RATE EXPRESSION AND REACTION MECHANISM RATE LAW CONCENTRATION






















- Slides: 33

RATE EXPRESSION AND REACTION MECHANISM

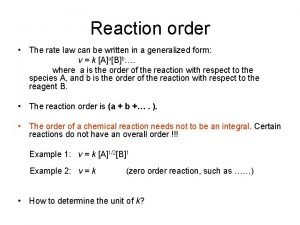
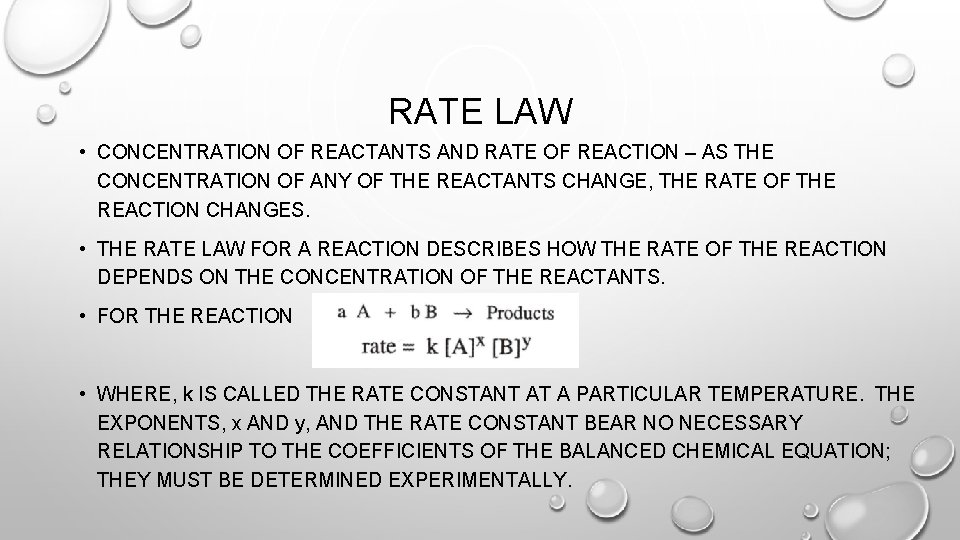
RATE LAW • CONCENTRATION OF REACTANTS AND RATE OF REACTION – AS THE CONCENTRATION OF ANY OF THE REACTANTS CHANGE, THE RATE OF THE REACTION CHANGES. • THE RATE LAW FOR A REACTION DESCRIBES HOW THE RATE OF THE REACTION DEPENDS ON THE CONCENTRATION OF THE REACTANTS. • FOR THE REACTION • WHERE, k IS CALLED THE RATE CONSTANT AT A PARTICULAR TEMPERATURE. THE EXPONENTS, x AND y, AND THE RATE CONSTANT BEAR NO NECESSARY RELATIONSHIP TO THE COEFFICIENTS OF THE BALANCED CHEMICAL EQUATION; THEY MUST BE DETERMINED EXPERIMENTALLY.

RATE LAW • THE EXPONENTS ARE USUALLY POSITIVE INTEGERS, OR ZERO, BUT THEY CAN OCCASIONALLY BE A FRACTION OR A NEGATIVE NUMBER. • FOR THE ABOVE RATE LAW,

