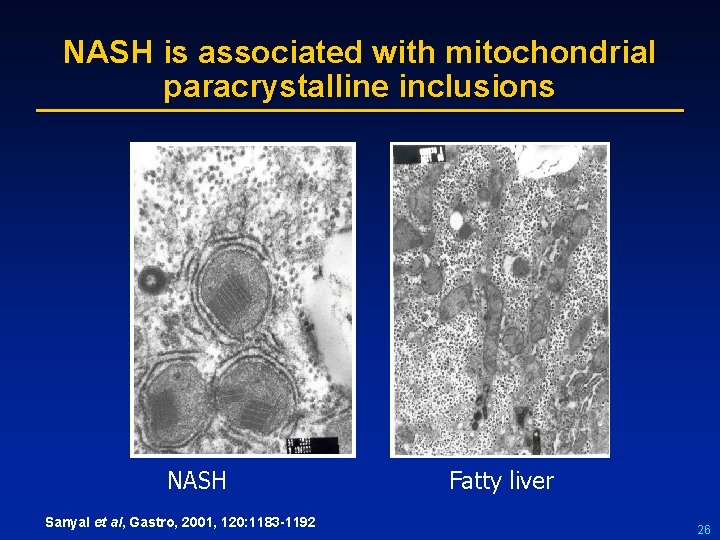
NASH is associated with mitochondrial paracrystalline inclusions NASH Sanyal et al, Gastro, 2001, 120: 1183 -1192 Fatty liver 26

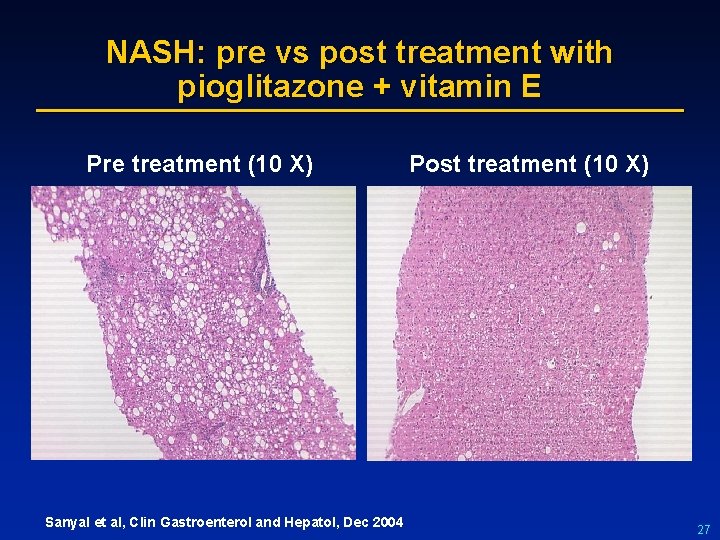
NASH: pre vs post treatment with pioglitazone + vitamin E Pre treatment (10 X) Sanyal et al, Clin Gastroenterol and Hepatol, Dec 2004 Post treatment (10 X) 27

% SVR in Genotype 1 Insulin Resistance and HCV: Effect on Response to Peg. IFN/RBV Therapy Homa: Homeostasis model of assessment – HOMA of insulin resistance Romero-Gomez, M et al. Gastroenterology 2005; 128: 636 -641. 28

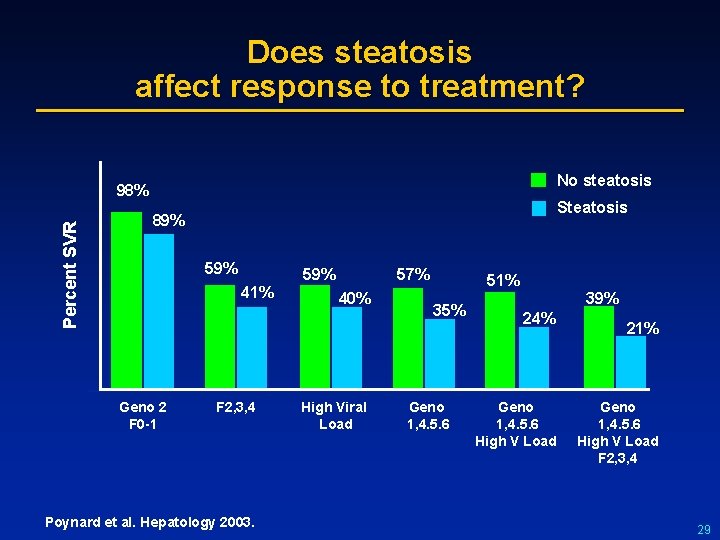
Does steatosis affect response to treatment? No steatosis Percent SVR 98% Steatosis 89% 59% 41% Geno 2 F 0 -1 F 2, 3, 4 Poynard et al. Hepatology 2003. 57% 40% High Viral Load 51% 35% Geno 1, 4. 5. 6 39% 24% Geno 1, 4. 5. 6 High V Load 21% Geno 1, 4. 5. 6 High V Load F 2, 3, 4 29

Insulin resistance and NAFLD in co-infection Results: In univariate analyses global and semi-quantitative measures of steatosis strongly associated with HOMA-IR (p<0. 0001) -see plot ALT (p=0. 003) AST (p=0. 003) genotype (p=0. 049) negative once genotype 3 excluded ethnicity (p=0. 024) Significant correlation between HOMA-IR and HAI fibrosis stage (p=0. 011) and steatohepatitis stage (p=0. 005) BMI correlated with HOMA-IR (p=0. 0085) but not histological parameters In logistic regression analyses only IR positively associated (p=0. 015 OR=1. 08 95%CI 1. 01 -1. 14) with moderate/severe steatosis 30

