Handling Concentration Time Data i Determining Elimination Rate

![AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-51.jpg)
![AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-52.jpg)
![AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-53.jpg)
![AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-54.jpg)
![AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-55.jpg)
![AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-56.jpg)
![AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-57.jpg)
![AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-58.jpg)
![AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-59.jpg)
![AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-60.jpg)
![AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-61.jpg)
![AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-62.jpg)
![AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-63.jpg)
![AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-64.jpg)
![AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-65.jpg)
![AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-69.jpg)
![AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-70.jpg)
![Dealing with [ ] –time Data (3) Back Extrapolation How do you calculate Volume Dealing with [ ] –time Data (3) Back Extrapolation How do you calculate Volume](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-85.jpg)
![Dealing with [ ] –time Data Back Extrap What happens if you do not Dealing with [ ] –time Data Back Extrap What happens if you do not](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-86.jpg)
![Dealing with [ ] –time Data Back Extrap What happens if you do not Dealing with [ ] –time Data Back Extrap What happens if you do not](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-87.jpg)
![Dealing with [ ] –time Data Back Extrap What happens if you do not Dealing with [ ] –time Data Back Extrap What happens if you do not](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-88.jpg)
![Back Extrap Dealing with [ ] –time Data What happens if you do not Back Extrap Dealing with [ ] –time Data What happens if you do not](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-89.jpg)
![Dealing with [ ] –time Data Back Extrap What happens if you do not Dealing with [ ] –time Data Back Extrap What happens if you do not](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-90.jpg)
![What happens if you do not have a time zero [ ]? Dose = What happens if you do not have a time zero [ ]? Dose =](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-91.jpg)
![Dealing with [ ] –time Data Back Extrap What is the volume of distribution Dealing with [ ] –time Data Back Extrap What is the volume of distribution](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-92.jpg)
![Dealing with [ ] –time Data Back Extrap What is the volume of distribution Dealing with [ ] –time Data Back Extrap What is the volume of distribution](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-93.jpg)
![Dealing with [ ] –time Data Back Extrap What is the volume of distribution Dealing with [ ] –time Data Back Extrap What is the volume of distribution](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-94.jpg)
![Dealing with [ ] –time Data Back Extrap What is the volume of distribution Dealing with [ ] –time Data Back Extrap What is the volume of distribution](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-95.jpg)
![Dealing with [ ] –time Data Back Extrap What is the volume of distribution Dealing with [ ] –time Data Back Extrap What is the volume of distribution](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-96.jpg)
- Slides: 102

Handling Concentration – Time Data (i) Determining Elimination Rate (K) (ii) AUC Calculations (iii) Back Calculation

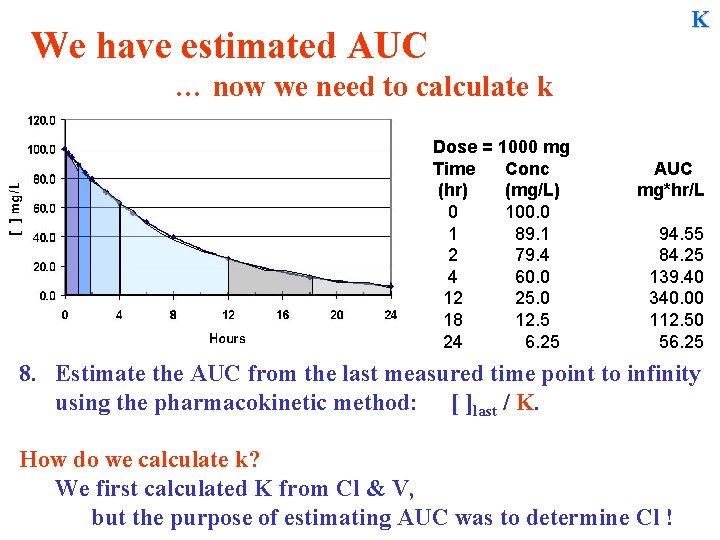
K We have estimated AUC … now we need to calculate k Dose = 1000 mg Time Conc (hr) (mg/L) 0 100. 0 1 89. 1 2 79. 4 4 60. 0 12 25. 0 18 12. 5 24 6. 25 AUC mg*hr/L 94. 55 84. 25 139. 40 340. 00 112. 50 56. 25 8. Estimate the AUC from the last measured time point to infinity using the pharmacokinetic method: [ ]last / K. How do we calculate k? We first calculated K from Cl & V, but the purpose of estimating AUC was to determine Cl !

Determining Elimination Rate Constant Drugs are cleared from the body. Clearance can occur by several pathways, urinary, biliary, excretion into the air, biotransformation in the liver… Ca Cv K

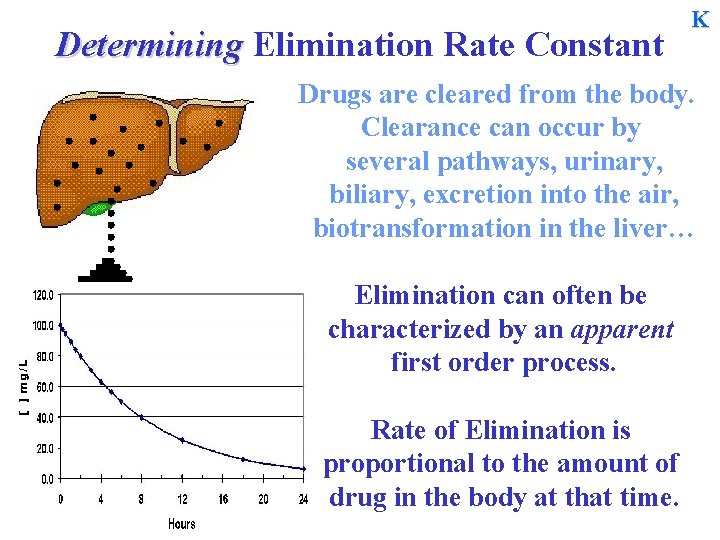
Determining Elimination Rate Constant K Drugs are cleared from the body. Clearance can occur by several pathways, urinary, biliary, excretion into the air, biotransformation in the liver… Elimination can often be characterized by an apparent first order process. Rate of Elimination is proportional to the amount of drug in the body at that time.

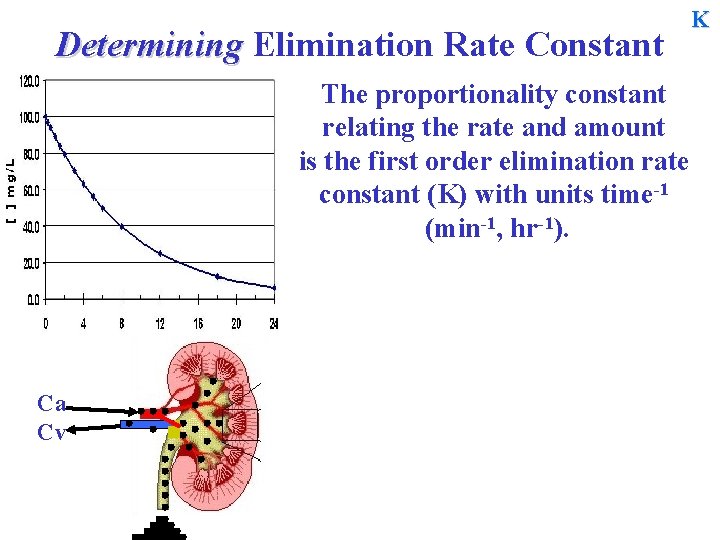
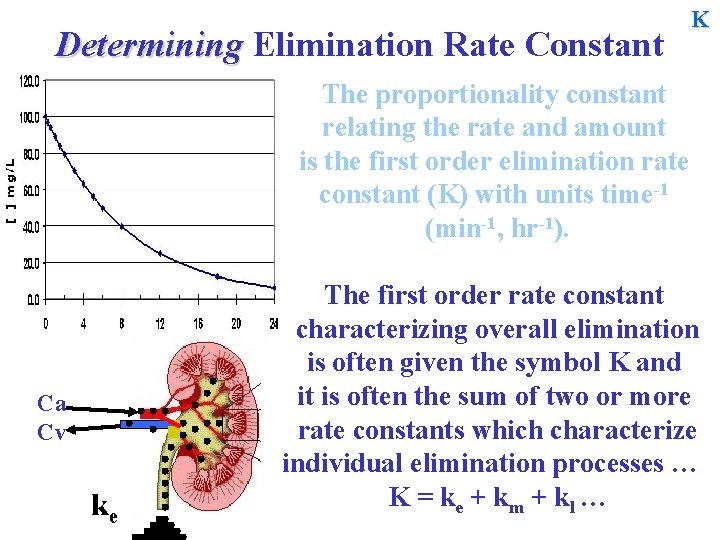
Determining Elimination Rate Constant The proportionality constant relating the rate and amount is the first order elimination rate constant (K) with units time-1 (min-1, hr-1). Ca Cv K

Determining Elimination Rate Constant K The proportionality constant relating the rate and amount is the first order elimination rate constant (K) with units time-1 (min-1, hr-1). Ca Cv ke The first order rate constant characterizing overall elimination is often given the symbol K and it is often the sum of two or more rate constants which characterize individual elimination processes … K = ke + k m + kl …

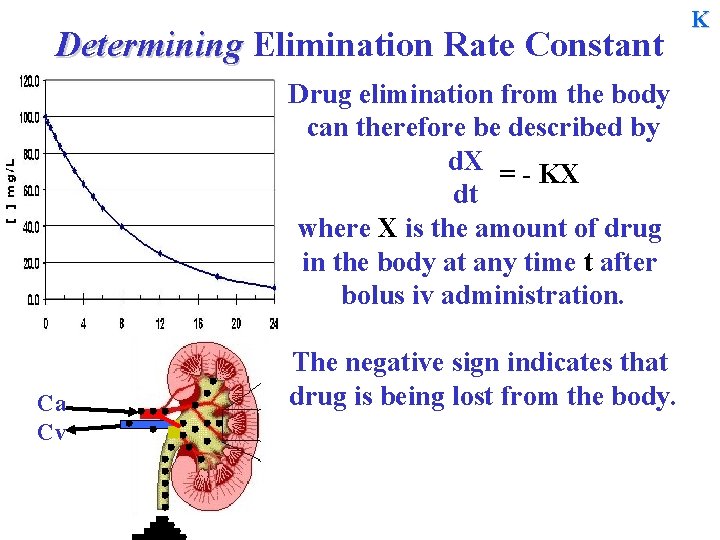
Determining Elimination Rate Constant Drug elimination from the body can therefore be described by d. X = - KX dt where X is the amount of drug in the body at any time t after bolus iv administration. Ca Cv The negative sign indicates that drug is being lost from the body. K

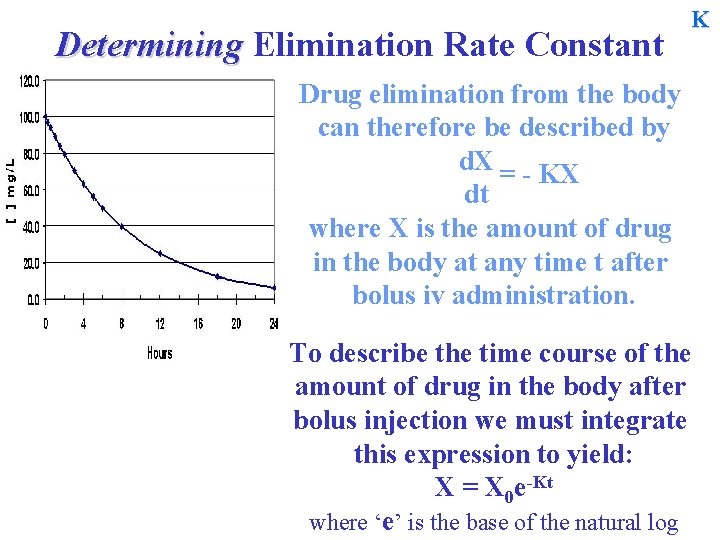
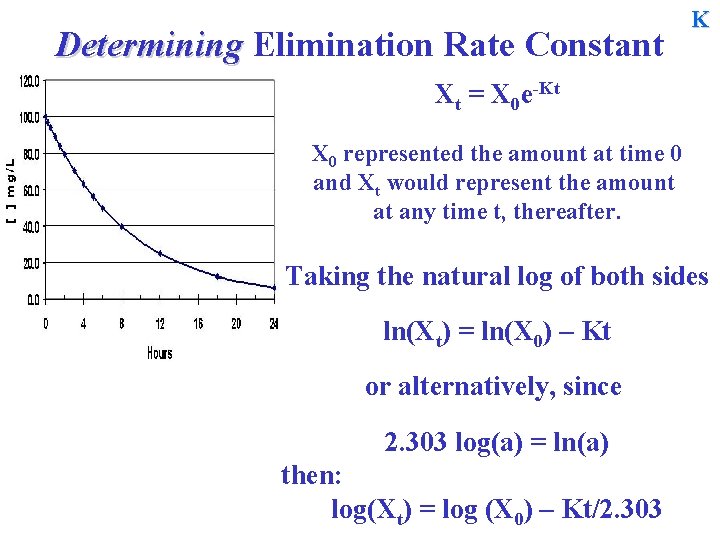
Determining Elimination Rate Constant K Drug elimination from the body can therefore be described by d. X = - KX dt where X is the amount of drug in the body at any time t after bolus iv administration. To describe the time course of the amount of drug in the body after bolus injection we must integrate this expression to yield: X = X 0 e-Kt where ‘e’ is the base of the natural log

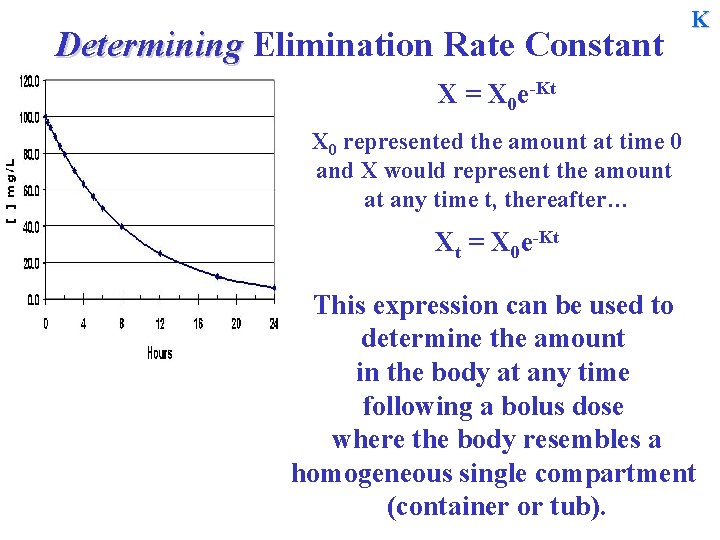
Determining Elimination Rate Constant K X = X 0 e-Kt X 0 represented the amount at time 0 and X would represent the amount at any time t, thereafter… Xt = X 0 e-Kt This expression can be used to determine the amount in the body at any time following a bolus dose where the body resembles a homogeneous single compartment (container or tub).