REMEMBER



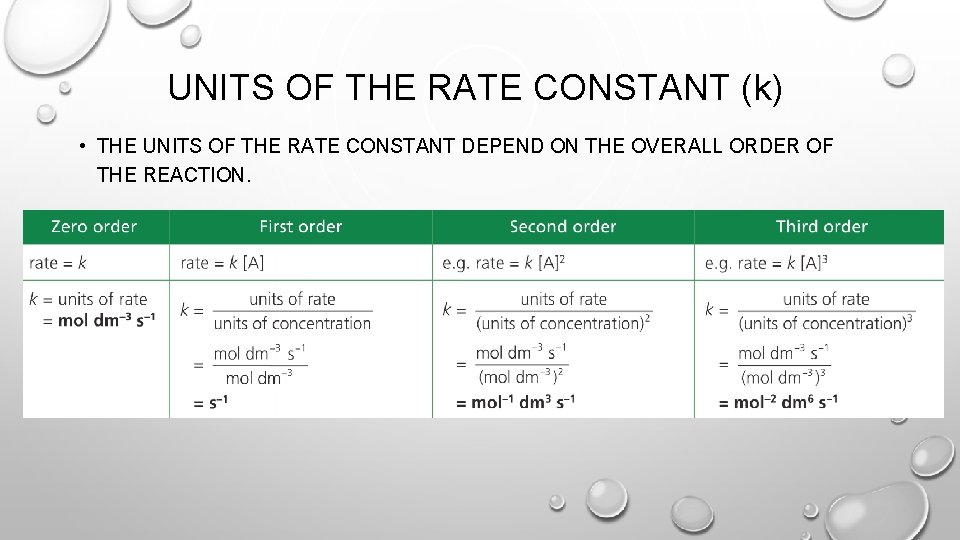
UNITS OF THE RATE CONSTANT (k) • THE UNITS OF THE RATE CONSTANT DEPEND ON THE OVERALL ORDER OF THE REACTION.




GRAPHICAL REPRESENTATIONS OF REACTION KINETICS • RATE VS. CONCENTRATION GRAPHS SHOW THE DIFFERENCE BETWEEN ZERO ORDER, FIRST ORDER, AND SECOND ORDER REACTIONS.

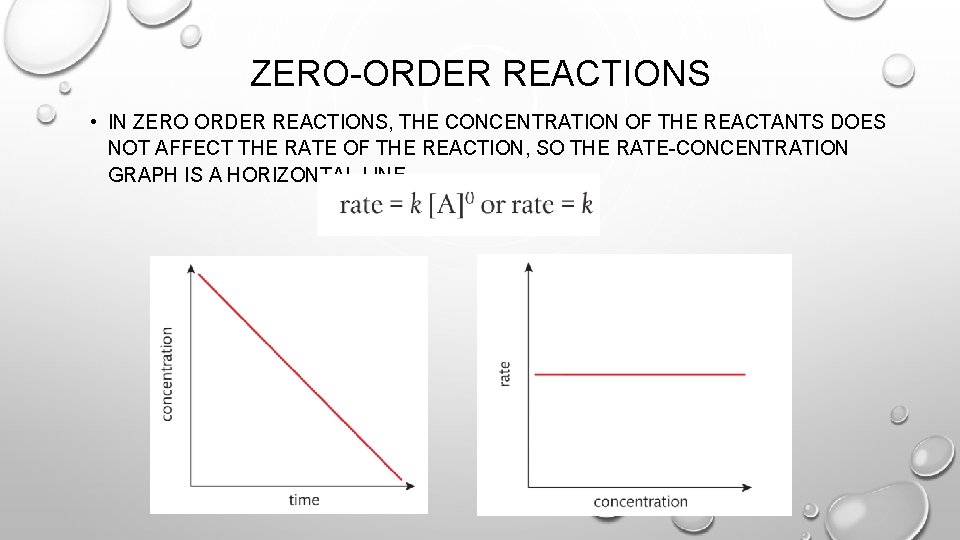
ZERO-ORDER REACTIONS • IN ZERO ORDER REACTIONS, THE CONCENTRATION OF THE REACTANTS DOES NOT AFFECT THE RATE OF THE REACTION, SO THE RATE-CONCENTRATION GRAPH IS A HORIZONTAL LINE