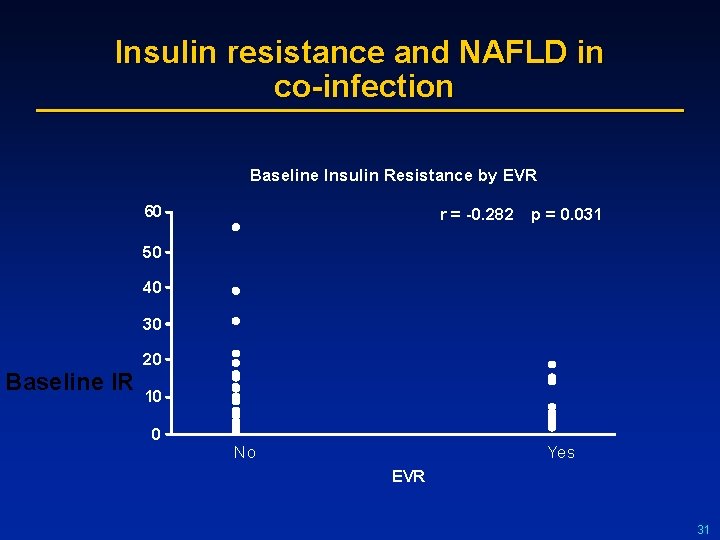
Insulin resistance and NAFLD in co-infection Baseline Insulin Resistance by EVR 60 r = -0. 282 p = 0. 031 50 40 30 Baseline IR 20 10 0 No Yes EVR 31

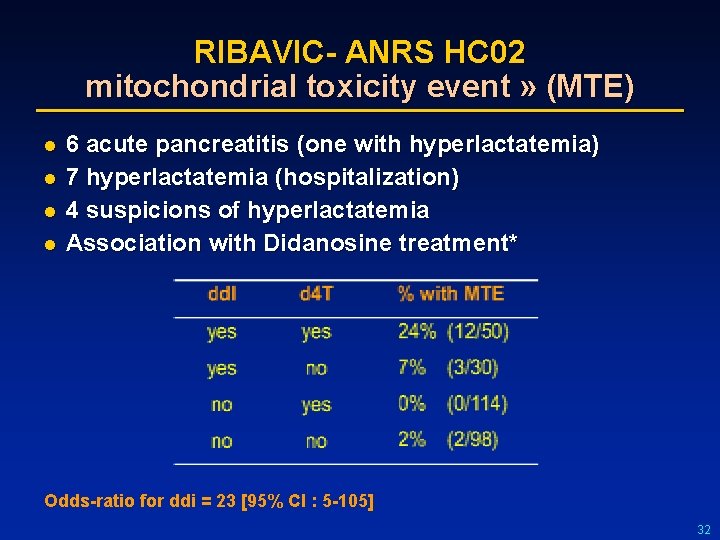
RIBAVIC- ANRS HC 02 mitochondrial toxicity event » (MTE) l l 6 acute pancreatitis (one with hyperlactatemia) 7 hyperlactatemia (hospitalization) 4 suspicions of hyperlactatemia Association with Didanosine treatment* Odds-ratio for ddi = 23 [95% CI : 5 -105] 32

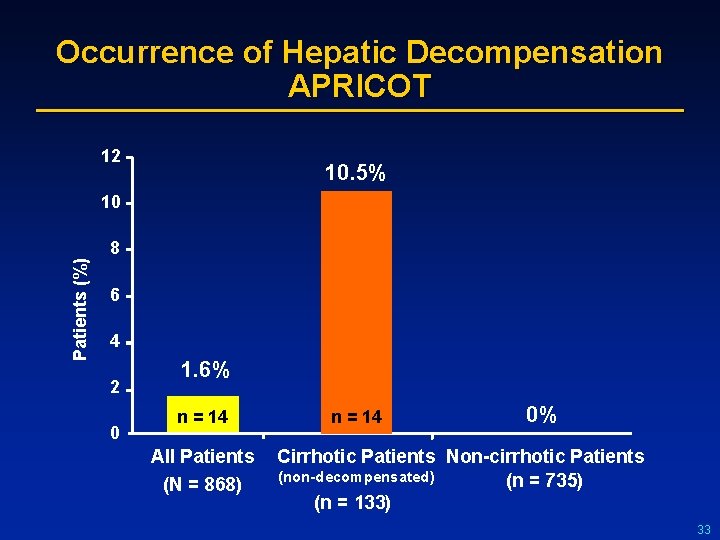
Occurrence of Hepatic Decompensation APRICOT 12 10. 5% 10 Patients (%) 8 6 4 2 0 1. 6% n = 14 All Patients (N = 868) n = 14 0% Cirrhotic Patients Non-cirrhotic Patients (non-decompensated) (n = 735) (n = 133) 33