Determining Elimination Rate Constant K Xt = X 0 e-Kt X 0 represented the amount at time 0 and Xt would represent the amount at any time t, thereafter. Taking the natural log of both sides ln(Xt) = ln(X 0) – Kt or alternatively, since 2. 303 log(a) = ln(a) then: log(Xt) = log (X 0) – Kt/2. 303

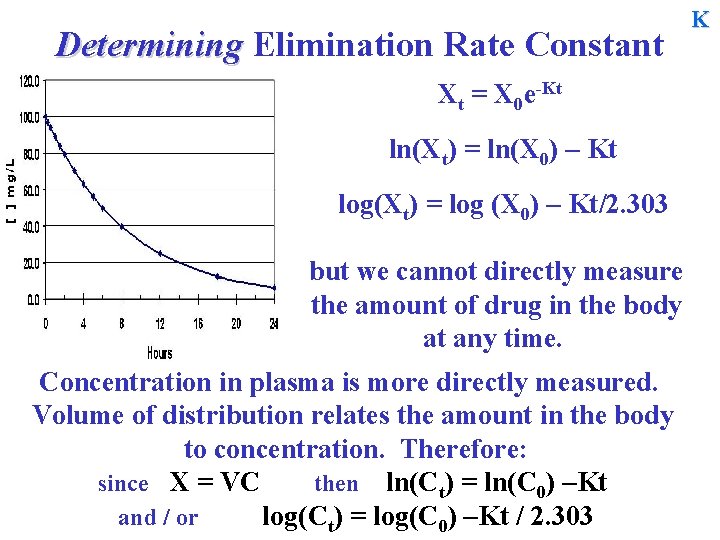
Determining Elimination Rate Constant Xt = X 0 e-Kt ln(Xt) = ln(X 0) – Kt log(Xt) = log (X 0) – Kt/2. 303 but we cannot directly measure the amount of drug in the body at any time. Concentration in plasma is more directly measured. Volume of distribution relates the amount in the body to concentration. Therefore: since X = VC then ln(Ct) = ln(C 0) –Kt and / or log(Ct) = log(C 0) –Kt / 2. 303 K

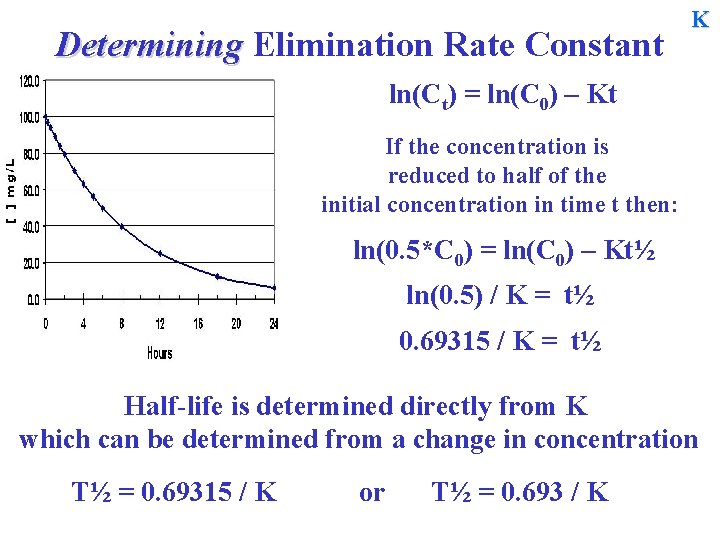
Determining Elimination Rate Constant K ln(Ct) = ln(C 0) – Kt If the concentration is reduced to half of the initial concentration in time t then: ln(0. 5*C 0) = ln(C 0) – Kt½ ln(0. 5) / K = t½ 0. 69315 / K = t½ Half-life is determined directly from K which can be determined from a change in concentration T½ = 0. 69315 / K or T½ = 0. 693 / K

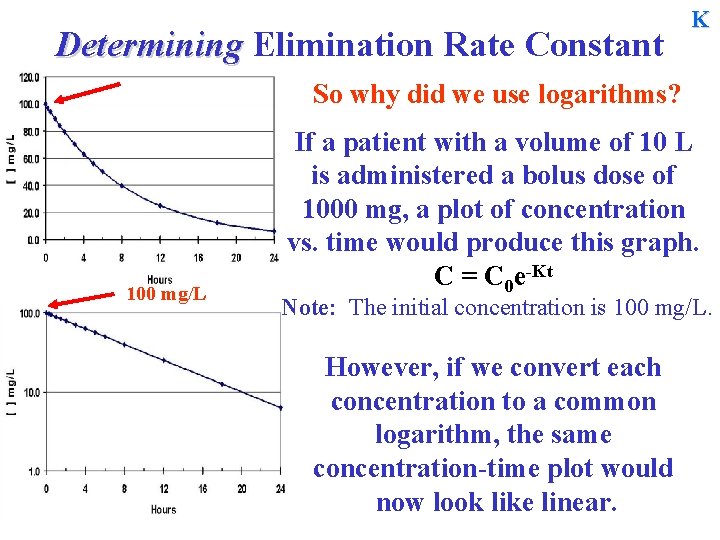
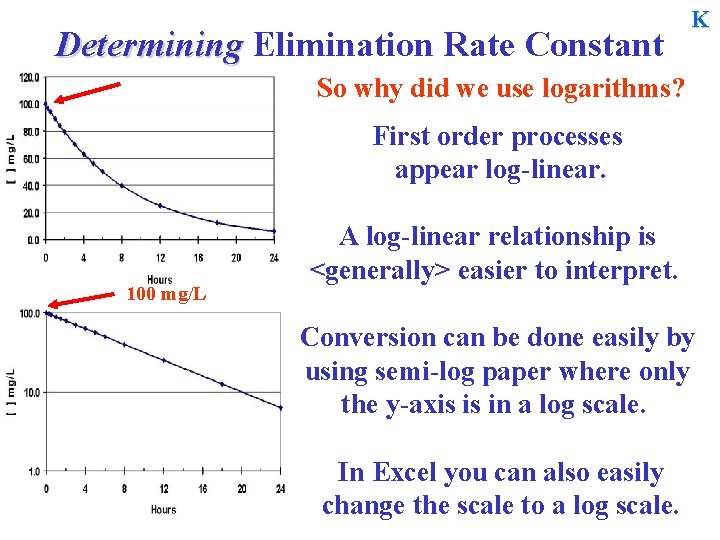
Determining Elimination Rate Constant K So why did we use logarithms? 100 mg/L If a patient with a volume of 10 L is administered a bolus dose of 1000 mg, a plot of concentration vs. time would produce this graph. C = C 0 e-Kt Note: The initial concentration is 100 mg/L. However, if we convert each concentration to a common logarithm, the same concentration-time plot would now look like linear.

Determining Elimination Rate Constant K So why did we use logarithms? First order processes appear log-linear. 100 mg/L A log-linear relationship is <generally> easier to interpret. Conversion can be done easily by using semi-log paper where only the y-axis is in a log scale. In Excel you can also easily change the scale to a log scale.

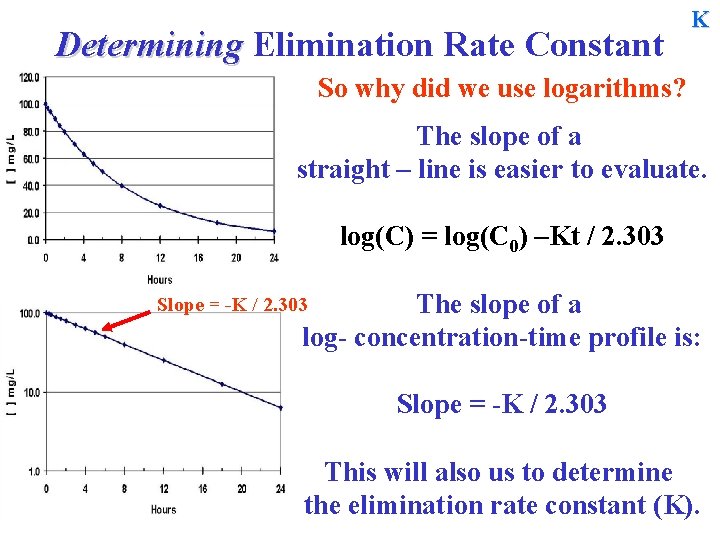
Determining Elimination Rate Constant K So why did we use logarithms? The slope of a straight – line is easier to evaluate. log(C) = log(C 0) –Kt / 2. 303 The slope of a log- concentration-time profile is: Slope = -K / 2. 303 This will also us to determine the elimination rate constant (K).

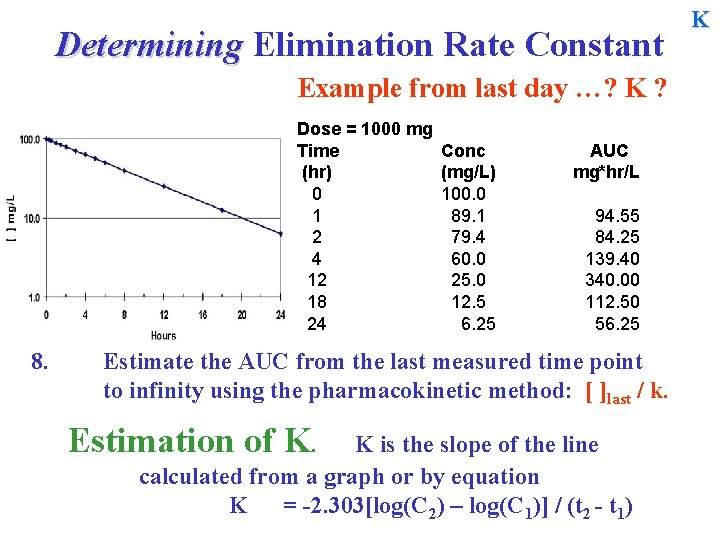
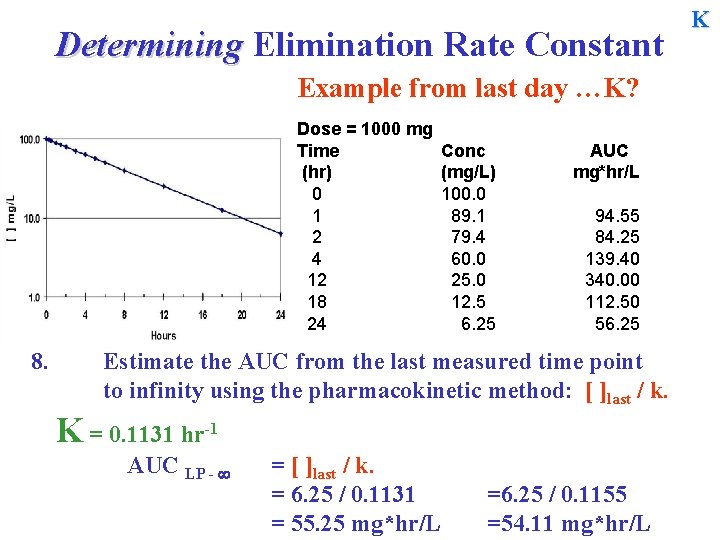
Determining Elimination Rate Constant Example from last day …? K ? Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 8. Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 AUC mg*hr/L 94. 55 84. 25 139. 40 340. 00 112. 50 56. 25 Estimate the AUC from the last measured time point to infinity using the pharmacokinetic method: [ ]last / k. Estimation of K. K is the slope of the line calculated from a graph or by equation K = -2. 303[log(C 2) – log(C 1)] / (t 2 - t 1) K

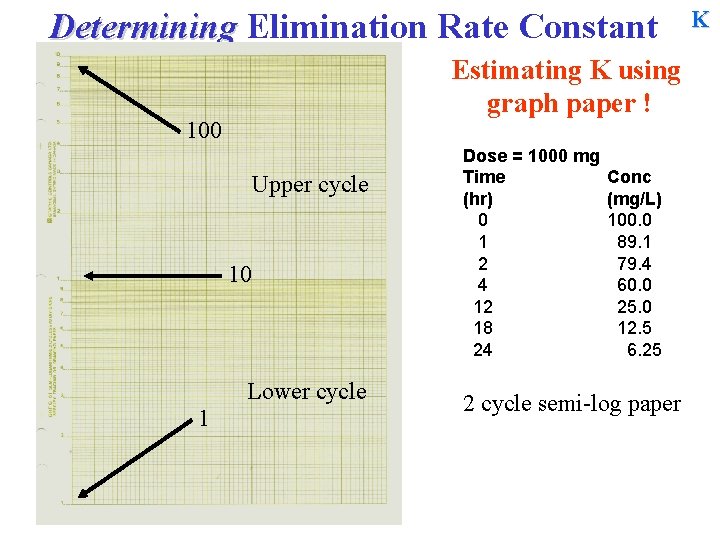
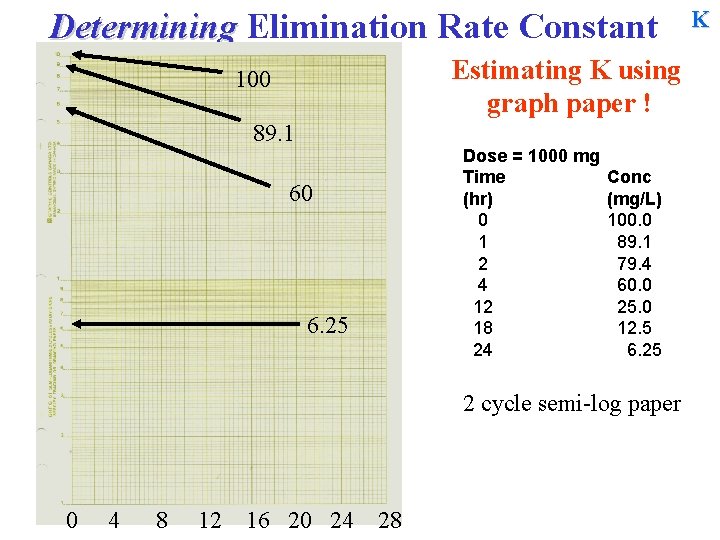
Determining Elimination Rate Constant Estimating K using graph paper ! 100 Upper cycle 10 Lower cycle 1 Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 2 cycle semi-log paper K

Determining Elimination Rate Constant Estimating K using graph paper ! 100 89. 1 Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 60 6. 25 Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 2 cycle semi-log paper 0 4 8 12 16 20 24 28 K

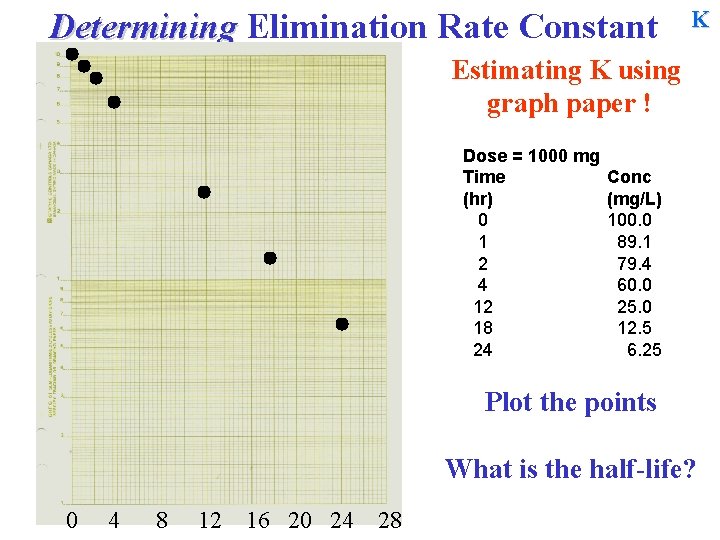
Determining Elimination Rate Constant K Estimating K using graph paper ! Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 Plot the points What is the half-life? 0 4 8 12 16 20 24 28

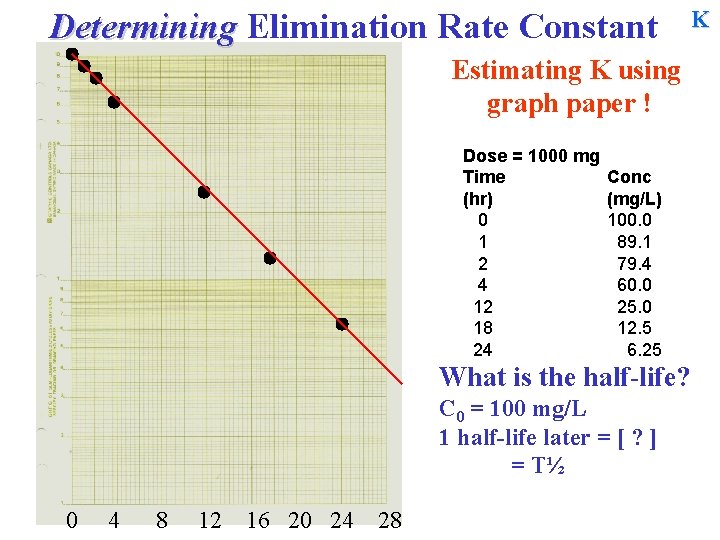
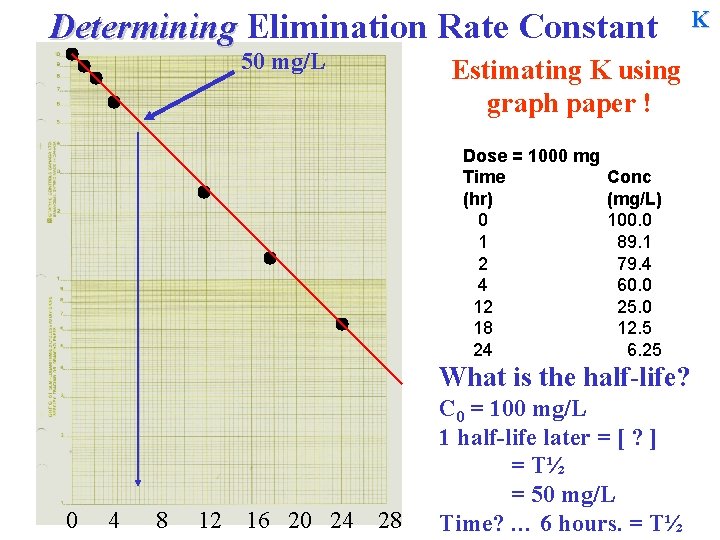
Determining Elimination Rate Constant Estimating K using graph paper ! Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 What is the half-life? C 0 = 100 mg/L 1 half-life later = [ ? ] = T½ 0 4 8 12 16 20 24 28 K

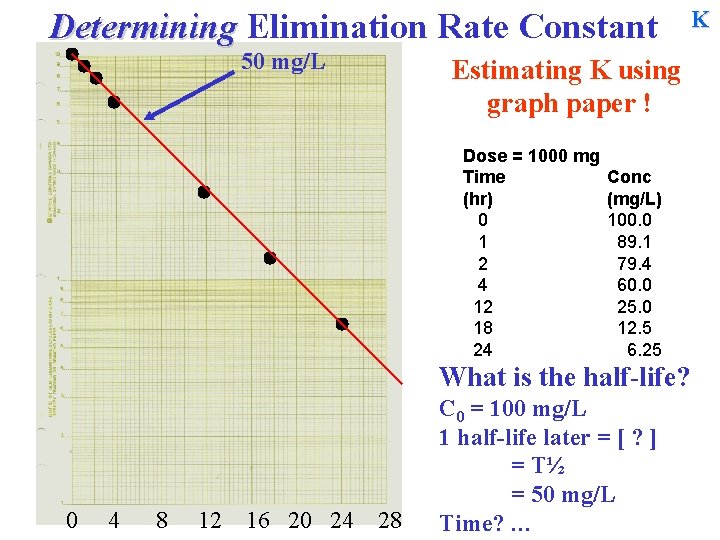
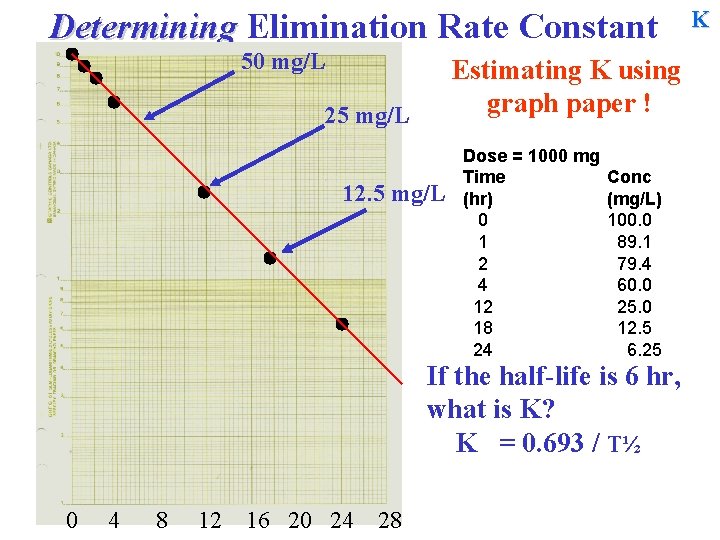
Determining Elimination Rate Constant 50 mg/L Estimating K using graph paper ! Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 What is the half-life? 0 4 8 12 16 20 24 28 C 0 = 100 mg/L 1 half-life later = [ ? ] = T½ = 50 mg/L Time? … K