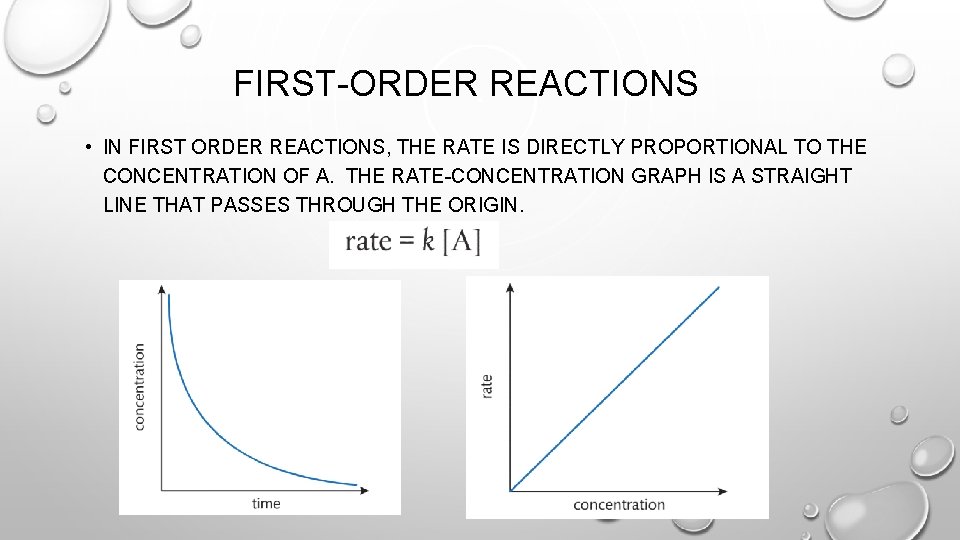
FIRST-ORDER REACTIONS • IN FIRST ORDER REACTIONS, THE RATE IS DIRECTLY PROPORTIONAL TO THE CONCENTRATION OF A. THE RATE-CONCENTRATION GRAPH IS A STRAIGHT LINE THAT PASSES THROUGH THE ORIGIN.

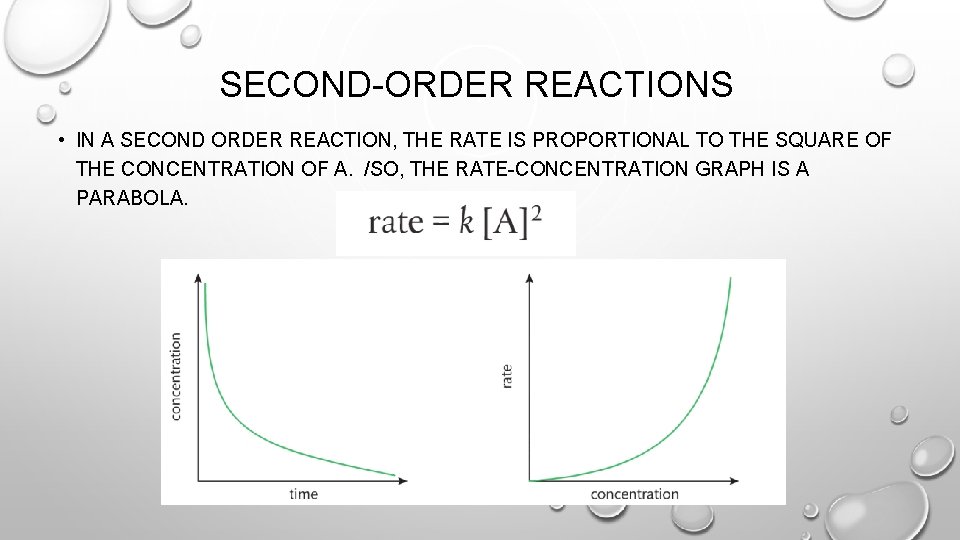
SECOND-ORDER REACTIONS • IN A SECOND ORDER REACTION, THE RATE IS PROPORTIONAL TO THE SQUARE OF THE CONCENTRATION OF A. /SO, THE RATE-CONCENTRATION GRAPH IS A PARABOLA.