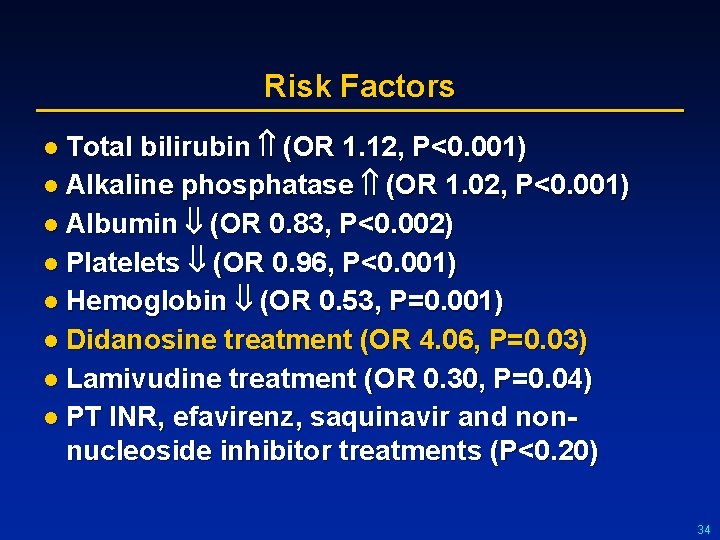
Risk Factors l Total bilirubin (OR 1. 12, P<0. 001) l Alkaline phosphatase (OR 1. 02, P<0. 001) l Albumin (OR 0. 83, P<0. 002) l Platelets (OR 0. 96, P<0. 001) l Hemoglobin (OR 0. 53, P=0. 001) l Didanosine treatment (OR 4. 06, P=0. 03) l Lamivudine treatment (OR 0. 30, P=0. 04) l PT INR, efavirenz, saquinavir and non- nucleoside inhibitor treatments (P<0. 20) 34

Ribavirin and dd. I = “don’t do it” dd. I Inositol IMP XMP dehydrogenase dd. I-MP dd. A-DP dd. A-TP (-) RT g-Pol GTP (-) Ribavirin-MP Adenosine kinase Ribavirin increases IMP • Increases dd. ATP • Increases inhibition of HIV RT • Increases inhibition of host g-pol 35

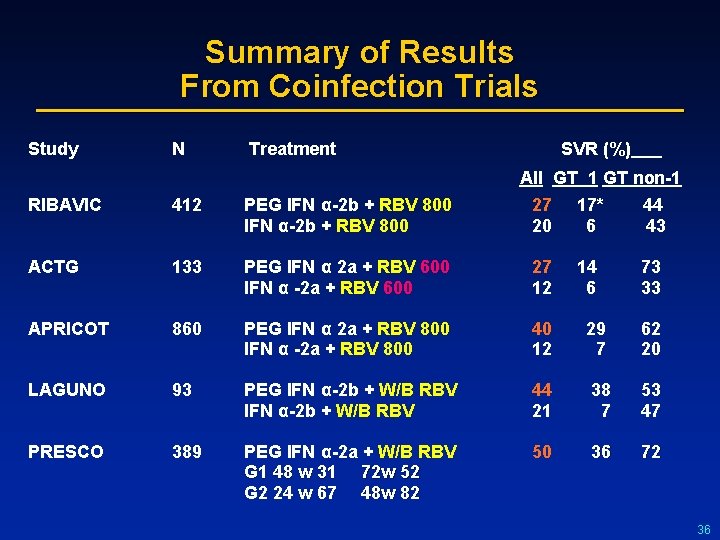
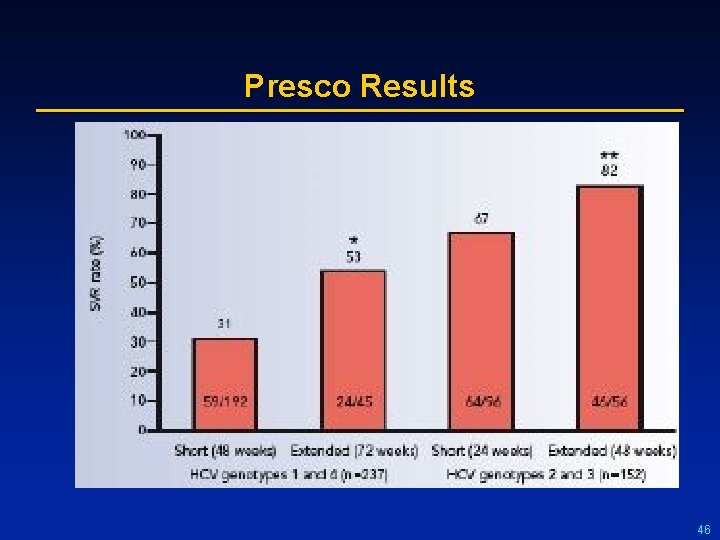
Summary of Results From Coinfection Trials Study N Treatment SVR (%) All GT 1 GT non-1 RIBAVIC 412 PEG IFN α-2 b + RBV 800 27 17* 44 20 6 43 ACTG 133 PEG IFN α 2 a + RBV 600 IFN α -2 a + RBV 600 27 14 73 12 6 33 APRICOT 860 PEG IFN α 2 a + RBV 800 IFN α -2 a + RBV 800 40 29 62 12 7 20 LAGUNO 93 PEG IFN α-2 b + W/B RBV 44 38 53 21 7 47 PRESCO 389 PEG IFN α-2 a + W/B RBV G 1 48 w 31 72 w 52 G 2 24 w 67 48 w 82 50 36 72 36