Determining Elimination Rate Constant 50 mg/L Estimating K using graph paper ! Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 What is the half-life? 0 4 8 12 16 20 24 28 C 0 = 100 mg/L 1 half-life later = [ ? ] = T½ = 50 mg/L Time? … 6 hours. = T½ K

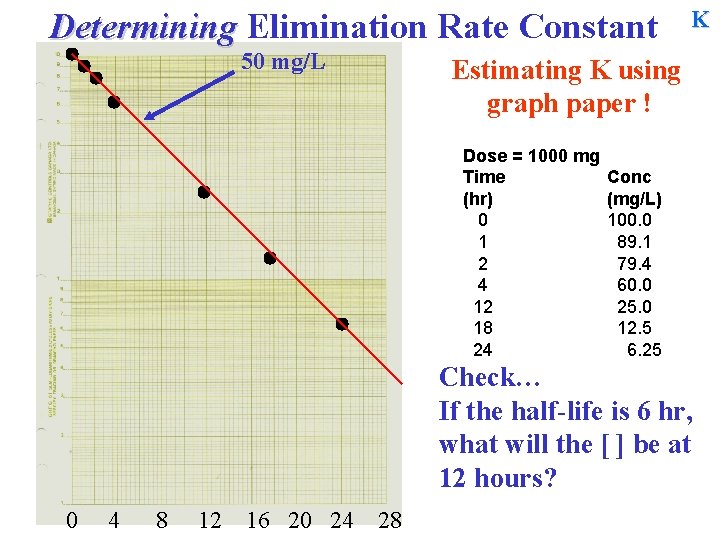
Determining Elimination Rate Constant 50 mg/L K Estimating K using graph paper ! Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 Check… If the half-life is 6 hr, what will the [ ] be at 12 hours? 0 4 8 12 16 20 24 28

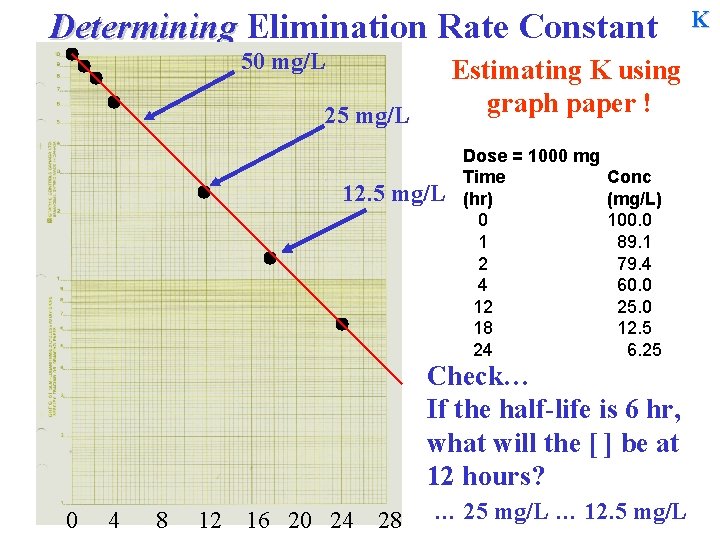
Determining Elimination Rate Constant 50 mg/L Estimating K using graph paper ! 25 mg/L 12. 5 mg/L Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 Check… If the half-life is 6 hr, what will the [ ] be at 12 hours? 0 4 8 12 16 20 24 28 … 25 mg/L … 12. 5 mg/L K

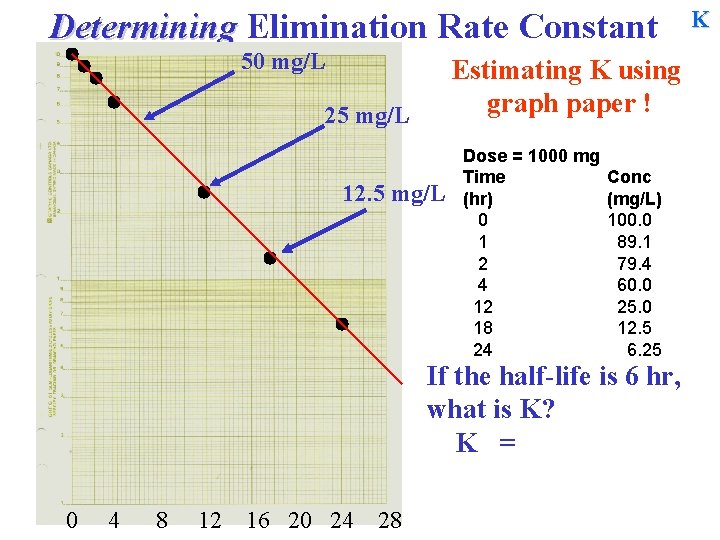
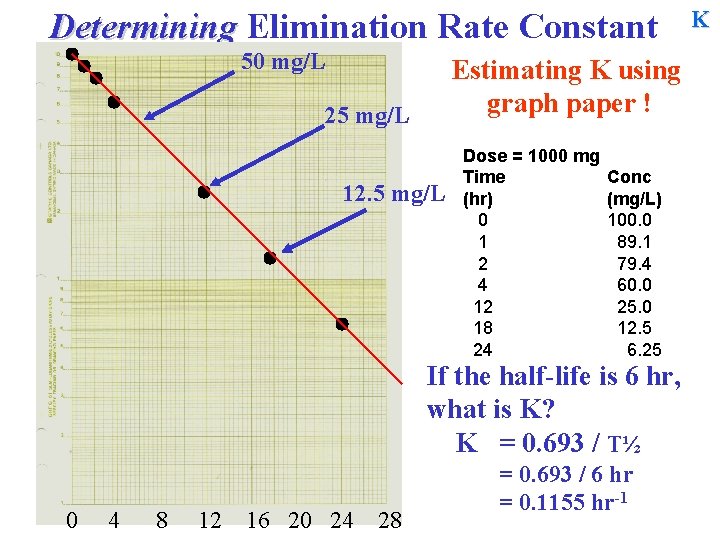
Determining Elimination Rate Constant 50 mg/L Estimating K using graph paper ! 25 mg/L 12. 5 mg/L Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 If the half-life is 6 hr, what is K? K = 0 4 8 12 16 20 24 28 K

Determining Elimination Rate Constant 50 mg/L Estimating K using graph paper ! 25 mg/L 12. 5 mg/L Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 If the half-life is 6 hr, what is K? K = 0. 693 / T½ 0 4 8 12 16 20 24 28 K

Determining Elimination Rate Constant 50 mg/L Estimating K using graph paper ! 25 mg/L 12. 5 mg/L Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 If the half-life is 6 hr, what is K? K = 0. 693 / T½ 0 4 8 12 16 20 24 28 = 0. 693 / 6 hr = 0. 1155 hr-1 K

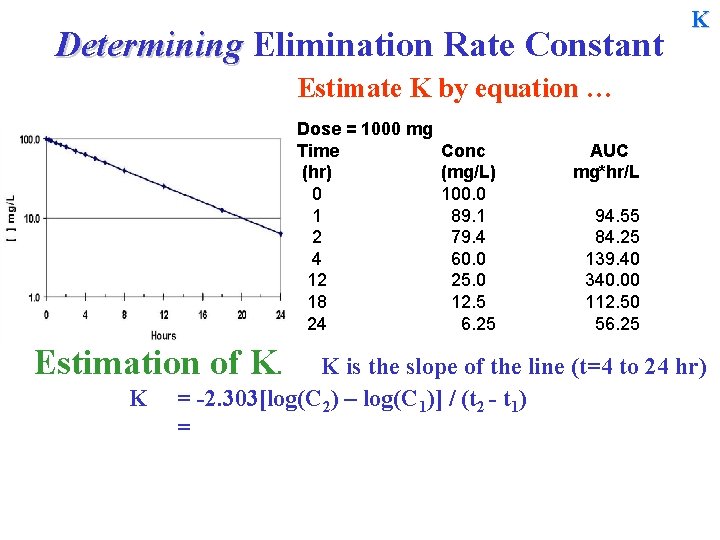
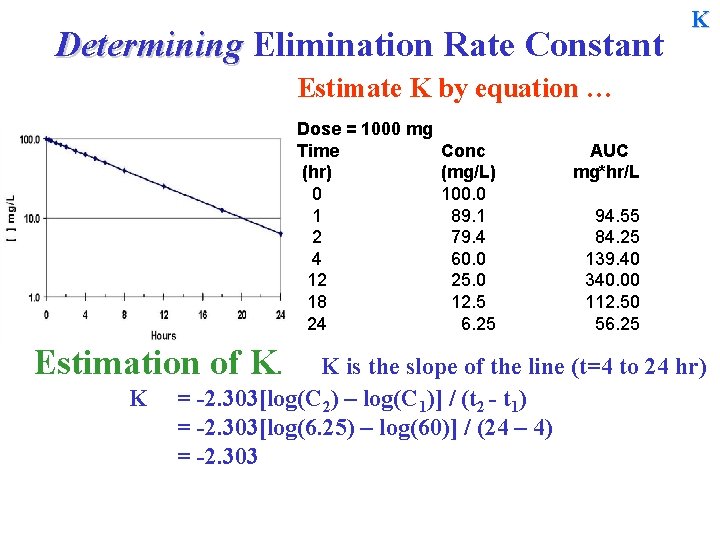
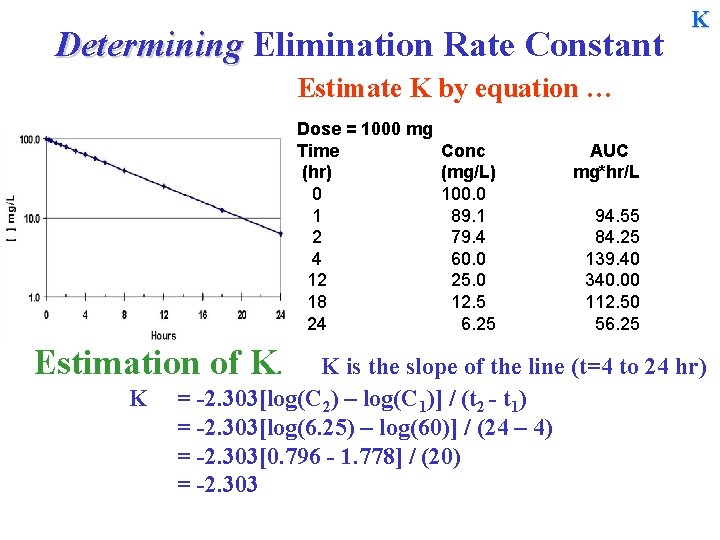
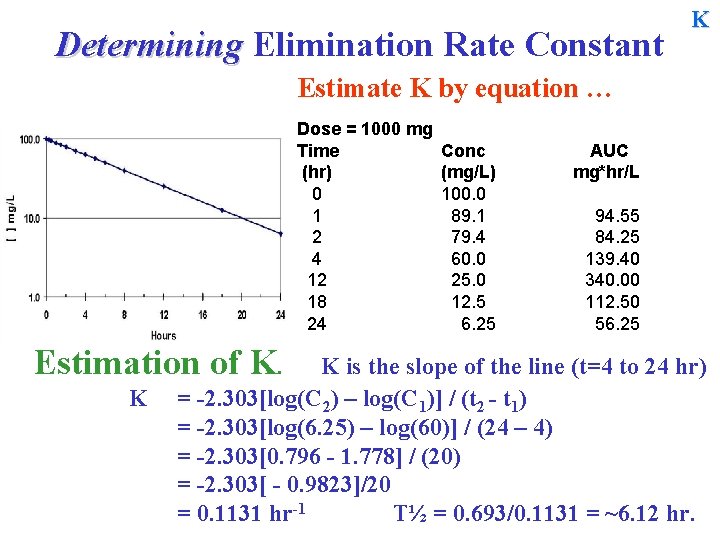
Determining Elimination Rate Constant K Estimate K by equation … Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 Estimation of K. K Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 AUC mg*hr/L 94. 55 84. 25 139. 40 340. 00 112. 50 56. 25 K is the slope of the line (t=4 to 24 hr) = -2. 303[log(C 2) – log(C 1)] / (t 2 - t 1) =

Determining Elimination Rate Constant K Estimate K by equation … Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 Estimation of K. K Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 AUC mg*hr/L 94. 55 84. 25 139. 40 340. 00 112. 50 56. 25 K is the slope of the line (t=4 to 24 hr) = -2. 303[log(C 2) – log(C 1)] / (t 2 - t 1) = -2. 303[log(6. 25) – log(60)] / (24 – 4) = -2. 303

Determining Elimination Rate Constant K Estimate K by equation … Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 Estimation of K. K Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 AUC mg*hr/L 94. 55 84. 25 139. 40 340. 00 112. 50 56. 25 K is the slope of the line (t=4 to 24 hr) = -2. 303[log(C 2) – log(C 1)] / (t 2 - t 1) = -2. 303[log(6. 25) – log(60)] / (24 – 4) = -2. 303[0. 796 - 1. 778] / (20) = -2. 303

Determining Elimination Rate Constant K Estimate K by equation … Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 Estimation of K. K Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 AUC mg*hr/L 94. 55 84. 25 139. 40 340. 00 112. 50 56. 25 K is the slope of the line (t=4 to 24 hr) = -2. 303[log(C 2) – log(C 1)] / (t 2 - t 1) = -2. 303[log(6. 25) – log(60)] / (24 – 4) = -2. 303[0. 796 - 1. 778] / (20) = -2. 303[ - 0. 9823]/20 = 0. 1131 hr-1 T½ = 0. 693/0. 1131 = ~6. 12 hr.

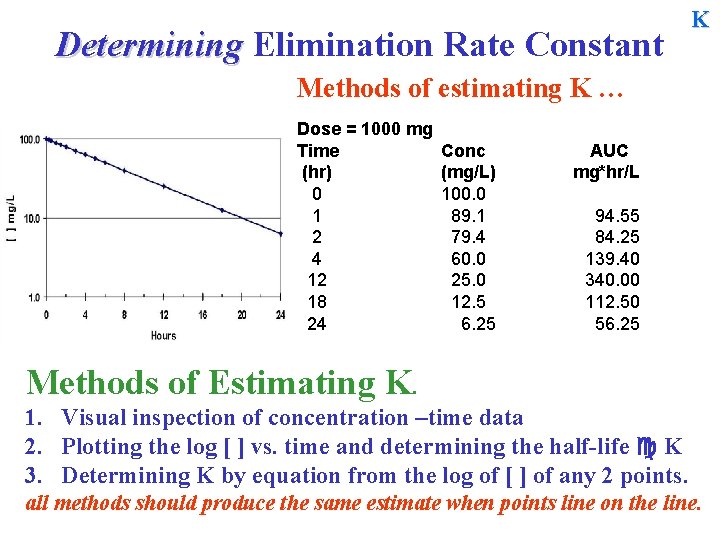
Determining Elimination Rate Constant K Methods of estimating K … Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 AUC mg*hr/L 94. 55 84. 25 139. 40 340. 00 112. 50 56. 25 Methods of Estimating K. 1. Visual inspection of concentration –time data 2. Plotting the log [ ] vs. time and determining the half-life K 3. Determining K by equation from the log of [ ] of any 2 points. all methods should produce the same estimate when points line on the line.