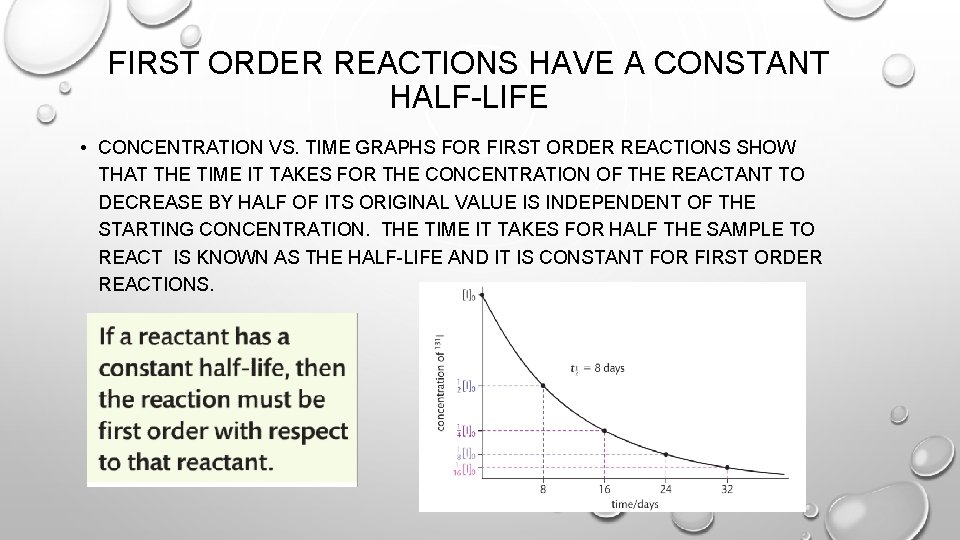
FIRST ORDER REACTIONS HAVE A CONSTANT HALF-LIFE • CONCENTRATION VS. TIME GRAPHS FOR FIRST ORDER REACTIONS SHOW THAT THE TIME IT TAKES FOR THE CONCENTRATION OF THE REACTANT TO DECREASE BY HALF OF ITS ORIGINAL VALUE IS INDEPENDENT OF THE STARTING CONCENTRATION. THE TIME IT TAKES FOR HALF THE SAMPLE TO REACT IS KNOWN AS THE HALF-LIFE AND IT IS CONSTANT FOR FIRST ORDER REACTIONS.

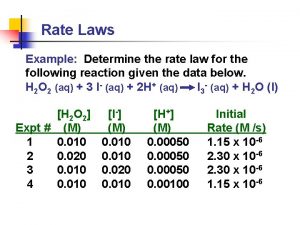
DETERMINING THE ORDER OF THE REACTION • THE ORDER OF THE REACTION CAN BE DETERMINED BY THE INITIAL RATES METHOD. • THIS METHOD INVOLVES CARRYING OUT SEPARATE EXPERIMENTS WHERE THE CONCENTRATION OF ONE OF THE REACTANTS IS KEPT CONSTANT WHILE THE CONCENTRATION OF THE OTHER REACTANT IS CHANGED. • THE PROCESS IS THEN REPEATED CHANGING THE CONCENTRATION OF THE OTHER REACTANT WHILE KEEPING THE CONCENTRATION OF THE FIRST REACTANT CONSTANT.








REACTION MECHANISMS • A REACTION MECHANISM IS THE STEP-BY STEP PATHWAY BY WHICH A REACTION OCCURS. • SOME REACTIONS TAKE PLACE IN A SINGLE STEP, HOWEVER, MOST REACTIONS OCCUR IN A SERIES OF ELEMENTARY STEPS. • EVIDENCE CAN SUPPORT A REACTION MECHANISM, BUT IT CANNOT PROVE IT TO BE CORRECT- ONLY THAT IT IS CONSISTENT WITH THE OBSERVED DATA.