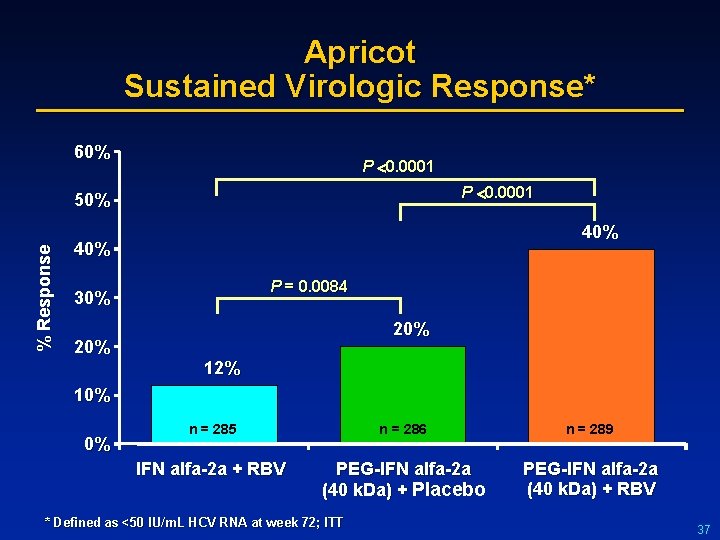
Apricot Sustained Virologic Response* 60% P 0. 0001 % Response 50% 40% P = 0. 0084 30% 20% 12% 10% 0% n = 285 n = 286 n = 289 IFN alfa-2 a + RBV PEG-IFN alfa-2 a (40 k. Da) + Placebo PEG-IFN alfa-2 a (40 k. Da) + RBV * Defined as <50 IU/ m. L HCV RNA at week 72; ITT 37

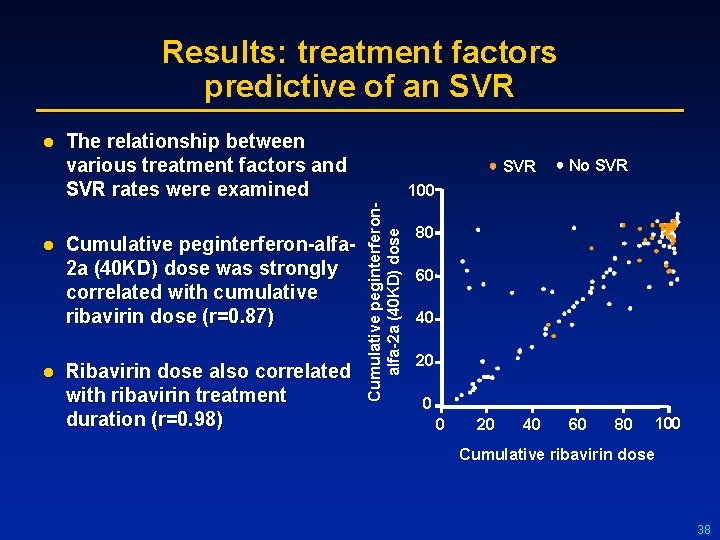
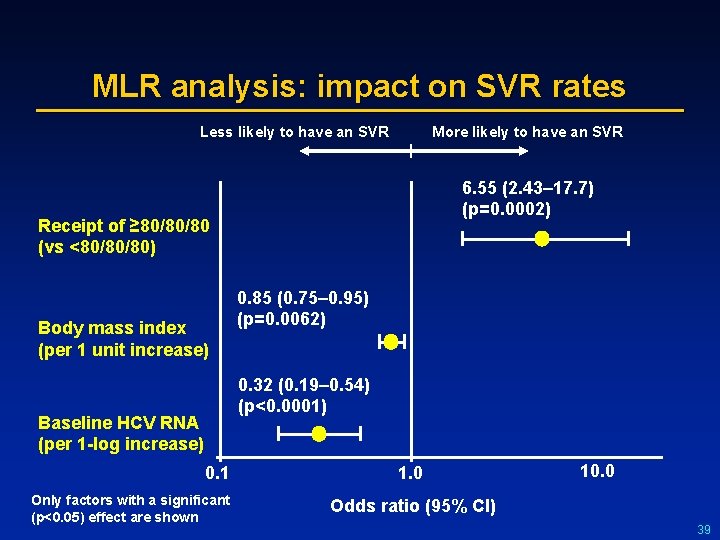
Results: treatment factors predictive of an SVR l l The relationship between various treatment factors and SVR rates were examined Cumulative peginterferon-alfa 2 a (40 KD) dose was strongly correlated with cumulative ribavirin dose (r=0. 87) Ribavirin dose also correlated with ribavirin treatment duration (r=0. 98) SVR No SVR 100 Cumulative peginterferonalfa-2 a (40 KD) dose l 80 60 40 20 0 0 20 40 60 80 100 Cumulative ribavirin dose 38

MLR analysis: impact on SVR rates Less likely to have an SVR More likely to have an SVR 6. 55 (2. 43– 17. 7) (p=0. 0002) Receipt of ≥ 80/80/80 (vs <80/80/80) Body mass index (per 1 unit increase) 0. 85 (0. 75– 0. 95) (p=0. 0062) 0. 32 (0. 19– 0. 54) (p<0. 0001) Baseline HCV RNA (per 1 -log increase) 0. 1 Only factors with a significant (p<0. 05) effect are shown 1. 0 10. 0 Odds ratio (95% CI) 39