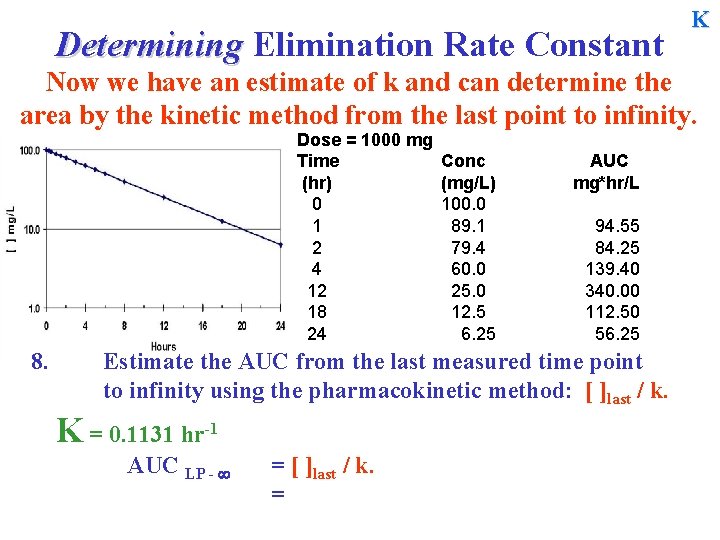
Determining Elimination Rate Constant K Now we have an estimate of k and can determine the area by the kinetic method from the last point to infinity. Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 8. Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 AUC mg*hr/L 94. 55 84. 25 139. 40 340. 00 112. 50 56. 25 Estimate the AUC from the last measured time point to infinity using the pharmacokinetic method: [ ]last / k. K = 0. 1131 hr-1 AUC LP - = [ ]last / k. =

Determining Elimination Rate Constant Example from last day …K? Dose = 1000 mg Time (hr) 0 1 2 4 12 18 24 8. Conc (mg/L) 100. 0 89. 1 79. 4 60. 0 25. 0 12. 5 6. 25 AUC mg*hr/L 94. 55 84. 25 139. 40 340. 00 112. 50 56. 25 Estimate the AUC from the last measured time point to infinity using the pharmacokinetic method: [ ]last / k. K = 0. 1131 hr-1 AUC LP - = [ ]last / k. = 6. 25 / 0. 1131 = 55. 25 mg*hr/L =6. 25 / 0. 1155 =54. 11 mg*hr/L K

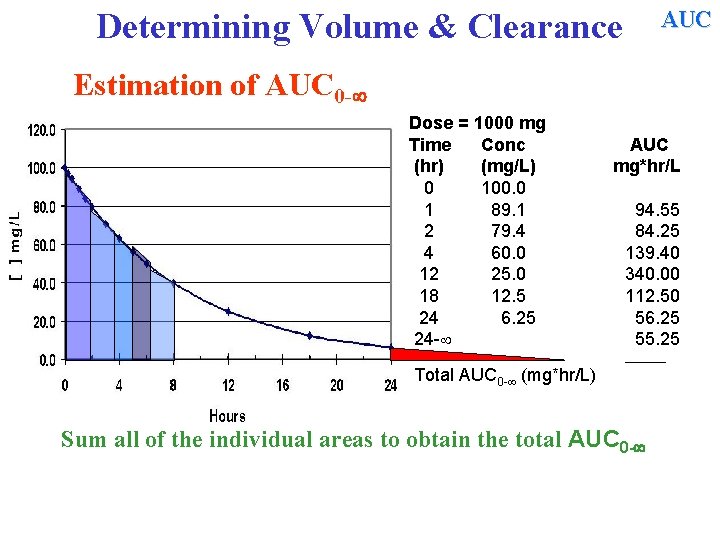
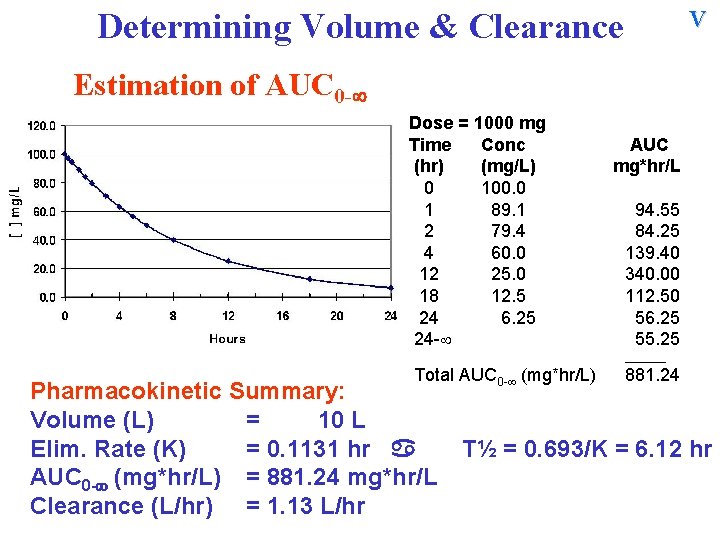
AUC Determining Volume & Clearance Estimation of AUC 0 - Dose = 1000 mg Time Conc (hr) (mg/L) 0 100. 0 1 89. 1 2 79. 4 4 60. 0 12 25. 0 18 12. 5 24 6. 25 24 - AUC mg*hr/L 94. 55 84. 25 139. 40 340. 00 112. 50 56. 25 55. 25 ______ Total AUC 0 - (mg*hr/L) Sum all of the individual areas to obtain the total AUC 0 -

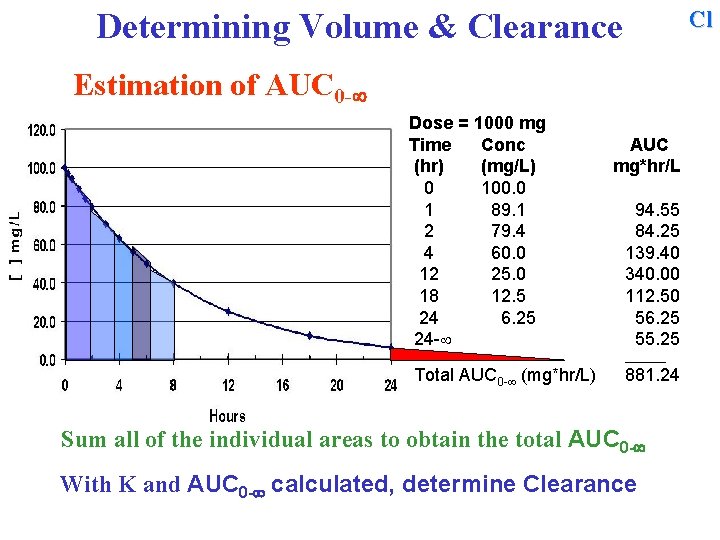
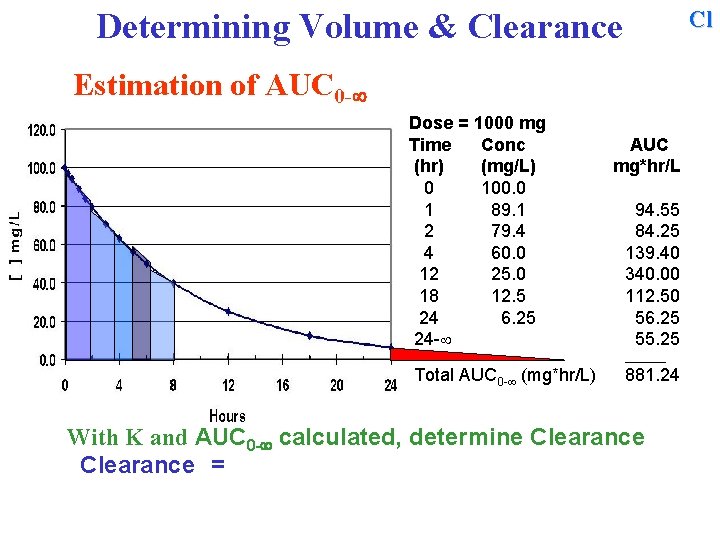
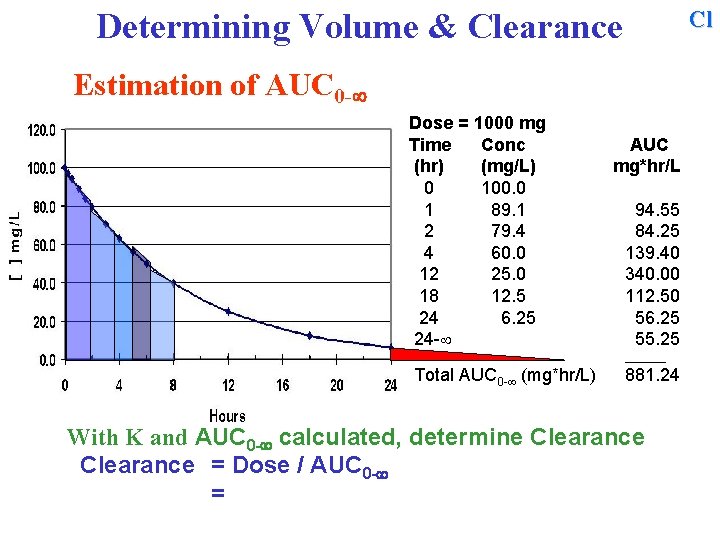
Cl Determining Volume & Clearance Estimation of AUC 0 - Dose = 1000 mg Time Conc (hr) (mg/L) 0 100. 0 1 89. 1 2 79. 4 4 60. 0 12 25. 0 18 12. 5 24 6. 25 24 - AUC mg*hr/L 94. 55 84. 25 139. 40 340. 00 112. 50 56. 25 55. 25 ______ Total AUC 0 - (mg*hr/L) 881. 24 Sum all of the individual areas to obtain the total AUC 0 - With K and AUC 0 - calculated, determine Clearance

Cl Determining Volume & Clearance Estimation of AUC 0 - Dose = 1000 mg Time Conc (hr) (mg/L) 0 100. 0 1 89. 1 2 79. 4 4 60. 0 12 25. 0 18 12. 5 24 6. 25 24 - AUC mg*hr/L 94. 55 84. 25 139. 40 340. 00 112. 50 56. 25 55. 25 ______ Total AUC 0 - (mg*hr/L) 881. 24 With K and AUC 0 - calculated, determine Clearance =

Cl Determining Volume & Clearance Estimation of AUC 0 - Dose = 1000 mg Time Conc (hr) (mg/L) 0 100. 0 1 89. 1 2 79. 4 4 60. 0 12 25. 0 18 12. 5 24 6. 25 24 - AUC mg*hr/L 94. 55 84. 25 139. 40 340. 00 112. 50 56. 25 55. 25 ______ Total AUC 0 - (mg*hr/L) 881. 24 With K and AUC 0 - calculated, determine Clearance = Dose / AUC 0 - =

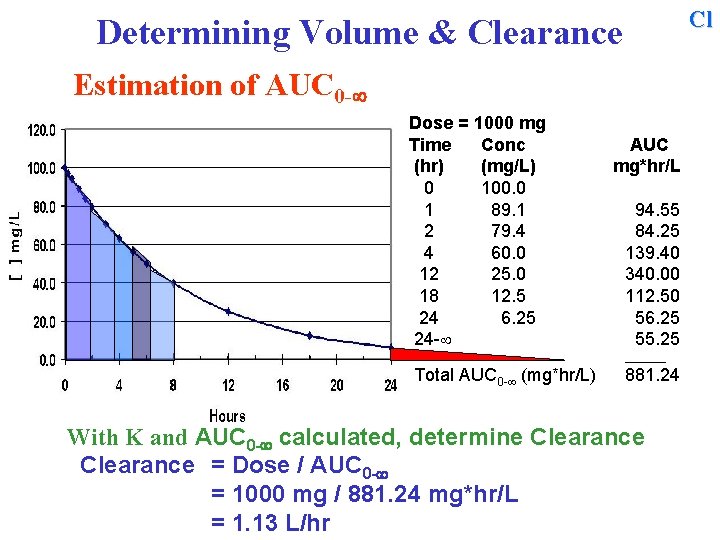
Cl Determining Volume & Clearance Estimation of AUC 0 - Dose = 1000 mg Time Conc (hr) (mg/L) 0 100. 0 1 89. 1 2 79. 4 4 60. 0 12 25. 0 18 12. 5 24 6. 25 24 - AUC mg*hr/L 94. 55 84. 25 139. 40 340. 00 112. 50 56. 25 55. 25 ______ Total AUC 0 - (mg*hr/L) 881. 24 With K and AUC 0 - calculated, determine Clearance = Dose / AUC 0 - = 1000 mg / 881. 24 mg*hr/L = 1. 13 L/hr

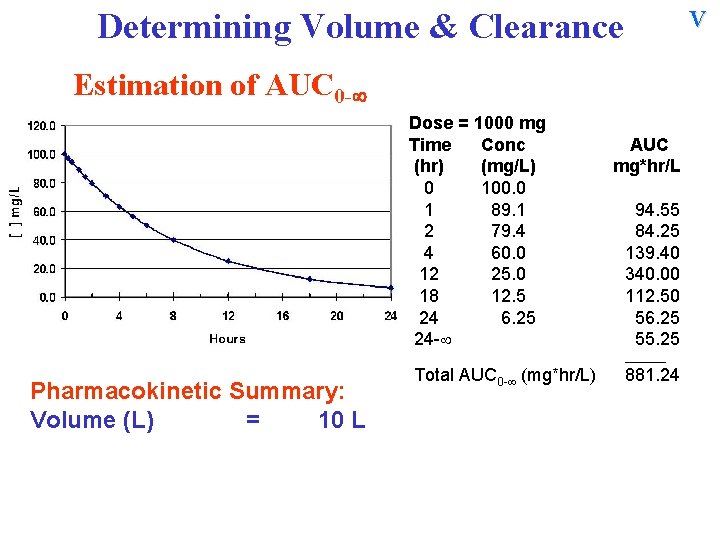
V Determining Volume & Clearance Estimation of AUC 0 - Dose = 1000 mg Time Conc (hr) (mg/L) 0 100. 0 1 89. 1 2 79. 4 4 60. 0 12 25. 0 18 12. 5 24 6. 25 24 - AUC mg*hr/L 94. 55 84. 25 139. 40 340. 00 112. 50 56. 25 55. 25 ______ Pharmacokinetic Summary: Volume (L) = 10 L Total AUC 0 - (mg*hr/L) 881. 24

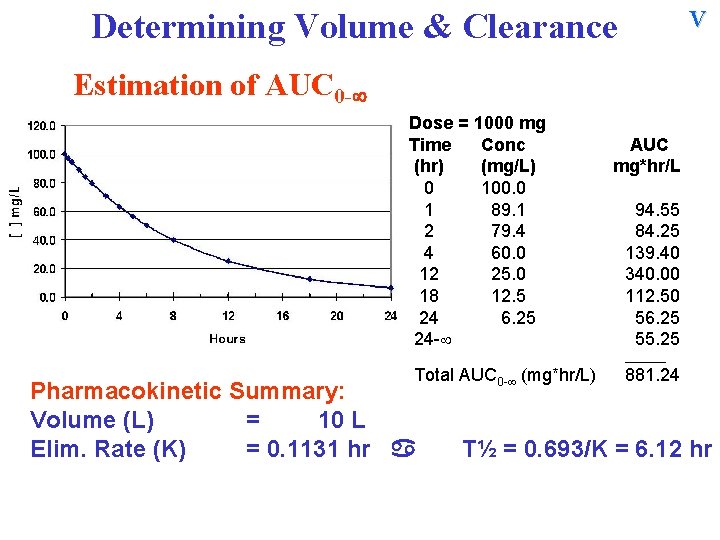
V Determining Volume & Clearance Estimation of AUC 0 - Dose = 1000 mg Time Conc (hr) (mg/L) 0 100. 0 1 89. 1 2 79. 4 4 60. 0 12 25. 0 18 12. 5 24 6. 25 24 - AUC mg*hr/L 94. 55 84. 25 139. 40 340. 00 112. 50 56. 25 55. 25 ______ Total AUC 0 - (mg*hr/L) Pharmacokinetic Summary: Volume (L) = 10 L Elim. Rate (K) = 0. 1131 hr 881. 24 T½ = 0. 693/K = 6. 12 hr

V Determining Volume & Clearance Estimation of AUC 0 - Dose = 1000 mg Time Conc (hr) (mg/L) 0 100. 0 1 89. 1 2 79. 4 4 60. 0 12 25. 0 18 12. 5 24 6. 25 24 - AUC mg*hr/L 94. 55 84. 25 139. 40 340. 00 112. 50 56. 25 55. 25 ______ Total AUC 0 - (mg*hr/L) Pharmacokinetic Summary: Volume (L) = 10 L Elim. Rate (K) = 0. 1131 hr AUC 0 - (mg*hr/L) = 881. 24 mg*hr/L Clearance (L/hr) = 1. 13 L/hr 881. 24 T½ = 0. 693/K = 6. 12 hr