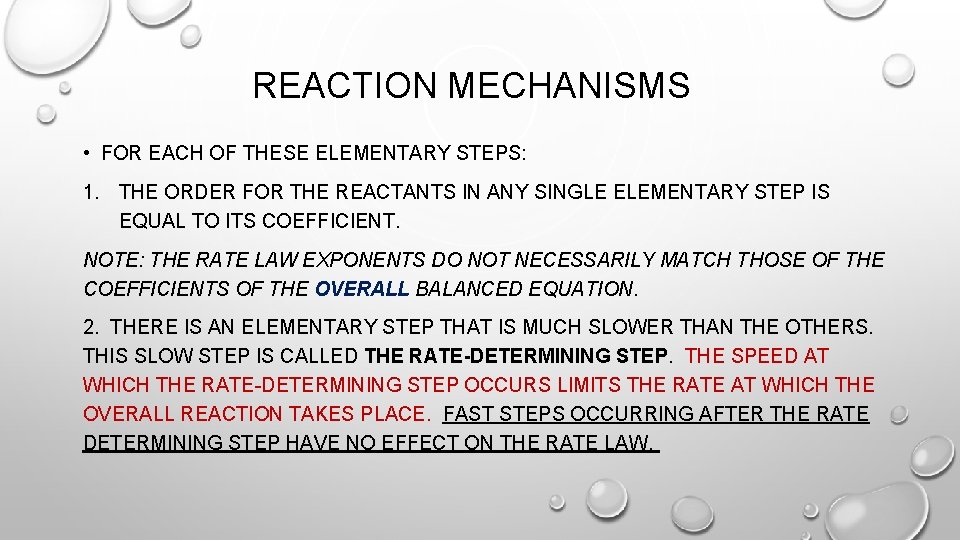
REACTION MECHANISMS • FOR EACH OF THESE ELEMENTARY STEPS: 1. THE ORDER FOR THE REACTANTS IN ANY SINGLE ELEMENTARY STEP IS EQUAL TO ITS COEFFICIENT. NOTE: THE RATE LAW EXPONENTS DO NOT NECESSARILY MATCH THOSE OF THE COEFFICIENTS OF THE OVERALL BALANCED EQUATION. 2. THERE IS AN ELEMENTARY STEP THAT IS MUCH SLOWER THAN THE OTHERS. THIS SLOW STEP IS CALLED THE RATE-DETERMINING STEP. THE SPEED AT WHICH THE RATE-DETERMINING STEP OCCURS LIMITS THE RATE AT WHICH THE OVERALL REACTION TAKES PLACE. FAST STEPS OCCURRING AFTER THE RATE DETERMINING STEP HAVE NO EFFECT ON THE RATE LAW.


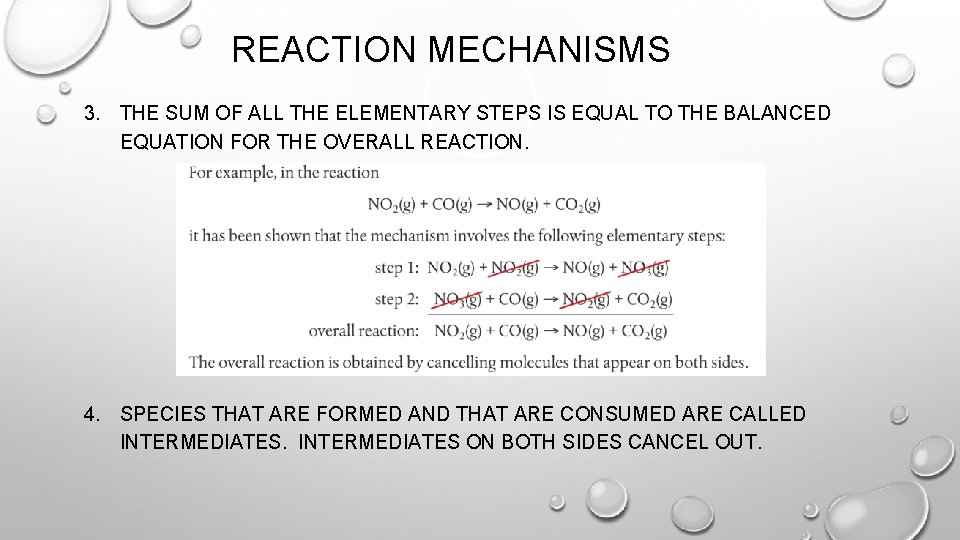
REACTION MECHANISMS 3. THE SUM OF ALL THE ELEMENTARY STEPS IS EQUAL TO THE BALANCED EQUATION FOR THE OVERALL REACTION. 4. SPECIES THAT ARE FORMED AND THAT ARE CONSUMED ARE CALLED INTERMEDIATES ON BOTH SIDES CANCEL OUT.