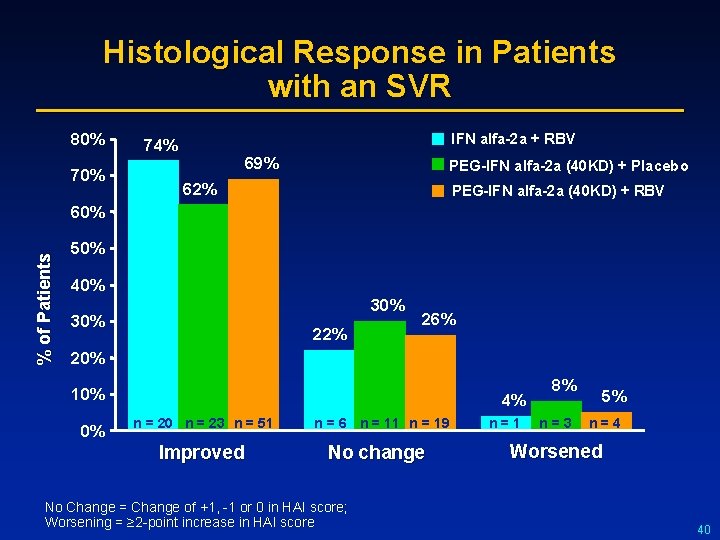
Histological Response in Patients with an SVR 80% 70% IFN alfa-2 a + RBV 74% 69% PEG-IFN alfa-2 a (40 KD) + Placebo 62% PEG-IFN alfa-2 a (40 KD) + RBV % of Patients 60% 50% 40% 30% 22% 26% 20% 10% 0% 4% n = 20 n = 23 n = 51 Improved n = 6 n = 11 n = 19 No change No Change = Change of +1, -1 or 0 in HAI score; Worsening = ≥ 2 -point increase in HAI score n = 1 8% n = 3 5% n = 4 Worsened 40

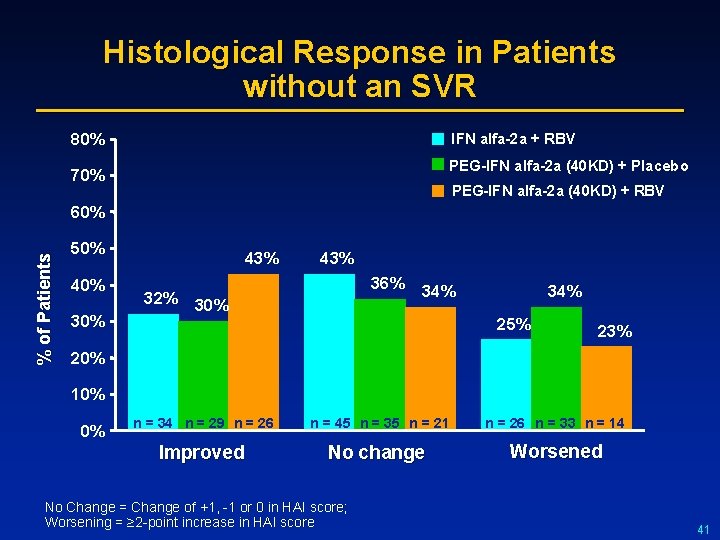
Histological Response in Patients without an SVR 80% IFN alfa-2 a + RBV PEG-IFN alfa-2 a (40 KD) + Placebo 70% PEG-IFN alfa-2 a (40 KD) + RBV % of Patients 60% 50% 40% 30% 43% 36% 34% 32% 30% 34% 25% 23% 20% 10% 0% n = 34 n = 29 n = 26 n = 45 n = 35 n = 21 n = 26 n = 33 n = 14 Improved No change Worsened No Change = Change of +1, -1 or 0 in HAI score; Worsening = ≥ 2 -point increase in HAI score 41

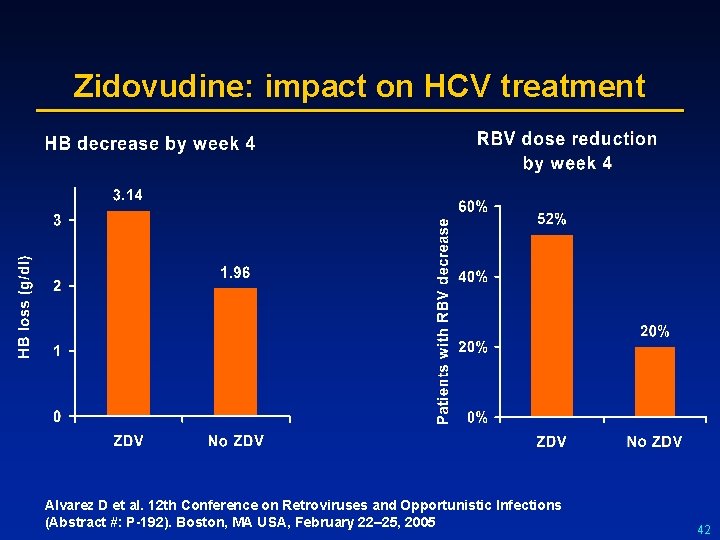
Zidovudine: impact on HCV treatment Alvarez D et al. 12 th Conference on Retroviruses and Opportunistic Infections (Abstract #: P-192). Boston, MA USA, February 22– 25, 2005 42

