Reaction Mechanisms and Catalysts Reaction Mechanism Pathway or








- Slides: 16

Reaction Mechanisms and Catalysts

Reaction Mechanism Pathway or series of steps through which reactants converted to products Not all steps proceed at the same rate Sum of the steps = overall reaction Rate of any step is directly proportional to the reactant concentrations in the step.

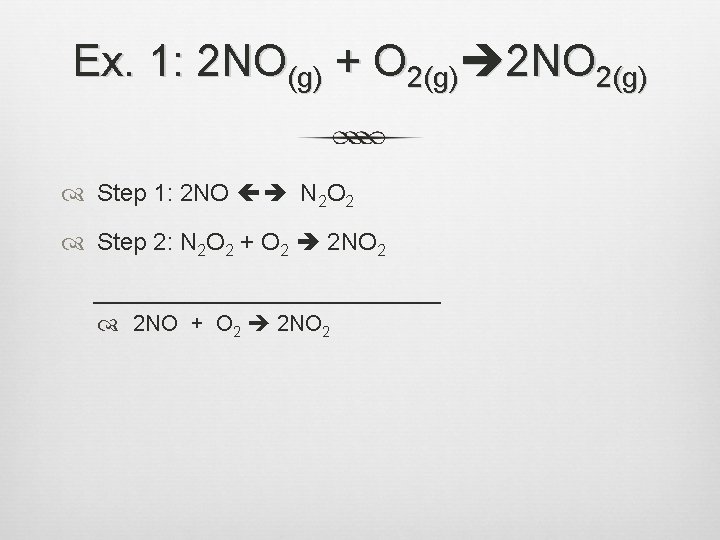
Ex. 1: 2 NO(g) + O 2(g) 2 NO 2(g) Step 1: 2 NO N 2 O 2 Step 2: N 2 O 2 + O 2 2 NO 2 _____________ 2 NO + O 2 2 NO 2

Rate-limiting Step in a reaction mechanism that “limits” how fast products are formed. “limits” the rate of reactants converting to products

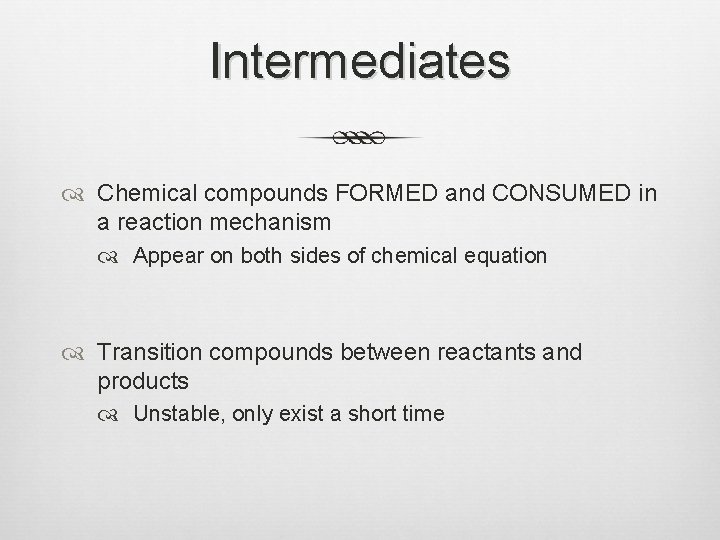
Intermediates Chemical compounds FORMED and CONSUMED in a reaction mechanism Appear on both sides of chemical equation Transition compounds between reactants and products Unstable, only exist a short time

Determining the rate law for a reaction using a reaction mechanism 1) Overall Reaction Rate Law Only determine experimentally 2) Overall Reaction Rate Law can be applied to find the steps in a reaction mechanism Rate law indicates slowest step in the mechanism Determine the fast steps remaining in the mechanism

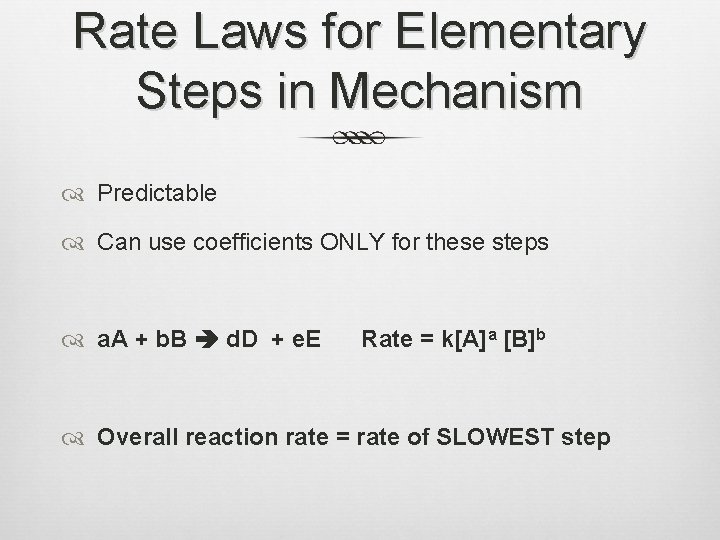
Rate Laws for Elementary Steps in Mechanism Predictable Can use coefficients ONLY for these steps a. A + b. B d. D + e. E Rate = k[A]a [B]b Overall reaction rate = rate of SLOWEST step