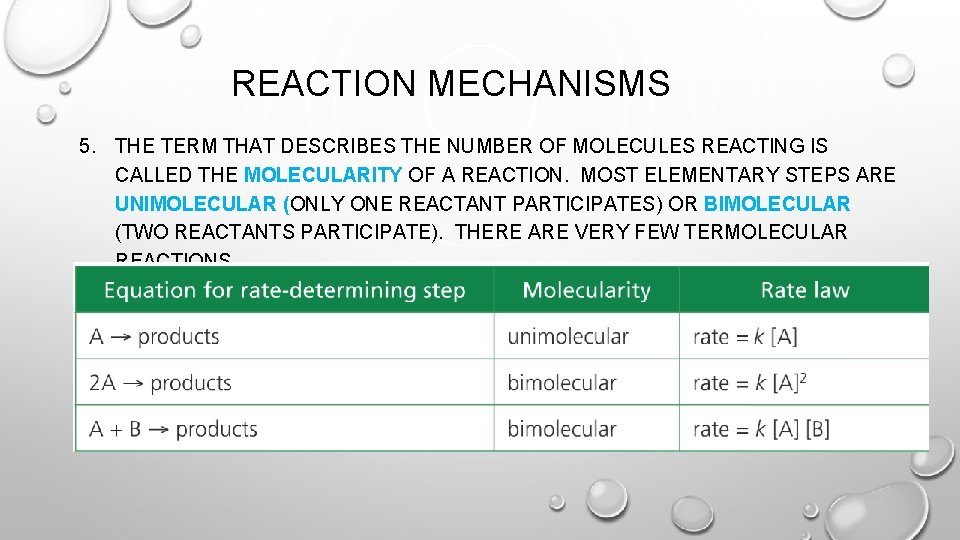
REACTION MECHANISMS 5. THE TERM THAT DESCRIBES THE NUMBER OF MOLECULES REACTING IS CALLED THE MOLECULARITY OF A REACTION. MOST ELEMENTARY STEPS ARE UNIMOLECULAR (ONLY ONE REACTANT PARTICIPATES) OR BIMOLECULAR (TWO REACTANTS PARTICIPATE). THERE ARE VERY FEW TERMOLECULAR REACTIONS.