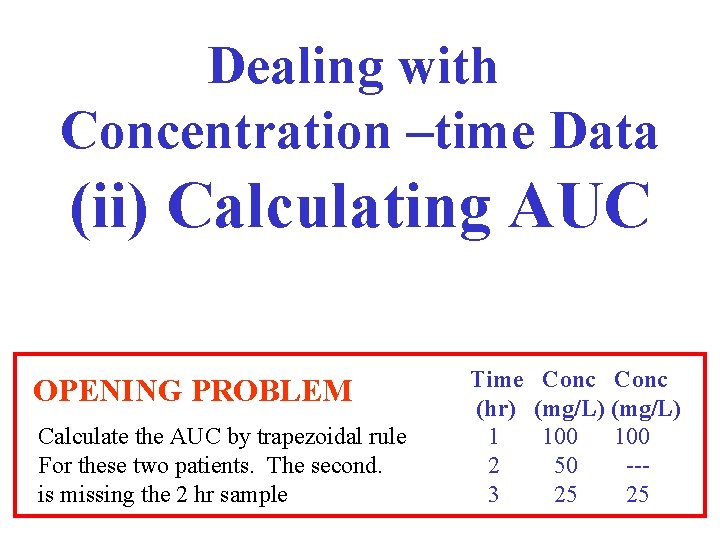
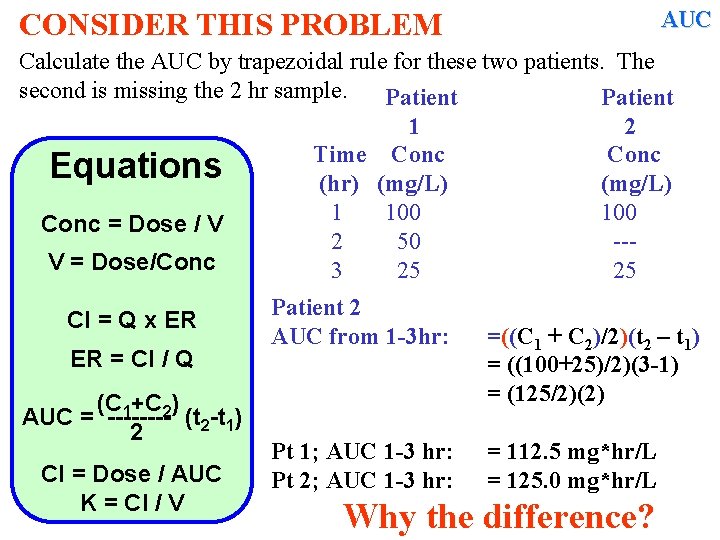
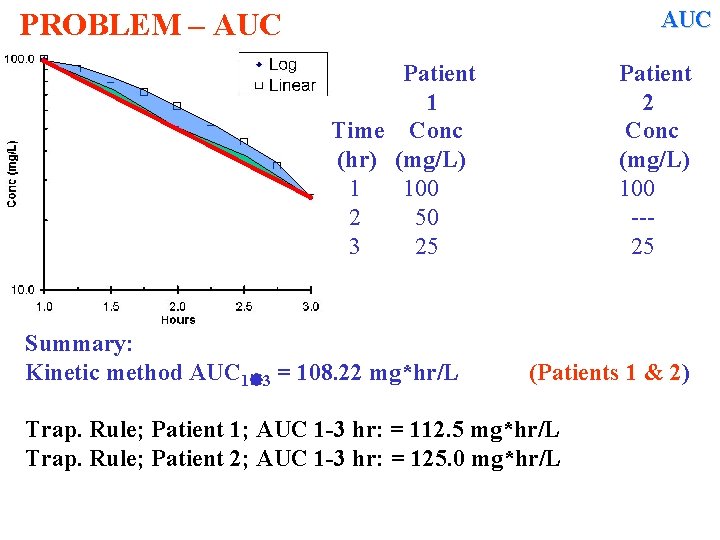
Dealing with Concentration –time Data (ii) Calculating AUC OPENING PROBLEM Calculate the AUC by trapezoidal rule For these two patients. The second. is missing the 2 hr sample Time Conc (hr) (mg/L) 1 100 2 50 --3 25 25

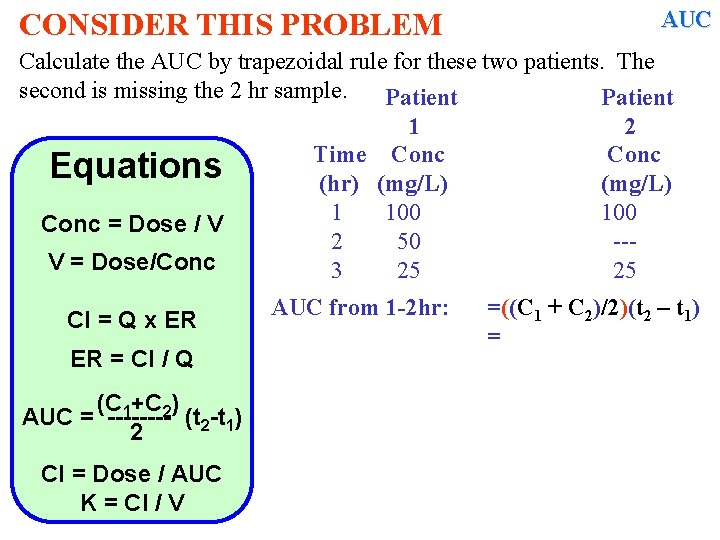
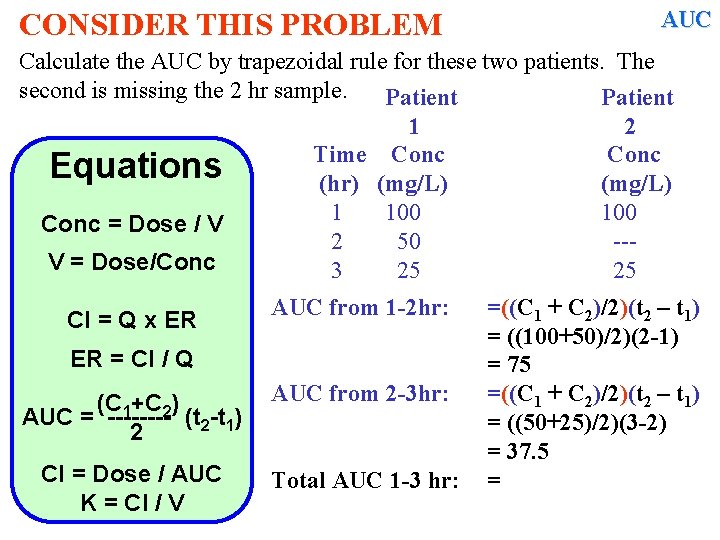
CONSIDER THIS PROBLEM AUC Calculate the AUC by trapezoidal rule for these two patients. The second is missing the 2 hr sample. Patient 1 2 Time Conc Equations (hr) (mg/L) 1 100 Conc = Dose / V 2 50 --V = Dose/Conc 3 25 25 Cl = Q x ER ER = Cl / Q (C 1+C 2) AUC = ---- (t 2 -t 1) 2 Cl = Dose / AUC K = Cl / V AUC from 1 -2 hr: =((C 1 + C 2)/2)(t 2 – t 1) =

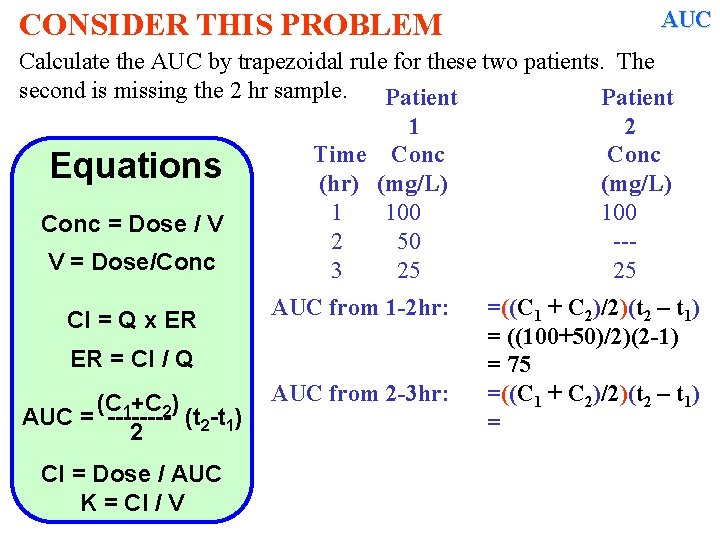
CONSIDER THIS PROBLEM AUC Calculate the AUC by trapezoidal rule for these two patients. The second is missing the 2 hr sample. Patient 1 2 Time Conc Equations (hr) (mg/L) 1 100 Conc = Dose / V 2 50 --V = Dose/Conc 3 25 25 Cl = Q x ER AUC from 1 -2 hr: ER = Cl / Q (C 1+C 2) AUC = ---- (t 2 -t 1) 2 Cl = Dose / AUC K = Cl / V AUC from 2 -3 hr: =((C 1 + C 2)/2)(t 2 – t 1) = ((100+50)/2)(2 -1) = 75 =((C 1 + C 2)/2)(t 2 – t 1) =

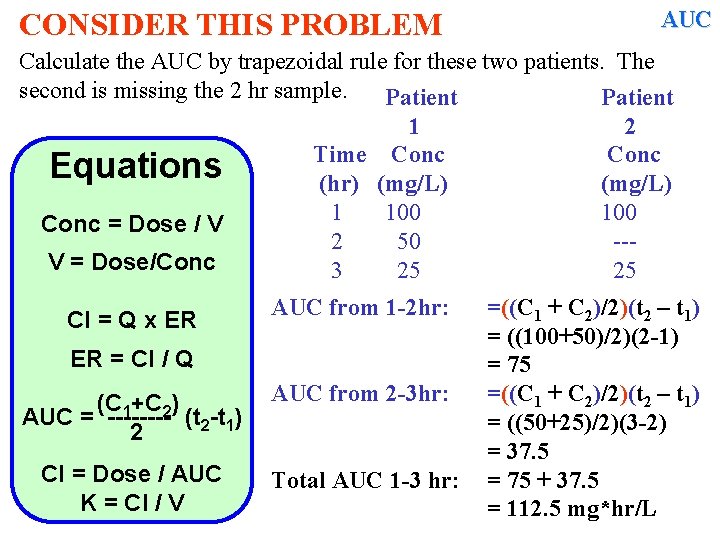
CONSIDER THIS PROBLEM AUC Calculate the AUC by trapezoidal rule for these two patients. The second is missing the 2 hr sample. Patient 1 2 Time Conc Equations (hr) (mg/L) 1 100 Conc = Dose / V 2 50 --V = Dose/Conc 3 25 25 Cl = Q x ER AUC from 1 -2 hr: ER = Cl / Q (C 1+C 2) AUC = ---- (t 2 -t 1) 2 AUC from 2 -3 hr: Cl = Dose / AUC K = Cl / V Total AUC 1 -3 hr: =((C 1 + C 2)/2)(t 2 – t 1) = ((100+50)/2)(2 -1) = 75 =((C 1 + C 2)/2)(t 2 – t 1) = ((50+25)/2)(3 -2) = 37. 5 =

CONSIDER THIS PROBLEM AUC Calculate the AUC by trapezoidal rule for these two patients. The second is missing the 2 hr sample. Patient 1 2 Time Conc Equations (hr) (mg/L) 1 100 Conc = Dose / V 2 50 --V = Dose/Conc 3 25 25 Cl = Q x ER AUC from 1 -2 hr: ER = Cl / Q (C 1+C 2) AUC = ---- (t 2 -t 1) 2 AUC from 2 -3 hr: Cl = Dose / AUC K = Cl / V Total AUC 1 -3 hr: =((C 1 + C 2)/2)(t 2 – t 1) = ((100+50)/2)(2 -1) = 75 =((C 1 + C 2)/2)(t 2 – t 1) = ((50+25)/2)(3 -2) = 37. 5 = 75 + 37. 5 = 112. 5 mg*hr/L

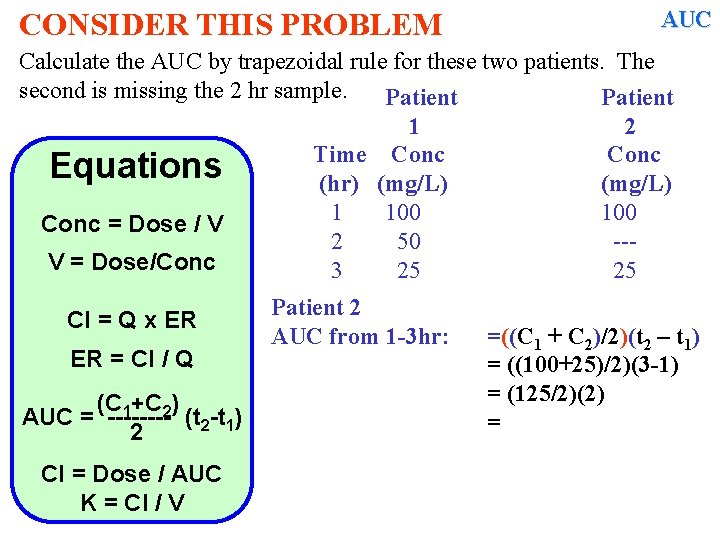
CONSIDER THIS PROBLEM AUC Calculate the AUC by trapezoidal rule for these two patients. The second is missing the 2 hr sample. Patient 1 2 Time Conc Equations (hr) (mg/L) 1 100 Conc = Dose / V 2 50 --V = Dose/Conc 3 25 25 Cl = Q x ER ER = Cl / Q (C 1+C 2) AUC = ---- (t 2 -t 1) 2 Cl = Dose / AUC K = Cl / V Patient 2 AUC from 1 -3 hr: =((C 1 + C 2)/2)(t 2 – t 1) = ((100+25)/2)(3 -1) = (125/2)(2) =

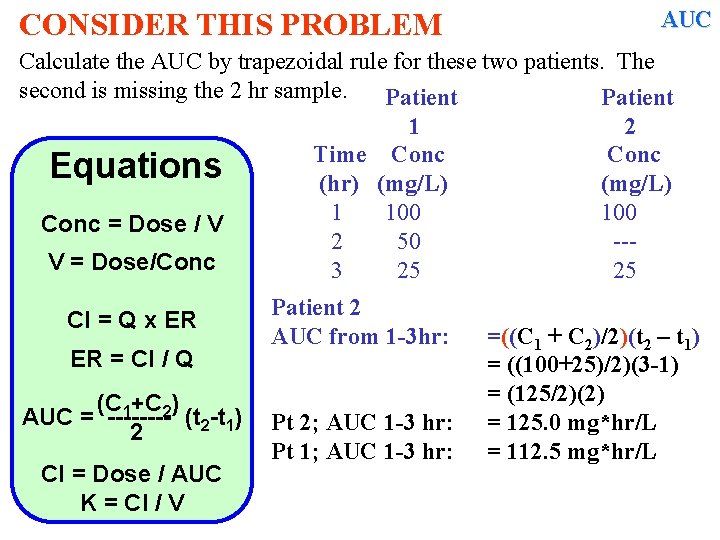
CONSIDER THIS PROBLEM AUC Calculate the AUC by trapezoidal rule for these two patients. The second is missing the 2 hr sample. Patient 1 2 Time Conc Equations (hr) (mg/L) 1 100 Conc = Dose / V 2 50 --V = Dose/Conc 3 25 25 Cl = Q x ER ER = Cl / Q (C 1+C 2) AUC = ---- (t 2 -t 1) 2 Cl = Dose / AUC K = Cl / V Patient 2 AUC from 1 -3 hr: Pt 2; AUC 1 -3 hr: Pt 1; AUC 1 -3 hr: =((C 1 + C 2)/2)(t 2 – t 1) = ((100+25)/2)(3 -1) = (125/2)(2) = 125. 0 mg*hr/L = 112. 5 mg*hr/L