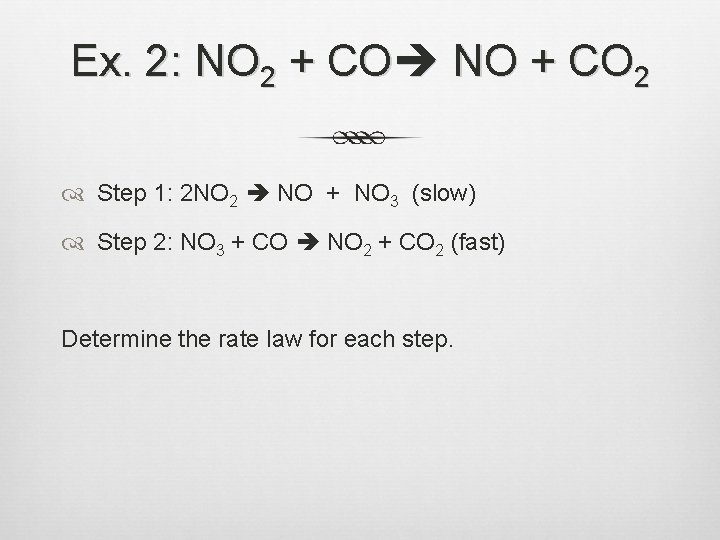
Ex. 2: NO 2 + CO NO + CO 2 Step 1: 2 NO 2 NO + NO 3 (slow) Step 2: NO 3 + CO NO 2 + CO 2 (fast) Determine the rate law for each step.

Ex. 3 Based on the following reaction mechanism…. F 2 + NO 2 F + F (slow) NO 2 + F NO 2 F (fast) a) Write the overall reaction. b) Determine the rate law for each step c) What is the rate law for the overall reaction?

Catalysts and Reaction Rates

Catalysts Chemical compounds (atoms, molecules, ions) that increase ONLY the reaction rate. Increases both forward and reverse rates for a chemical reaction Not consumed in the reaction, not altered Present at the start and end of reaction Have no effect on equilibrium constants (K), ΔH, or ΔS

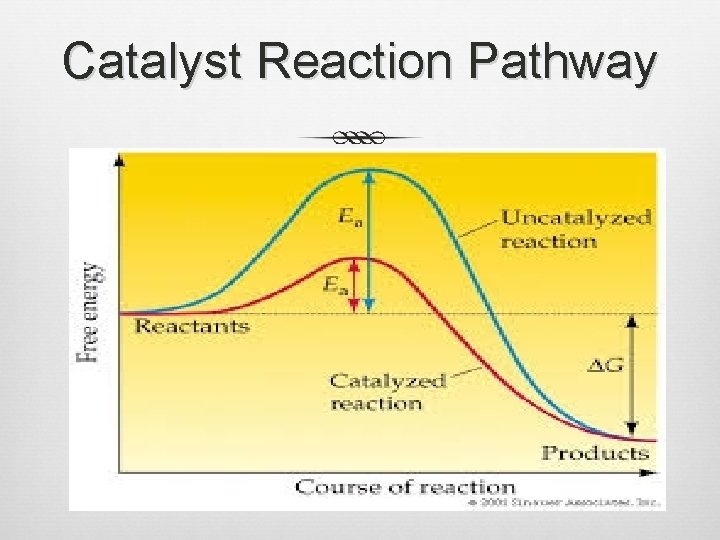
Catalysts (cont. ) Change the PATH a chemical reaction takes to get reactants to products Lowers activation energy (Ea) or energy needed for the reaction to start Only small amount of the compound needed to increase the rate for a reaction with a lot of reactant Recycle and Reuse

Catalyst Reaction Pathway

4 qualifications for a catalyst 1) Increase reaction rate 2) Not consumed in reaction 3) Only a small amount required 4) Does not alter equilibrium constant, enthalpy, or entropy. ONLY alters reaction path.

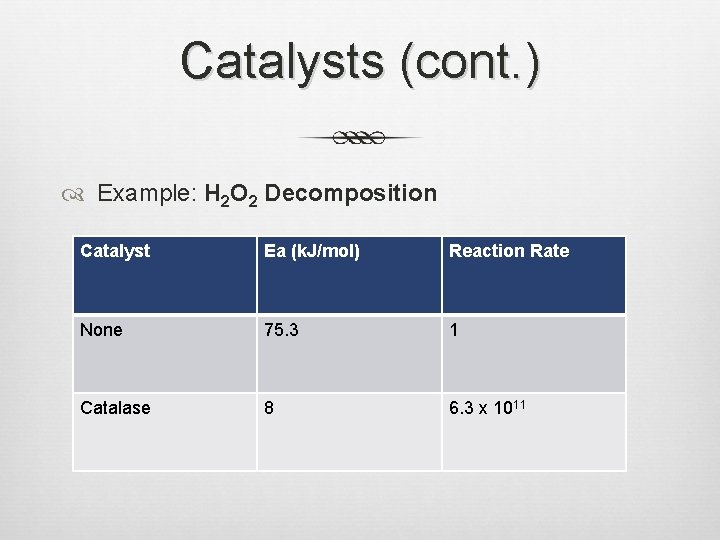
Catalysts (cont. ) Example: H 2 O 2 Decomposition Catalyst Ea (k. J/mol) Reaction Rate None 75. 3 1 Catalase 8 6. 3 x 1011

Homework Kinetics II Worksheet