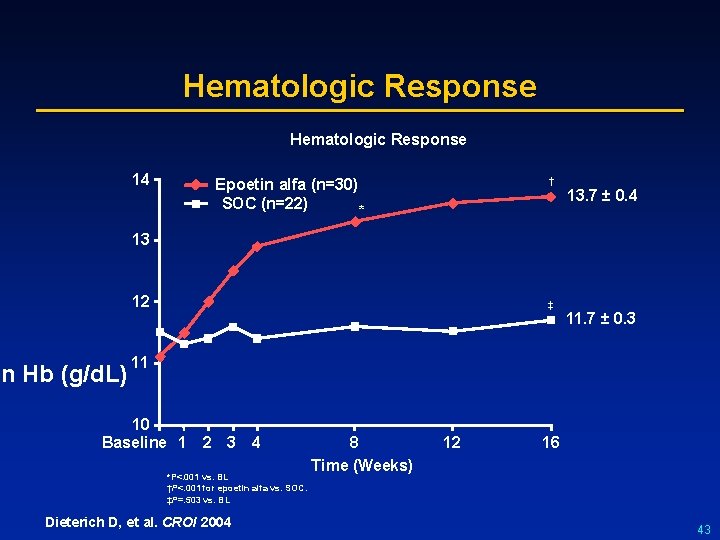
Hematologic Response 14 † Epoetin alfa (n=30) SOC (n=22) * 13. 7 ± 0. 4 13 12 an Hb (g/d. L) ‡ 11. 7 ± 0. 3 11 10 Baseline 1 2 3 4 *P<. 001 vs. BL †P<. 001 for epoetin alfa vs. SOC. ‡P=. 503 vs. BL Dieterich D, et al. CROI 2004 8 Time (Weeks) 12 16 43

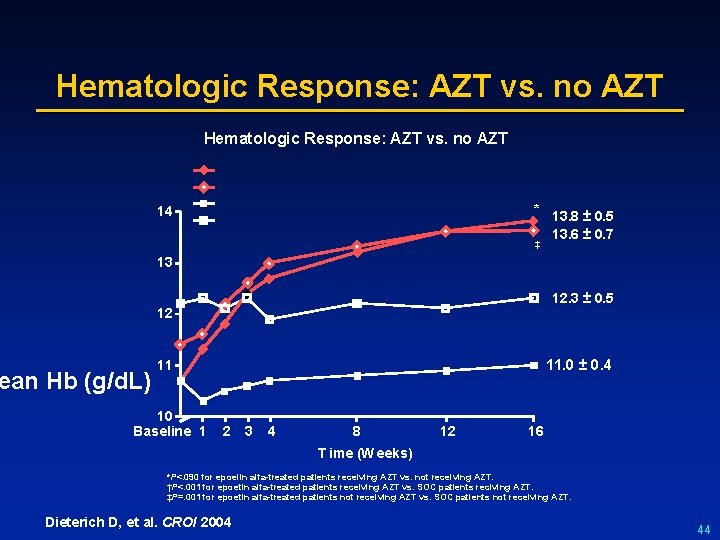
Hematologic Response: AZT vs. no AZT * 13. 8 ± 0. 5 13. 6 ± 0. 7 14 ‡ 13 12. 3 ± 0. 5 12 ean Hb (g/d. L) 11. 0 ± 0. 4 11 10 Baseline 1 2 3 4 8 12 16 T ime (W eeks) *P<. 090 for epoelin alfa-treated patients receiving AZT vs. not receiving AZT. †P<. 001 for epoetin alfa-treated patients receiving AZT vs. SOC patients reciving AZT. ‡P=. 001 for epoetin alfa-treated patients not receiving AZT vs. SOC patients not receiving AZT. Dieterich D, et al. CROI 2004 44

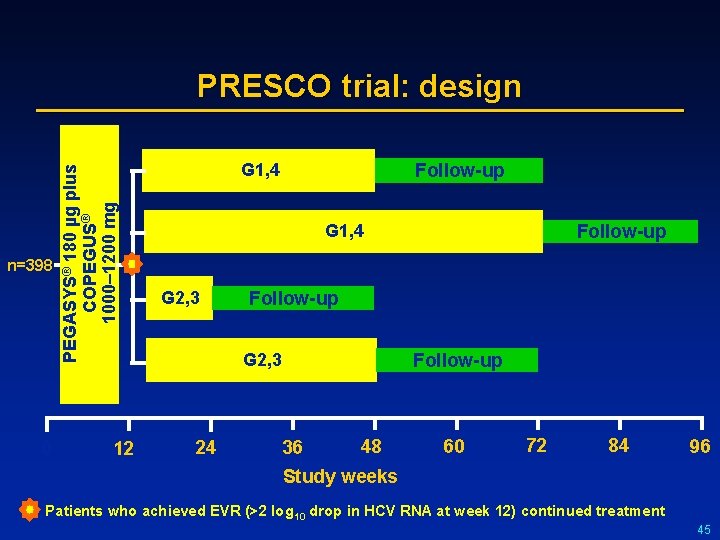
n=398 0 PEGASYS® 180 µg plus COPEGUS® 1000– 1200 mg PRESCO trial: design 12 G 1, 4 Follow-up G 1, 4 G 2, 3 Follow-up G 2, 3 24 Follow-up 36 48 60 72 84 96 Study weeks Patients who achieved EVR (>2 log 10 drop in HCV RNA at week 12) continued treatment 45