AUC CONSIDER THIS PROBLEM Calculate the AUC by trapezoidal rule for these two patients. The second is missing the 2 hr sample. Patient 1 2 Time Conc Equations (hr) (mg/L) 1 100 Conc = Dose / V 2 50 --V = Dose/Conc 3 25 25 Cl = Q x ER ER = Cl / Q (C 1+C 2) AUC = ---- (t 2 -t 1) 2 Cl = Dose / AUC K = Cl / V Patient 2 AUC from 1 -3 hr: Pt 1; AUC 1 -3 hr: Pt 2; AUC 1 -3 hr: =((C 1 + C 2)/2)(t 2 – t 1) = ((100+25)/2)(3 -1) = (125/2)(2) = 112. 5 mg*hr/L = 125. 0 mg*hr/L Why the difference?
![AUC PROBLEM AUC Trapezoidal rule assumes a linear decline in with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-51.jpg)
AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with time. Examine Patient 1 Data: Given Actual Time Conc Calc (hr) (mg/L) Conc 1. 00 100 1. 25 84. 1 1. 50 70. 7 1. 75 59. 5 2. 00 50 50. 0 2. 25 42. 1 2. 50 35. 4 2. 75 29. 7 3. 00 25 25. 0
![AUC PROBLEM AUC Trapezoidal rule assumes a linear decline in with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-52.jpg)
AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with time. Examine Patient 1 Data: Given Actual Arith Time Conc Calc (hr) (mg/L) Conc 1. 00 100 1. 25 84. 1 87. 5 1. 50 70. 7 75. 0 1. 75 59. 5 62. 5 2. 00 50 50. 0 2. 25 42. 1 43. 75 2. 50 35. 4 37. 50 2. 75 29. 7 31. 25 3. 00 25 25. 00
![AUC PROBLEM AUC Trapezoidal rule assumes a linear decline in with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-53.jpg)
AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with time. 50 mg/L 25 mg/L Examine Patient 1 Data: Given Actual Arith Time Conc Calc (hr) (mg/L) Conc 1. 00 100 1. 25 84. 1 87. 5 1. 50 70. 7 75. 0 1. 75 59. 5 62. 5 2. 00 50 50. 0 2. 25 42. 1 43. 75 2. 50 35. 4 37. 50 2. 75 29. 7 31. 25 3. 00 25 25. 00
![AUC PROBLEM AUC Trapezoidal rule assumes a linear decline in with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-54.jpg)
AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with time. 50 mg/L 25 mg/L Examine Patient 1 Data: Given Actual Arith Time Conc Calc (hr) (mg/L) Conc. Line in Conc 1. 00 100 red shows 100 the actual 87. 5 1. 25 84. 1 concentration 1. 50 70. 7 75. 0 that would 62. 5 be 1. 75 59. 5 2. 00 50 50. 0 present 50. 0 given 2. 25 42. 1 the initial 43. 75 concentration 2. 50 35. 4 37. 50 and half-life. 2. 75 29. 7 31. 25 3. 00 25 25. 00
![AUC PROBLEM AUC Trapezoidal rule assumes a linear decline in with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-55.jpg)
AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with time. 50 mg/L 25 mg/L Examine Patient 1 Data: Given Actual Arith Time Conc Calc (hr) (mg/L) Conc 1. 00 100 1. 25 84. 1 87. 5 1. 50 70. 7 75. 0 1. 75 59. 5 62. 5 2. 00 50 50. 0 2. 25 42. 1 43. 75 2. 50 35. 4 37. 50 2. 75 29. 7 31. 25 3. 00 25 25. 00 Calculated concentration given by red line in previous slide
![AUC PROBLEM AUC Trapezoidal rule assumes a linear decline in with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-56.jpg)
AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with time. 50 mg/L 25 mg/L Examine Patient 1 Data: Given Actual Arith Time Conc Calc (hr) (mg/L) Conc 1. 00 100 1. 25 84. 1 87. 5 1. 50 70. 7 75. 0 1. 75 59. 5 62. 5 2. 00 50 50. 0 2. 25 42. 1 43. 75 2. 50 35. 4 37. 50 2. 75 29. 7 31. 25 3. 00 25 25. 00 Calculated concentration using Ct = Co e(-Kt)
![AUC PROBLEM AUC Trapezoidal rule assumes a linear decline in with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-57.jpg)
AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with time. 50 mg/L Notice 37. 5 vs. 35. 4 Examine Patient 1 Data: Given Actual Arith Time Conc Calc (hr) (mg/L) Conc 1. 00 100 1. 25 84. 1 87. 5 1. 50 70. 7 75. 0 1. 75 59. 5 62. 5 2. 00 50 50. 0 2. 25 42. 1 43. 75 2. 50 35. 4 37. 50 2. 75 29. 7 31. 25 3. 00 25 25. 00 Arithmetically calculated concentration
![AUC PROBLEM AUC Trapezoidal rule assumes a linear decline in with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-58.jpg)
AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with time. 50 mg/L Notice 37. 5 vs. 35. 4 Examine Patient 1 Data: Given Actual Arith Time Conc Calc (hr) (mg/L) Conc 1. 00 100 1. 25 84. 1 87. 5 1. 50 70. 7 75. 0 1. 75 59. 5 62. 5 2. 00 50 50. 0 2. 25 42. 1 43. 75 2. 50 35. 4 37. 50 2. 75 29. 7 31. 25 3. 00 25 25. 00 recall AUC Calc from 2 -3 hr AUC = ((C 1 + C 2)/2)(t 2 – t 1) = ((50+25)/2)(3 -2) = 37. 5
![AUC PROBLEM AUC Trapezoidal rule assumes a linear decline in with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-59.jpg)
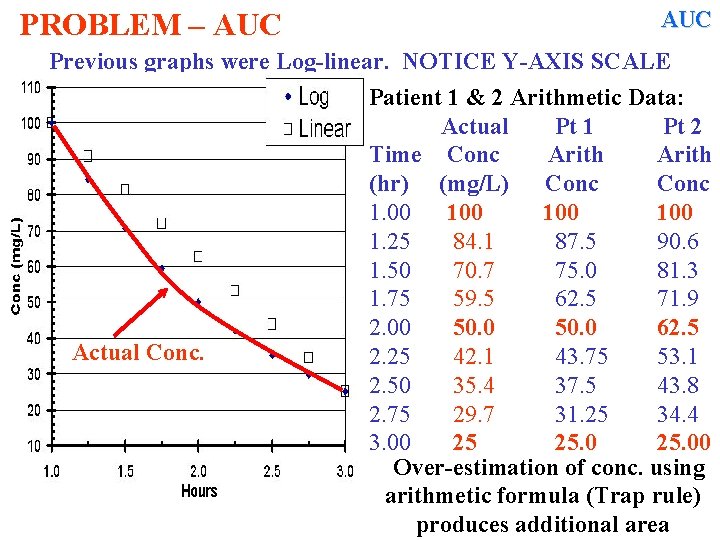
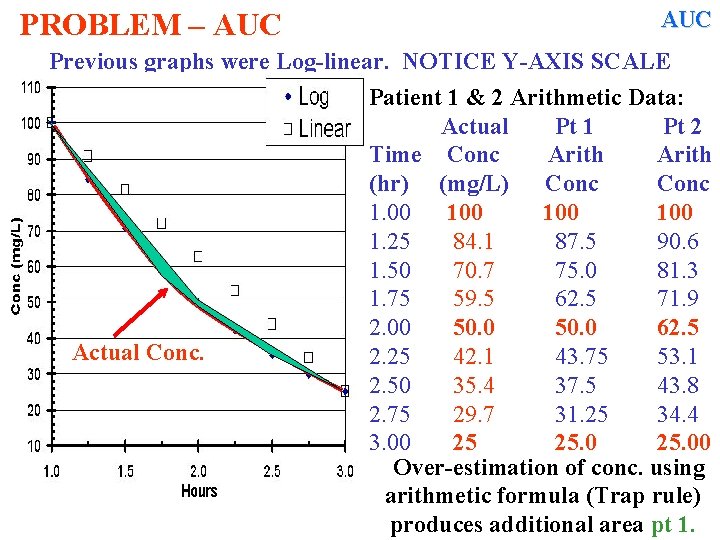
AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with time. 50 mg/L Notice 37. 5 vs. 35. 4 Examine Patient 1 Data: Given Actual Arith Time Conc Calc (hr) (mg/L) Conc 1. 00 100 1. 25 84. 1 87. 5 1. 50 70. 7 75. 0 1. 75 59. 5 62. 5 2. 00 50 50. 0 2. 25 42. 1 43. 75 2. 50 35. 4 37. 50 2. 75 29. 7 31. 25 3. 00 25 25. 00 Over-estimation of conc. using arithmetic formula Trap rule produces additional (green) area
![AUC PROBLEM AUC Trapezoidal rule assumes a linear decline in with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-60.jpg)
AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with time. 100 mg/L 25 mg/L Examine Patient 2 Data: Given Actual Arith Time Conc Calc (hr) (mg/L) Conc 1. 00 100 1. 25 84. 1 90. 6 1. 50 70. 7 81. 3 1. 75 59. 5 71. 9 2. 00 -50. 0 62. 5 2. 25 42. 1 53. 1 2. 50 35. 4 43. 8 2. 75 29. 7 34. 4 3. 00 25 25. 00
![AUC PROBLEM AUC Trapezoidal rule assumes a linear decline in with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-61.jpg)
AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with time. 100 mg/L 25 mg/L Examine Patient 2 Data: Given Actual Arith Again, Time Conc Calc RED Line (hr) (mg/L) Conc shows 1. 00 100 100 the actual 1. 25 84. 1 90. 6 concentration 1. 50 70. 7 81. 3 that would be 1. 75 59. 5 71. 9 present 2. 00 -50. 0 62. 5 given the initial 2. 25 42. 1 53. 1 concentration 2. 50 35. 4 43. 8 and half-life. 2. 75 29. 7 34. 4 3. 00 25 25. 00
![AUC PROBLEM AUC Trapezoidal rule assumes a linear decline in with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-62.jpg)
AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with time. 100 mg/L 25 mg/L Examine Patient 2 Data: Given Actual Arith Time Conc Calc Conc (hr) (mg/L) Conc calc 1. 00 100 100 Using 1. 25 84. 1 90. 6 Ct=Coe(-Kt) 1. 50 70. 7 81. 3 1. 75 59. 5 71. 9 This 2. 00 -50. 0 set 62. 5 of 2. 25 42. 1 conc 53. 1 is 2. 50 35. 4 identical 43. 8 2. 75 29. 7 to Pt 34. 4 1. 3. 00 25 25. 00
![AUC PROBLEM AUC Trapezoidal rule assumes a linear decline in with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-63.jpg)
AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with time. Notice 62. 5 vs. 50. 0 25 mg/L Examine Patient 2 Data: Given Actual Arith Time Conc Calc (hr) (mg/L) Conc 1. 00 100 1. 25 84. 1 90. 6 1. 50 70. 7 81. 3 1. 75 59. 5 71. 9 2. 00 -50. 0 62. 5 2. 25 42. 1 53. 1 2. 50 35. 4 43. 8 2. 75 29. 7 34. 4 3. 00 25 25. 00 Arithmetically calculated concentrations
![AUC PROBLEM AUC Trapezoidal rule assumes a linear decline in with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-64.jpg)
AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with time. Notice 62. 5 vs. 50. 0 25 mg/L Examine Patient 2 Data: Given Actual Arith Time Conc Calc (hr) (mg/L) Conc 1. 00 100 1. 25 84. 1 90. 6 1. 50 70. 7 81. 3 1. 75 59. 5 71. 9 2. 00 -50. 0 62. 5 2. 25 42. 1 53. 1 2. 50 35. 4 43. 8 2. 75 29. 7 34. 4 3. 00 25 25. 00 Over-estimation of conc. using arithmetic formula (Trap rule) produces additional (blue) area
![AUC PROBLEM AUC Trapezoidal rule assumes a linear decline in with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-65.jpg)
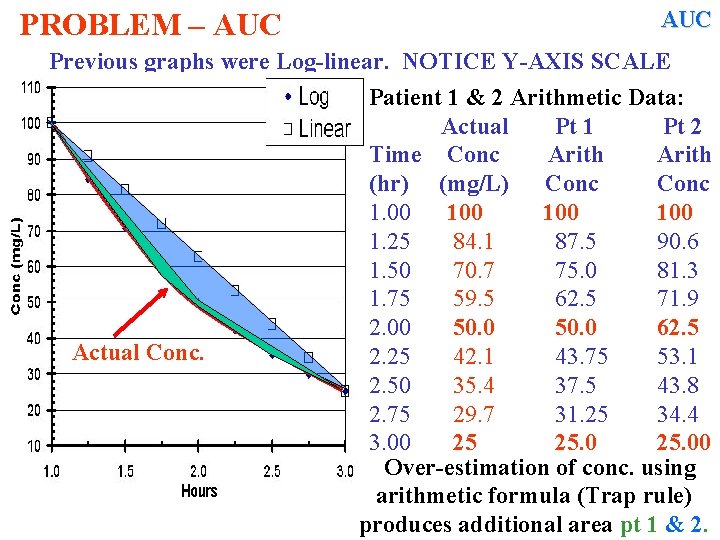
AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with time. Patient 1 & 2 Arithmetic Data: Actual Pt 1 Pt 2 Time Conc Arith (hr) (mg/L) Conc 1. 00 100 1. 25 84. 1 87. 5 90. 6 1. 50 70. 7 75. 0 81. 3 1. 75 59. 5 62. 5 71. 9 2. 00 50. 0 62. 5 2. 25 42. 1 43. 75 53. 1 2. 50 35. 4 37. 5 43. 8 2. 75 29. 7 31. 25 34. 4 3. 00 25 25. 00 Over-estimation of conc. using arithmetic formula (Trap rule) produces additional area

PROBLEM – AUC Previous graphs were Log-linear. NOTICE Y-AXIS SCALE Actual Conc. Patient 1 & 2 Arithmetic Data: Actual Pt 1 Pt 2 Time Conc Arith (hr) (mg/L) Conc 1. 00 100 1. 25 84. 1 87. 5 90. 6 1. 50 70. 7 75. 0 81. 3 1. 75 59. 5 62. 5 71. 9 2. 00 50. 0 62. 5 2. 25 42. 1 43. 75 53. 1 2. 50 35. 4 37. 5 43. 8 2. 75 29. 7 31. 25 34. 4 3. 00 25 25. 00 Over-estimation of conc. using arithmetic formula (Trap rule) produces additional area

PROBLEM – AUC Previous graphs were Log-linear. NOTICE Y-AXIS SCALE Actual Conc. Patient 1 & 2 Arithmetic Data: Actual Pt 1 Pt 2 Time Conc Arith (hr) (mg/L) Conc 1. 00 100 1. 25 84. 1 87. 5 90. 6 1. 50 70. 7 75. 0 81. 3 1. 75 59. 5 62. 5 71. 9 2. 00 50. 0 62. 5 2. 25 42. 1 43. 75 53. 1 2. 50 35. 4 37. 5 43. 8 2. 75 29. 7 31. 25 34. 4 3. 00 25 25. 00 Over-estimation of conc. using arithmetic formula (Trap rule) produces additional area pt 1.

PROBLEM – AUC Previous graphs were Log-linear. NOTICE Y-AXIS SCALE Actual Conc. Patient 1 & 2 Arithmetic Data: Actual Pt 1 Pt 2 Time Conc Arith (hr) (mg/L) Conc 1. 00 100 1. 25 84. 1 87. 5 90. 6 1. 50 70. 7 75. 0 81. 3 1. 75 59. 5 62. 5 71. 9 2. 00 50. 0 62. 5 2. 25 42. 1 43. 75 53. 1 2. 50 35. 4 37. 5 43. 8 2. 75 29. 7 31. 25 34. 4 3. 00 25 25. 00 Over-estimation of conc. using arithmetic formula (Trap rule) produces additional area pt 1 & 2.
![AUC PROBLEM AUC Trapezoidal rule assumes a linear decline in with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-69.jpg)
AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with time. Patient 1 Time Conc (hr) (mg/L) 1 100 2 50 3 25 Patient 2 Conc (mg/L) 100 --25 Trapezoidal rule assumes a linear decline in [ ] with time and overestimates AUC. Patient 1; AUC 1 -3 hr: = 112. 5 mg*hr/L Patient 2; AUC 1 -3 hr: = 125. 0 mg*hr/L
![AUC PROBLEM AUC Trapezoidal rule assumes a linear decline in with AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-70.jpg)
AUC PROBLEM – AUC Trapezoidal rule assumes a linear decline in [ ] with time. Time (hr) 1 2 3 Patient 1 Conc (mg/L) 100 50 25 Patient 2 Conc (mg/L) 100 --25 AUC 112. 5 125. 0 mg*hr/L So … if concentrations are declining in log-linear fashion, can we not estimate AUC by a method which more closely approximates the change in concentration? … PCK method ?

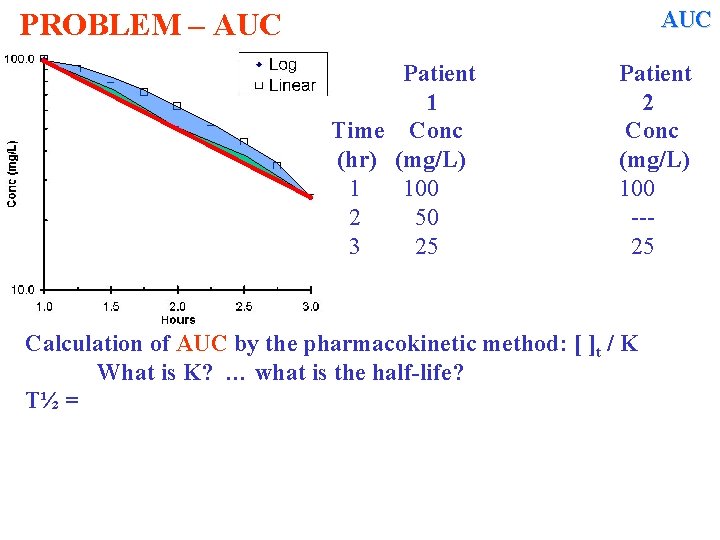
AUC PROBLEM – AUC Patient 1 Time Conc (hr) (mg/L) 1 100 2 50 3 25 Patient 2 Conc (mg/L) 100 --25 Calculation of AUC by the pharmacokinetic method: [ ]t / K What is K? … what is the half-life? T½ =

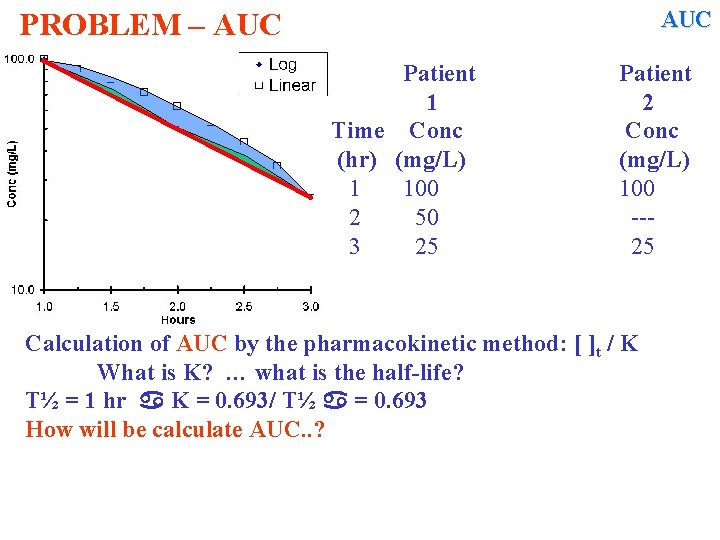
AUC PROBLEM – AUC Patient 1 Time Conc (hr) (mg/L) 1 100 2 50 3 25 Patient 2 Conc (mg/L) 100 --25 Calculation of AUC by the pharmacokinetic method: [ ]t / K What is K? … what is the half-life? T½ = 1 hr K = 0. 693/ T½ = 0. 693 How will be calculate AUC. . ?