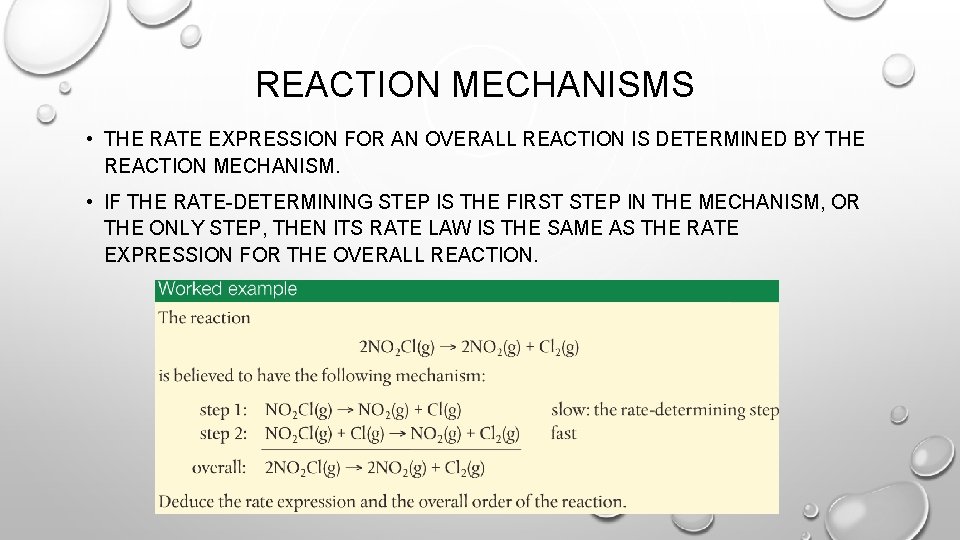
REACTION MECHANISMS • THE RATE EXPRESSION FOR AN OVERALL REACTION IS DETERMINED BY THE REACTION MECHANISM. • IF THE RATE-DETERMINING STEP IS THE FIRST STEP IN THE MECHANISM, OR THE ONLY STEP, THEN ITS RATE LAW IS THE SAME AS THE RATE EXPRESSION FOR THE OVERALL REACTION.

REACTION MECHANISMS

• WHEN THE RATE-DETERMINING STEP IS NOT THE FIRST STEP IN THE MECHANISM, IT IS NECESSARY TO TAKE INTO ACCOUNT THE EARLIER STEPS.


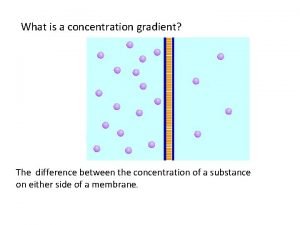
 Whats a concentration gradient
Whats a concentration gradient Movement of high concentration to low concentration
Movement of high concentration to low concentration Rate of reaction
Rate of reaction Quantitative expression of solution concentration
Quantitative expression of solution concentration Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law
Newton's first law How to determine the rate law of a reaction
How to determine the rate law of a reaction Molecularity of reaction
Molecularity of reaction How to find the rate law of a reaction
How to find the rate law of a reaction Rate law for first order reaction
Rate law for first order reaction Overall rate law of a reaction
Overall rate law of a reaction How to determine the rate law of a reaction
How to determine the rate law of a reaction Chemical energy
Chemical energy Concentration in chemical reaction
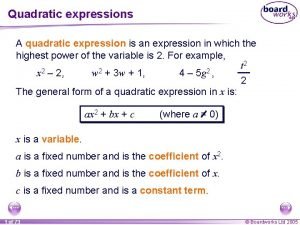
Concentration in chemical reaction A quadratic expression is an expression of
A quadratic expression is an expression of Ictahedron
Ictahedron Neutron emission
Neutron emission Performance equation of batch reactor
Performance equation of batch reactor Boyles law
Boyles law Avogadro's law constant
Avogadro's law constant Reaction formation defense mechanism
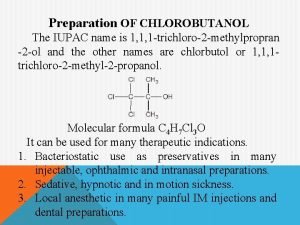
Reaction formation defense mechanism Chlorobutanol is
Chlorobutanol is Ch3 cl2
Ch3 cl2 Catalytic converter reaction mechanism
Catalytic converter reaction mechanism Alkyl halide
Alkyl halide Mechanism of haloform reaction
Mechanism of haloform reaction No2 + co reaction mechanism
No2 + co reaction mechanism Barnum effect
Barnum effect Reaction mechanism
Reaction mechanism Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction What is catalyst and how it affects reaction rate
What is catalyst and how it affects reaction rate Reaction rate and stoichiometry
Reaction rate and stoichiometry According to the third law of motion action and reaction
According to the third law of motion action and reaction Normal force
Normal force