Presco Results 46

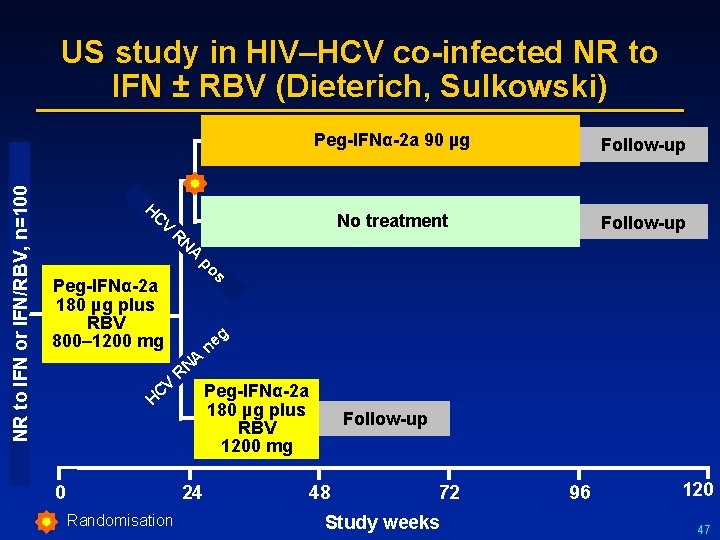
NR to IFN or IFN/RBV, n=100 US study in HIV–HCV co-infected NR to IFN ± RBV (Dieterich, Sulkowski) Peg-IFNα-2 a 90 µg Follow-up No treatment Follow-up H C V R Peg-IFNα-2 a 180 µg plus RBV 800– 1200 mg N A p os e n A N R V C Peg-IFNα-2 a 180 µg plus RBV 1200 mg H 0 24 Randomisation g Follow-up 48 72 Study weeks 96 120 47

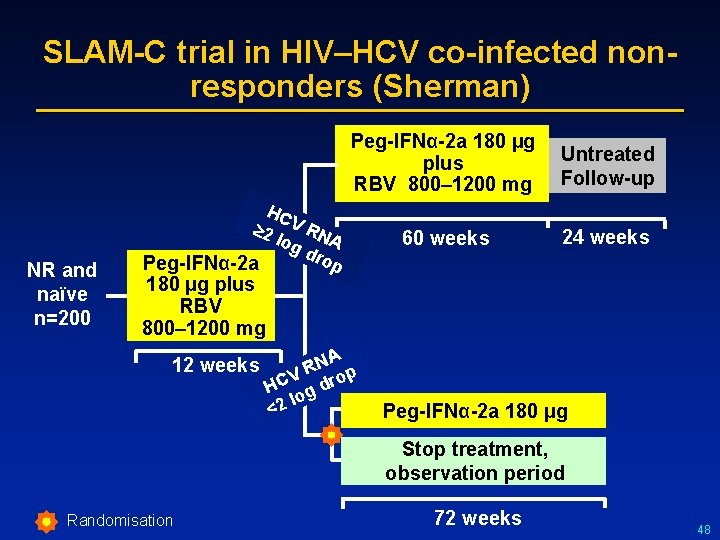
SLAM-C trial in HIV–HCV co-infected nonresponders (Sherman) Peg-IFNα-2 a 180 µg Untreated plus RBV 800– 1200 mg Follow-up NR and naïve n=200 HC 2 V RN log A dr op Peg-IFNα-2 a 60 weeks 24 weeks 180 µg plus RBV 800– 1200 mg 12 weeks A N R op V HC g dr lo 2 < Peg-IFNα-2 a 180 µg Stop treatment, observation period Randomisation 72 weeks 48

Approach to non-responders METAVIR 0– 1 l Wait for new treatment options (Helicase, protease inhibitors, polymerase inhibitors) Liver biopsy METAVIR 3– 4 l Retreat with Peg-IFN + RBV l Antifibrotic treatment strategy l Consider Peg 360 and wt based RBV l Treat with insulin sensitzer METAVIR 2– 3 l Retreat with Peg-IFN + RBV l Consider Peg 360 and wt based RBV l Treat with insulin sensitizer 49

Orthotopic Liver Transplantation l 23 HIV + compared to UNOS (11, 453 HIV -) l l l HCV, 14 and HBV, 9 CD 4, 200 (range 76 – 506) MELD 16 HCV worse than HBV Drug-drug interaction (tacrolimus) Outcome not associated with HIV RNA or CD 4 Ragni M. Survival in HIV-infected Liver Transplant Recipients. 10 th CROI #155 50

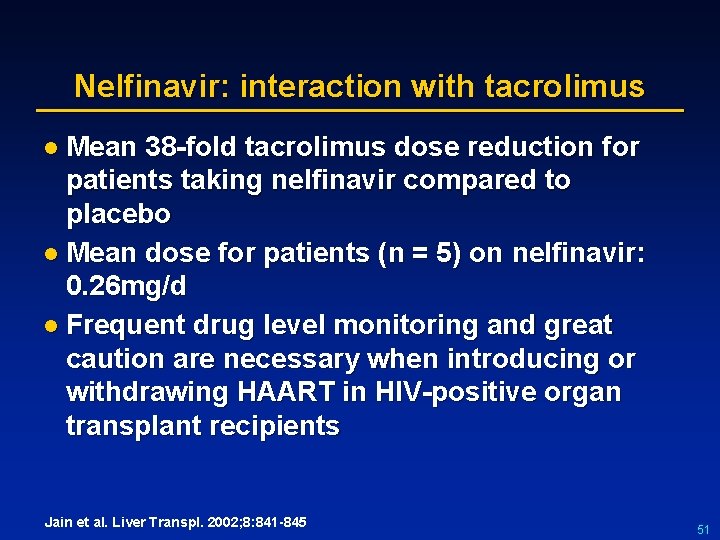
Nelfinavir: interaction with tacrolimus l Mean 38 -fold tacrolimus dose reduction for patients taking nelfinavir compared to placebo l Mean dose for patients (n = 5) on nelfinavir: 0. 26 mg/d l Frequent drug level monitoring and great caution are necessary when introducing or withdrawing HAART in HIV-positive organ transplant recipients Jain et al. Liver Transpl. 2002; 8: 841 -845 51