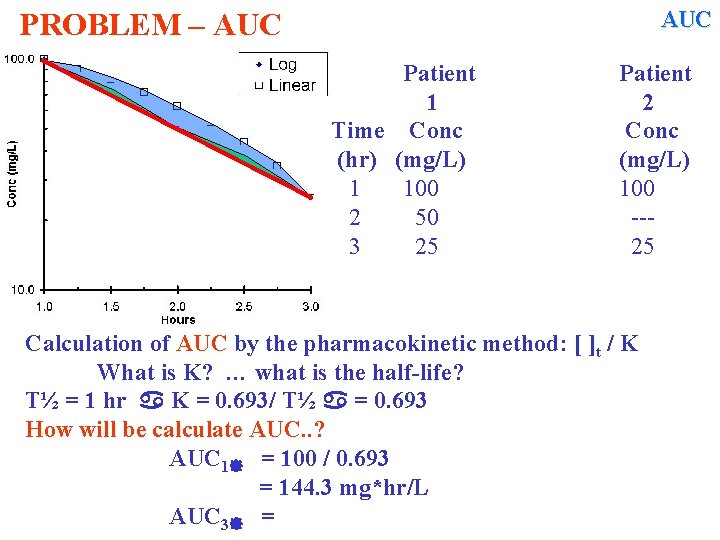
AUC PROBLEM – AUC Patient 1 Time Conc (hr) (mg/L) 1 100 2 50 3 25 Patient 2 Conc (mg/L) 100 --25 Calculation of AUC by the pharmacokinetic method: [ ]t / K What is K? … what is the half-life? T½ = 1 hr K = 0. 693/ T½ = 0. 693 How will be calculate AUC. . ? AUC 1 = 100 / 0. 693 = 144. 3 mg*hr/L AUC 3 =

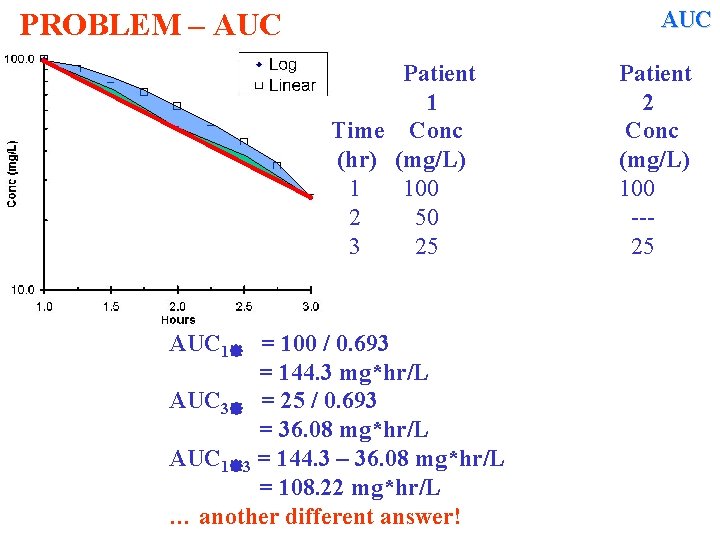
AUC PROBLEM – AUC Patient 1 Time Conc (hr) (mg/L) 1 100 2 50 3 25 AUC 1 = 100 / 0. 693 = 144. 3 mg*hr/L AUC 3 = 25 / 0. 693 = 36. 08 mg*hr/L AUC 1 3 = 144. 3 – 36. 08 mg*hr/L = 108. 22 mg*hr/L … another different answer! Patient 2 Conc (mg/L) 100 --25

AUC PROBLEM – AUC Patient 1 Time Conc (hr) (mg/L) 1 100 2 50 3 25 Summary: Kinetic method AUC 1 3 = 108. 22 mg*hr/L Patient 2 Conc (mg/L) 100 --25 (Patients 1 & 2) Trap. Rule; Patient 1; AUC 1 -3 hr: = 112. 5 mg*hr/L Trap. Rule; Patient 2; AUC 1 -3 hr: = 125. 0 mg*hr/L

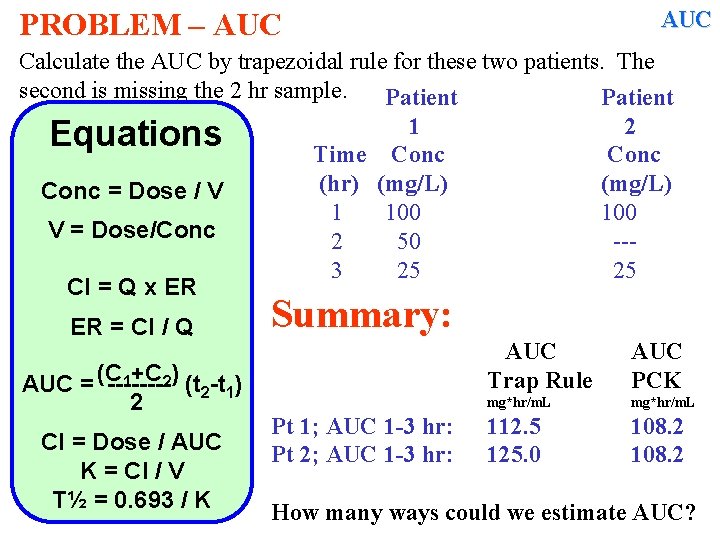
AUC PROBLEM – AUC Calculate the AUC by trapezoidal rule for these two patients. The second is missing the 2 hr sample. Patient 1 2 Equations Time Conc (hr) (mg/L) Conc = Dose / V 1 100 V = Dose/Conc 2 50 --3 25 25 Cl = Q x ER ER = Cl / Q 1+C 2) (t -t ) AUC = (C -------2 1 2 Cl = Dose / AUC K = Cl / V T½ = 0. 693 / K Summary: Pt 1; AUC 1 -3 hr: Pt 2; AUC 1 -3 hr: AUC Trap Rule AUC PCK mg*hr/m. L 112. 5 125. 0 108. 2 How many ways could we estimate AUC?

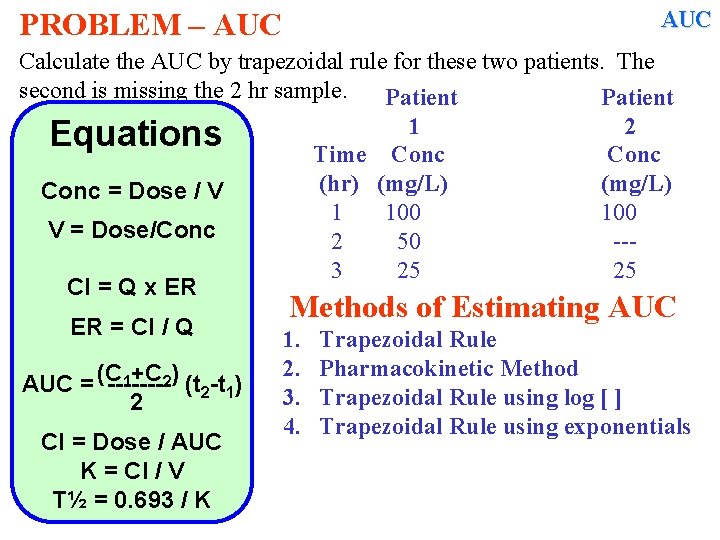
AUC PROBLEM – AUC Calculate the AUC by trapezoidal rule for these two patients. The second is missing the 2 hr sample. Patient 1 2 Equations Time Conc (hr) (mg/L) Conc = Dose / V 1 100 V = Dose/Conc 2 50 --3 25 25 Cl = Q x ER ER = Cl / Q 1+C 2) (t -t ) AUC = (C -------2 1 2 Cl = Dose / AUC K = Cl / V T½ = 0. 693 / K Methods of Estimating AUC 1. 2. 3. 4. Trapezoidal Rule Pharmacokinetic Method Trapezoidal Rule using log [ ] Trapezoidal Rule using exponentials

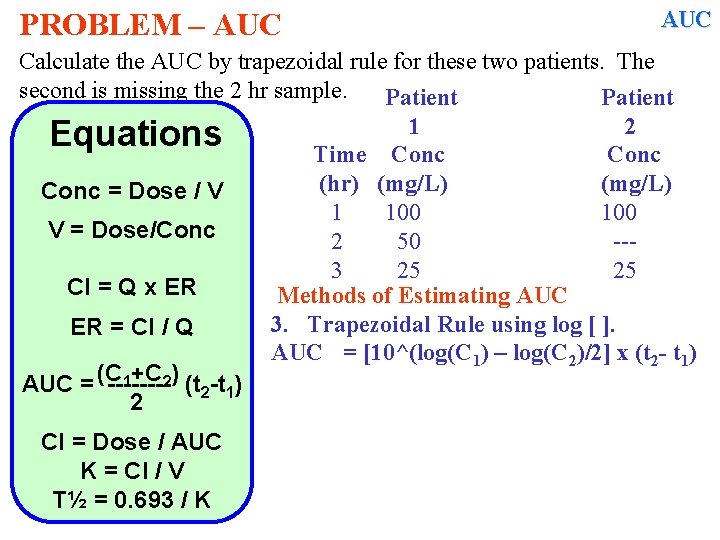
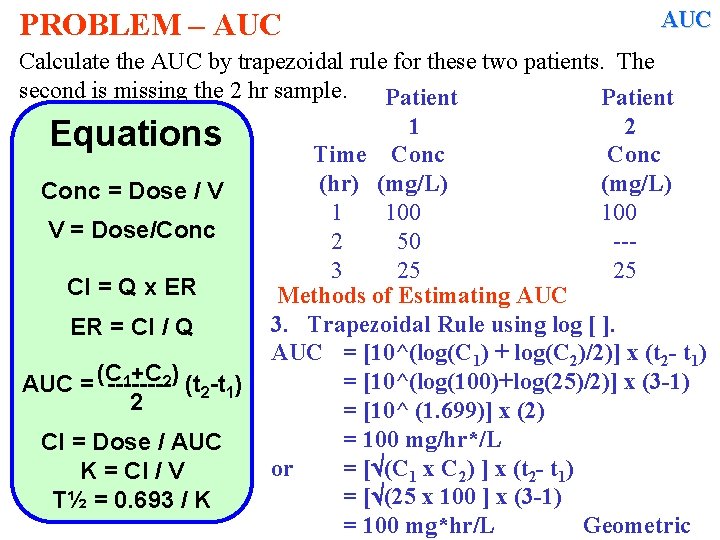
PROBLEM – AUC Calculate the AUC by trapezoidal rule for these two patients. The second is missing the 2 hr sample. Patient 1 2 Equations Time Conc (hr) (mg/L) Conc = Dose / V 1 100 V = Dose/Conc 2 50 --3 25 25 Cl = Q x ER Methods of Estimating AUC 3. Trapezoidal Rule using log [ ]. ER = Cl / Q AUC = [10^(log(C 1) – log(C 2)/2] x (t 2 - t 1) 1+C 2) (t -t ) AUC = (C -------2 1 2 Cl = Dose / AUC K = Cl / V T½ = 0. 693 / K

PROBLEM – AUC Calculate the AUC by trapezoidal rule for these two patients. The second is missing the 2 hr sample. Patient 1 2 Equations Time Conc (hr) (mg/L) Conc = Dose / V 1 100 V = Dose/Conc 2 50 --3 25 25 Cl = Q x ER Methods of Estimating AUC 3. Trapezoidal Rule using log [ ]. ER = Cl / Q AUC = [10^(log(C 1) + log(C 2)/2)] x (t 2 - t 1) 1+C 2) (t -t ) = [10^(log(100)+log(25)/2)] x (3 -1) AUC = (C -------2 1 2 = [10^ (1. 699)] x (2) = 100 mg/hr*/L Cl = Dose / AUC or = [ (C 1 x C 2) ] x (t 2 - t 1) K = Cl / V = [ (25 x 100 ] x (3 -1) T½ = 0. 693 / K = 100 mg*hr/L Geometric

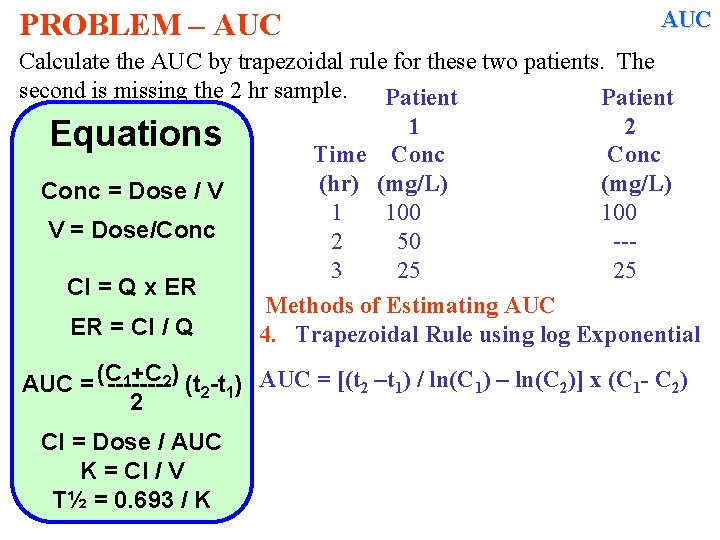
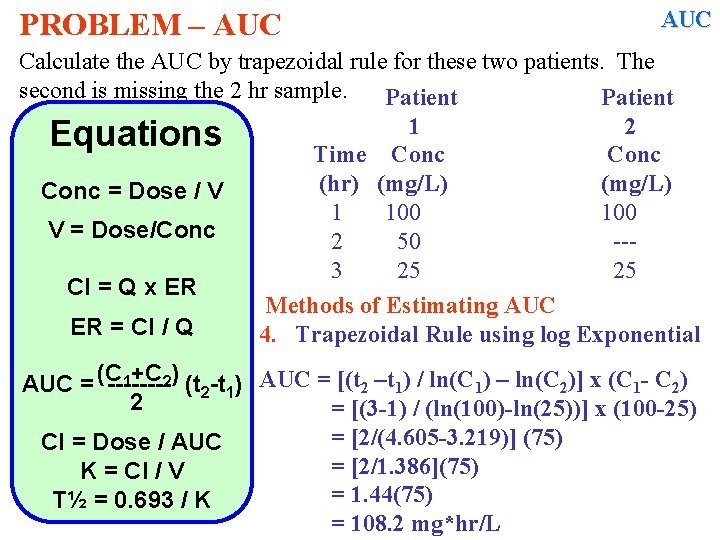
PROBLEM – AUC Calculate the AUC by trapezoidal rule for these two patients. The second is missing the 2 hr sample. Patient 1 2 Equations Time Conc (hr) (mg/L) Conc = Dose / V 1 100 V = Dose/Conc 2 50 --3 25 25 Cl = Q x ER Methods of Estimating AUC ER = Cl / Q 4. Trapezoidal Rule using log Exponential 1+C 2) (t -t ) AUC = [(t 2 –t 1) / ln(C 1) – ln(C 2)] x (C 1 - C 2) AUC = (C -------2 1 2 Cl = Dose / AUC K = Cl / V T½ = 0. 693 / K

PROBLEM – AUC Calculate the AUC by trapezoidal rule for these two patients. The second is missing the 2 hr sample. Patient 1 2 Equations Time Conc (hr) (mg/L) Conc = Dose / V 1 100 V = Dose/Conc 2 50 --3 25 25 Cl = Q x ER Methods of Estimating AUC ER = Cl / Q 4. Trapezoidal Rule using log Exponential 1+C 2) (t -t ) AUC = [(t 2 –t 1) / ln(C 1) – ln(C 2)] x (C 1 - C 2) AUC = (C -------2 1 2 = [(3 -1) / (ln(100)-ln(25))] x (100 -25) = [2/(4. 605 -3. 219)] (75) Cl = Dose / AUC = [2/1. 386](75) K = Cl / V = 1. 44(75) T½ = 0. 693 / K = 108. 2 mg*hr/L

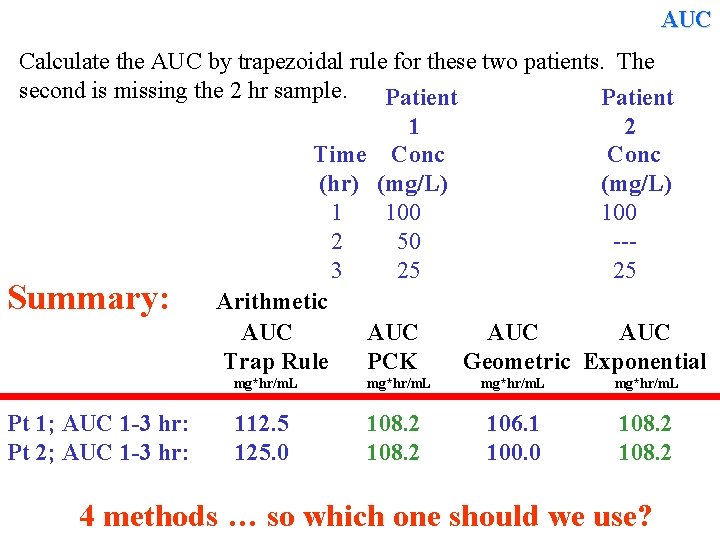
AUC Calculate the AUC by trapezoidal rule for these two patients. The second is missing the 2 hr sample. Patient 1 2 Time Conc (hr) (mg/L) 1 100 2 50 --3 25 25 Summary: Arithmetic AUC AUC Trap Rule PCK Geometric Exponential Pt 1; AUC 1 -3 hr: Pt 2; AUC 1 -3 hr: mg*hr/m. L 112. 5 125. 0 108. 2 mg*hr/m. L 106. 1 100. 0 108. 2 4 methods … so which one should we use?

AUC Summary: AUC Trap Rule Pt 1; AUC 1 -3 hr: Pt 2; AUC 1 -3 hr: AUC PCK mg*hr/m. L 112. 5 125. 0 108. 2 AUC Geometric Exponential mg*hr/m. L 106. 1 100. 0 108. 2 So which one should we use? In log-linear regions the PCK method is accurate, simple and quick, but arithmetic trapezoidal rule is still a reasonable estimate. . In “other regions”, where true knowledge of the rate of change in concentration is not known, arithmetic trapezoidal rule is a simple, quick & a reasonable estimate of AUC and may be the best.

AUC Summary: So which one should we use? 1. If the conc. -time profile is log linear you can use the kinetic method… [ ]/k. 2. … but if it is not log-linear, if you are unsure, use the arithmetic trapezoidal rule. It is a simple, quick and a reasonable estimate of AUC. Use Arithmetic Trapezoidal Rule
![Dealing with time Data 3 Back Extrapolation How do you calculate Volume Dealing with [ ] –time Data (3) Back Extrapolation How do you calculate Volume](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-85.jpg)
Dealing with [ ] –time Data (3) Back Extrapolation How do you calculate Volume if you do not have an initial concentration? (a time-zero concentration)
![Dealing with time Data Back Extrap What happens if you do not Dealing with [ ] –time Data Back Extrap What happens if you do not](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-86.jpg)
Dealing with [ ] –time Data Back Extrap What happens if you do not have a time zero [ ]? How do you calculate V? Dose = 1000 mg Time Conc (hr) (mg/L) 0 1 2 4 60. 0 12 25. 0 18 12. 5 24 6. 25 Volume (L) = Dose / [ ]t=0 What is the concentration at time zero … or what would it have been?
![Dealing with time Data Back Extrap What happens if you do not Dealing with [ ] –time Data Back Extrap What happens if you do not](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-87.jpg)
Dealing with [ ] –time Data Back Extrap What happens if you do not have a time zero [ ]? How do you calculate V? Dose = 1000 mg Time Conc (hr) (mg/L) 0 1 2 4 60. 0 12 25. 0 18 12. 5 24 6. 25 Volume (L) = Dose / [ ]t=0 Plot the data to observe the rate of change in [ ]. Is it linear ? … log-linear? If so extrapolate or back-extrapolate to t=0.
![Dealing with time Data Back Extrap What happens if you do not Dealing with [ ] –time Data Back Extrap What happens if you do not](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-88.jpg)
Dealing with [ ] –time Data Back Extrap What happens if you do not have a time zero [ ]? How do you calculate V? Dose = 1000 mg Time Conc (hr) (mg/L) 0 1 2 4 60. 0 12 25. 0 18 12. 5 24 6. 25 Volume (L) = Dose / [ ]t=0 Extrapolate by one of two methods: Graphical, using semi-log paper … using slope or equation Or using Excel “Intercept” function.
![Back Extrap Dealing with time Data What happens if you do not Back Extrap Dealing with [ ] –time Data What happens if you do not](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-89.jpg)
Back Extrap Dealing with [ ] –time Data What happens if you do not have a time zero [ ]? How do you calculate V? Dose = 1000 mg Time Conc (hr) (mg/L) 0 1 2 4 60. 0 12 25. 0 18 12. 5 24 6. 25 Extrapolate by Equation: Ct = C 0 e-kt Equation determines concentration at any time following a given initial concentration C 12 = C 4 e-K(8) where K = 0. 1155 (T½ = 6 hr) C 12 = 25 mg/L Negative sign (-K) indicates loss of concentration
![Dealing with time Data Back Extrap What happens if you do not Dealing with [ ] –time Data Back Extrap What happens if you do not](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-90.jpg)
Dealing with [ ] –time Data Back Extrap What happens if you do not have a time zero [ ]? How do you calculate V? Dose = 1000 mg Time Conc (hr) (mg/L) 0 1 2 4 60. 0 12 25. 0 18 12. 5 24 6. 25 Extrapolate by Equation: Ct = C 0 e+kt A Positive sign (+K) would indicates INCREASING conc. C 0 = C 4 e+K(4) where K = 0. 1155 (T½ = 6 hr) C 0 = 100 mg/L An example is shown in the Excel tutorial slides 40 & 41.
![What happens if you do not have a time zero Dose What happens if you do not have a time zero [ ]? Dose =](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-91.jpg)
What happens if you do not have a time zero [ ]? Dose = 1000 mg Time Conc (hr) (mg/L) 0 1 2 4 60. 0 12 25. 0 18 12. 5 24 6. 25 60 mg/L Graphically …. Time zero Intercept should be exactly (very close) to 100 mg/L 0 4 8 12 16 20 24 28 Excel® example shown at the end of the slideshow.
![Dealing with time Data Back Extrap What is the volume of distribution Dealing with [ ] –time Data Back Extrap What is the volume of distribution](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-92.jpg)
Dealing with [ ] –time Data Back Extrap What is the volume of distribution following a 1000 mg dose, if the following conc. were observed? Dose = 1000 mg Time Conc (hr) (mg/L) 0 1 2 4 60. 0 12 25. 0 18 12. 5 24 6. 25 Step by Step: 1. What do we need to calculate first? Volume, AUC, Clearance, half-life or K?
![Dealing with time Data Back Extrap What is the volume of distribution Dealing with [ ] –time Data Back Extrap What is the volume of distribution](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-93.jpg)
Dealing with [ ] –time Data Back Extrap What is the volume of distribution following a 1000 mg dose, if the following conc. were observed? Dose = 1000 mg Time Conc (hr) (mg/L) 0 1 2 4 60. 0 12 25. 0 18 12. 5 24 6. 25 Step by Step: 2. K or T½, by either visual inspection of data or equation. T½ by visual inspection is 6 hr K = 0. 693/6=0. 1155 hr-1
![Dealing with time Data Back Extrap What is the volume of distribution Dealing with [ ] –time Data Back Extrap What is the volume of distribution](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-94.jpg)
Dealing with [ ] –time Data Back Extrap What is the volume of distribution following a 1000 mg dose, if the following conc. were observed? Dose = 1000 mg Time Conc (hr) (mg/L) 0 1 2 4 60. 0 12 25. 0 18 12. 5 24 6. 25 Step by Step: 3. Back – extrapolate using K to determine C 0. Ct = C 0 e+kt C 0 = C 4 e+K(4) where K = 0. 1155 hr-1 & C 4 = 60 mg/L C 0 = 100 mg/L
![Dealing with time Data Back Extrap What is the volume of distribution Dealing with [ ] –time Data Back Extrap What is the volume of distribution](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-95.jpg)
Dealing with [ ] –time Data Back Extrap What is the volume of distribution following a 1000 mg dose, if the following conc. were observed? Dose = 1000 mg Time Conc (hr) (mg/L) 0 1 2 4 60. 0 12 25. 0 18 12. 5 24 6. 25 Step by Step: 4. Determine volume using the Dose (1000 mg) and the back extrapolated concentration. (100 mg/L) Volume = Dose / Conc = 1000 mg / 100 mg/L = 10 L.
![Dealing with time Data Back Extrap What is the volume of distribution Dealing with [ ] –time Data Back Extrap What is the volume of distribution](https://slidetodoc.com/presentation_image_h/342f8a0974a0c9d7c4092a78194ab450/image-96.jpg)
Dealing with [ ] –time Data Back Extrap What is the volume of distribution following a 1000 mg dose, if the following conc. were observed? Dose = 1000 mg Time Conc (hr) (mg/L) 0 1 2 4 60. 0 12 25. 0 18 12. 5 24 6. 25 Step by Step: 5. You could now calculate AUC and then clearance. Remember, AUC MUST include the C 0 concentration. Do not start calculating AUC from 4 hours. !!

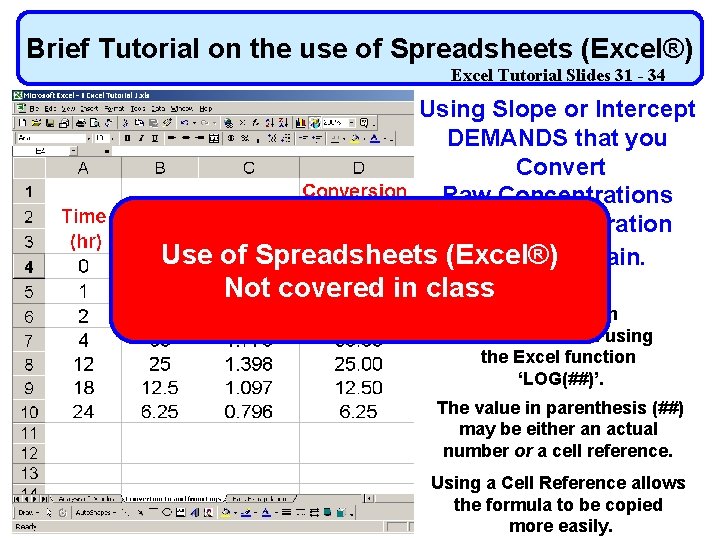
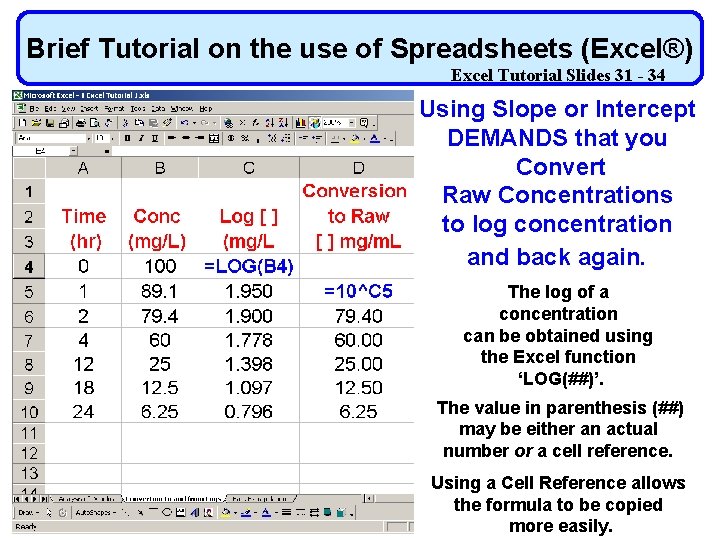
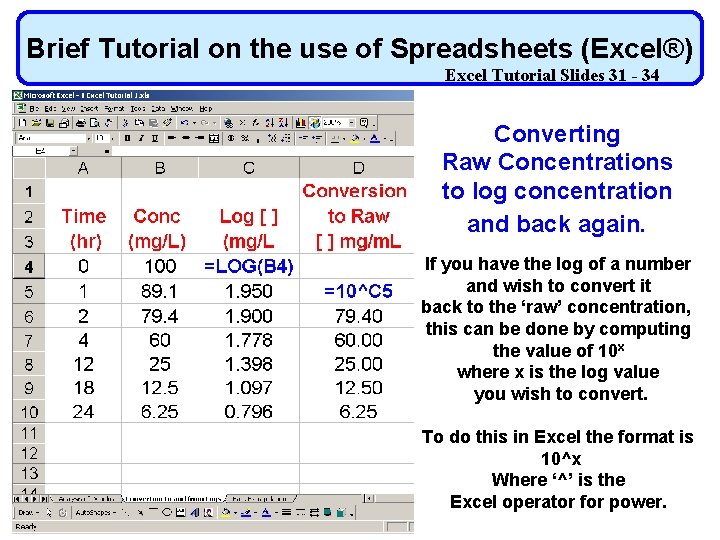
Brief Tutorial on the use of Spreadsheets (Excel®) Excel Tutorial Slides 31 - 34 Using Slope or Intercept DEMANDS that you Convert Raw Concentrations to log concentration Use of Spreadsheets (Excel®) and back again. Not covered in class The log of a concentration can be obtained using the Excel function ‘LOG(##)’. The value in parenthesis (##) may be either an actual number or a cell reference. Using a Cell Reference allows the formula to be copied more easily.

Brief Tutorial on the use of Spreadsheets (Excel®) Excel Tutorial Slides 31 - 34 Using Slope or Intercept DEMANDS that you Convert Raw Concentrations to log concentration and back again. The log of a concentration can be obtained using the Excel function ‘LOG(##)’. The value in parenthesis (##) may be either an actual number or a cell reference. Using a Cell Reference allows the formula to be copied more easily.

Brief Tutorial on the use of Spreadsheets (Excel®) Excel Tutorial Slides 31 - 34 Converting Raw Concentrations to log concentration and back again. If you have the log of a number and wish to convert it back to the ‘raw’ concentration, this can be done by computing the value of 10 x where x is the log value you wish to convert. To do this in Excel the format is 10^x Where ‘^’ is the Excel operator for power.

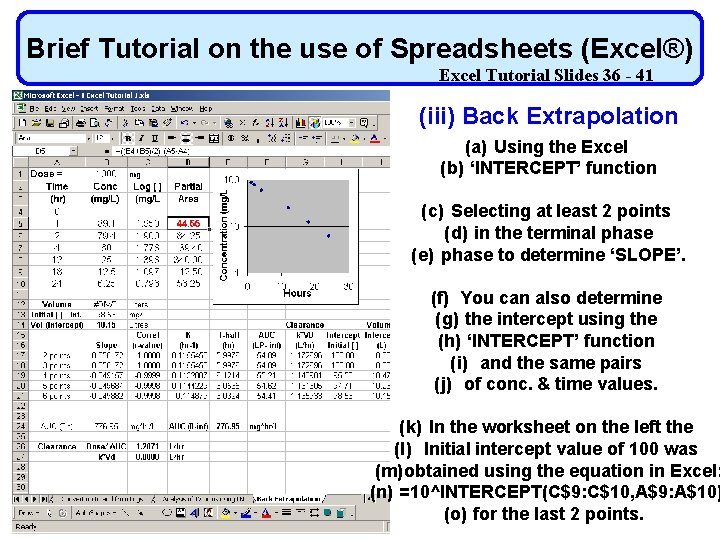
Brief Tutorial on the use of Spreadsheets (Excel®) Excel Tutorial Slides 36 - 41 (iii) Back Extrapolation (a) Using the Excel (b) ‘INTERCEPT’ function (c) Selecting at least 2 points (d) in the terminal phase (e) phase to determine ‘SLOPE’. (f) You can also determine (g) the intercept using the (h) ‘INTERCEPT’ function (i) and the same pairs (j) of conc. & time values. (k) In the worksheet on the left the (l) Initial intercept value of 100 was (m)obtained using the equation in Excel: (n) =10^INTERCEPT(C$9: C$10, A$9: A$10) (o) for the last 2 points.

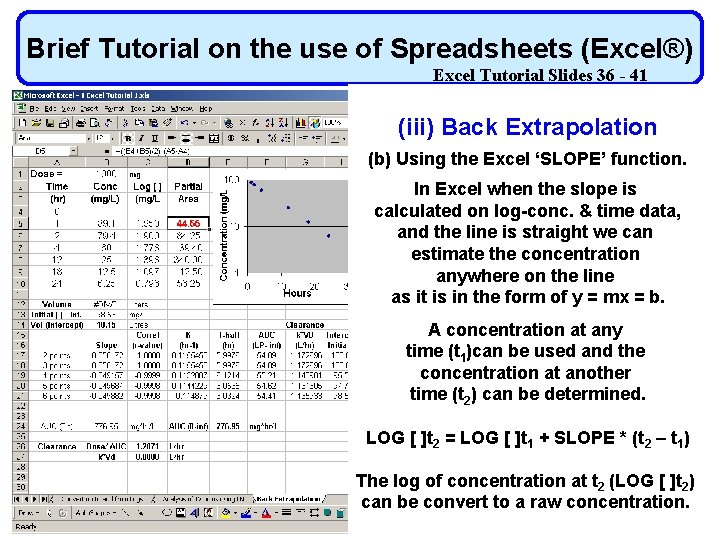
Brief Tutorial on the use of Spreadsheets (Excel®) Excel Tutorial Slides 36 - 41 (iii) Back Extrapolation (b) Using the Excel ‘SLOPE’ function. In Excel when the slope is calculated on log-conc. & time data, and the line is straight we can estimate the concentration anywhere on the line as it is in the form of y = mx = b. A concentration at any time (t 1)can be used and the concentration at another time (t 2) can be determined. LOG [ ]t 2 = LOG [ ]t 1 + SLOPE * (t 2 – t 1) The log of concentration at t 2 (LOG [ ]t 2) can be convert to a raw concentration.

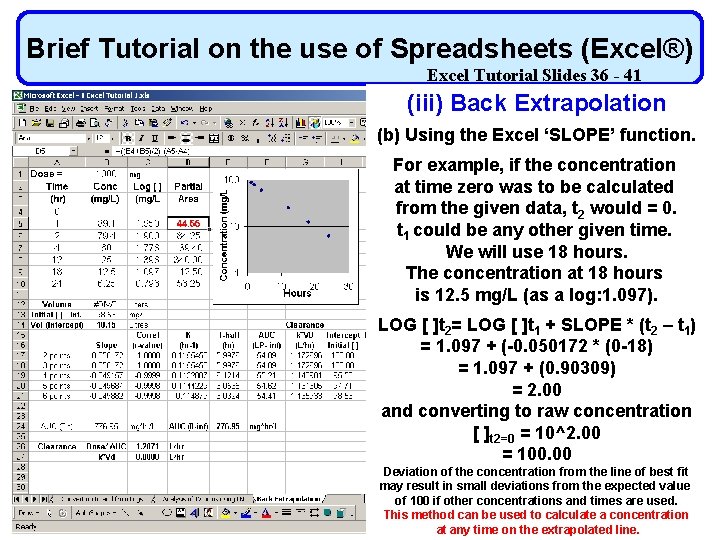
Brief Tutorial on the use of Spreadsheets (Excel®) Excel Tutorial Slides 36 - 41 (iii) Back Extrapolation (b) Using the Excel ‘SLOPE’ function. For example, if the concentration at time zero was to be calculated from the given data, t 2 would = 0. t 1 could be any other given time. We will use 18 hours. The concentration at 18 hours is 12. 5 mg/L (as a log: 1. 097). LOG [ ]t 2= LOG [ ]t 1 + SLOPE * (t 2 – t 1) = 1. 097 + (-0. 050172 * (0 -18) = 1. 097 + (0. 90309) = 2. 00 and converting to raw concentration [ ]t 2=0 = 10^2. 00 = 100. 00 Deviation of the concentration from the line of best fit may result in small deviations from the expected value of 100 if other concentrations and times are used. This method can be used to calculate a concentration at any time on the extrapolated line.