Patient Survival compared with OPTN Three Year Patient Survival One Year Patient Survival Liver All OPTN 65 years+ OPTN HIV-infected study subjects Kidney Liver 87. 6 95. 6 79. 9 (87. 0, 88. 2) (95. 4, 95. 8) (79. 3, 80. 5) 80. 5 90. 4 69. 6 (77. 9, 83. 0) (89. 4, 91. 3) (66. 9, 72. 2) 90. 9 93. 8 80. 8 (73. 9, 100) (81. 9, 100) (56. 8, 100) Kidney 90. 8 (90. 5, 91. 1) 78. 0 (76. 6, 79. 4) 93. 8 (81. 9, 100) Based on OPTN data as of February 4, 2005. One year survival is based on 1999 - 2001 transplants, and 3 year survival is based on 1996 - 1999 transplants. 52

Participating Centers (visit the study website for updated list of centers and contact information) Atlanta Baltimore Boston Charlottesville Chicago Cincinnati Cleveland Los Angeles Miami New Orleans New York Philadelphia Pittsburgh San Francisco Washington, D. C. Emory University (K) University of Maryland (K) Beth Israel Deaconess Medical University of Virginia (K, L) Center (K, L) University of Chicago (K, L, Peds K, Rush University (K, L) Peds L) University of Cincinnati (K, L) Cleveland Clinic (K, L) Cedars-Sinai (L) University of Miami (K) Tulane (K, L) Mount Sinai School of Medicine (K, L, Columbia University (L, Peds L) Peds K) Drexel University (K) University of Pennsylvania (K, L) University of Pittsburgh (K, L) University of California (K, L, Peds K, Washington Hospital Center (K) Peds L) Georgetown Medical Center (K, L) 53

Conclusions l HCV is a major, if not the major cause of morbidity and mortality in HIV+ patients today l Successful treatment of HCV (cure) in HIV+ patients is possible and even likely with pegylated interferon and ribavirin l Side effects can be effectively managed to ensure treatment success l Liver (and kidney) transplant is possible and is being investigated in HIV+ patients 54

Topics Timelines Registration: • Acute Hepatitis C- epidemiology and treatment Early reg. Feb. 15 - March 15, 2007 • Chronic Hepatitis C- kinetics, current treatment Regular reg. March 16 - April 30, 2007 and new therapies Late reg. May 1 - May 31, 2007 • Hepatitis B - Treatment strategies • Liver Transplantation- selection and management Abstract submission: • Steatosis in HIV and HCV Deadline April 30, 2007 • Non invasive measures of fibrosis • End stage of liver disease managment • Tayloring ART therapy in Hepatitis More information • Update on new drugs for HCV and HBV For more information, please visit www. virology-education. com • Clinical cases P-55
 Hcv treatment
Hcv treatment Hcv treatment
Hcv treatment Douglas t dietrich
Douglas t dietrich Hcv symptoms female
Hcv symptoms female Window period hiv
Window period hiv Otto hoffman's by product oven
Otto hoffman's by product oven Dietrich beitzke
Dietrich beitzke Brmer
Brmer Nvperf
Nvperf Madeleine dietrich
Madeleine dietrich Brain balance autism
Brain balance autism Yannick dietrich
Yannick dietrich Anderson dietrich
Anderson dietrich Kai übel
Kai übel Constanze dietrich
Constanze dietrich Dietrich bonhoeffer quotes on discipleship
Dietrich bonhoeffer quotes on discipleship Dietrich metal studs
Dietrich metal studs Dietrich belitz
Dietrich belitz Dietrich sabine
Dietrich sabine Dbg ahlhorn
Dbg ahlhorn Stewart douglas ametek
Stewart douglas ametek Anatomi vesika urinaria
Anatomi vesika urinaria Georgia blanche douglas camp johnson
Georgia blanche douglas camp johnson History of human computer interaction
History of human computer interaction Day trade school
Day trade school Hàm cobb-douglas toán cao cấp
Hàm cobb-douglas toán cao cấp Sigit
Sigit Leap program douglasville ga
Leap program douglasville ga Deep water by william douglas
Deep water by william douglas Douglas county mental health initiative
Douglas county mental health initiative Alexander gardner
Alexander gardner Douglas macarthur
Douglas macarthur Douglas valentine
Douglas valentine Douglas adams stopárov sprievodca galaxiou
Douglas adams stopárov sprievodca galaxiou Contoh soal metode douglas
Contoh soal metode douglas Douglas schrock
Douglas schrock Croissance douglas
Croissance douglas Douglas w diamond
Douglas w diamond Douglas vinci hoy
Douglas vinci hoy Douglas howry
Douglas howry Steven douglas slides
Steven douglas slides Mrts cobb douglas
Mrts cobb douglas Douglas krupp
Douglas krupp Douglas bell ucla
Douglas bell ucla Douglas seraphim james de couto
Douglas seraphim james de couto Douglas
Douglas Anteflexion of uterus
Anteflexion of uterus Douglas guiffrida
Douglas guiffrida Parede abdominal
Parede abdominal Tara douglas-williams
Tara douglas-williams Stars douglas
Stars douglas Douglas wilhelm harder
Douglas wilhelm harder Douglas merritte
Douglas merritte Douglas prasher
Douglas prasher Douglas pereira da silva
Douglas pereira da silva Michael douglas
Michael douglas