Rate and Concentration Determining the rate A reaction




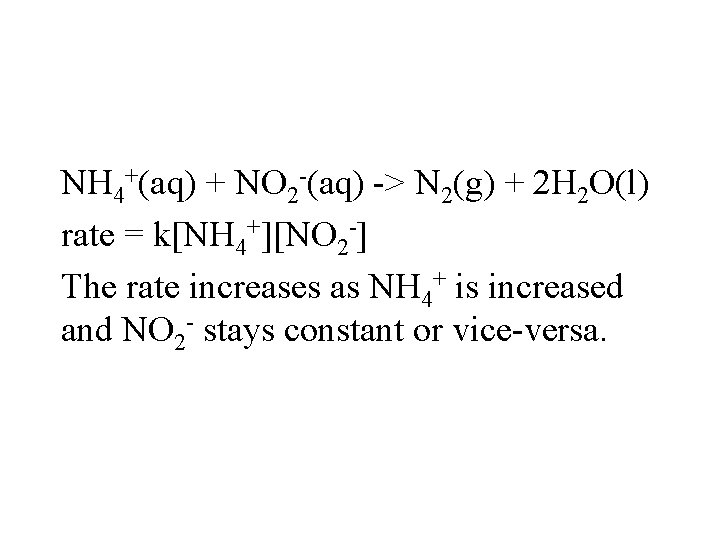
![The general rate law equation is: rate = k [reactant 1]m [reactant 2]n. . The general rate law equation is: rate = k [reactant 1]m [reactant 2]n. .](https://slidetodoc.com/presentation_image_h/099cdca299829da9ff7335301dd05d8b/image-8.jpg)

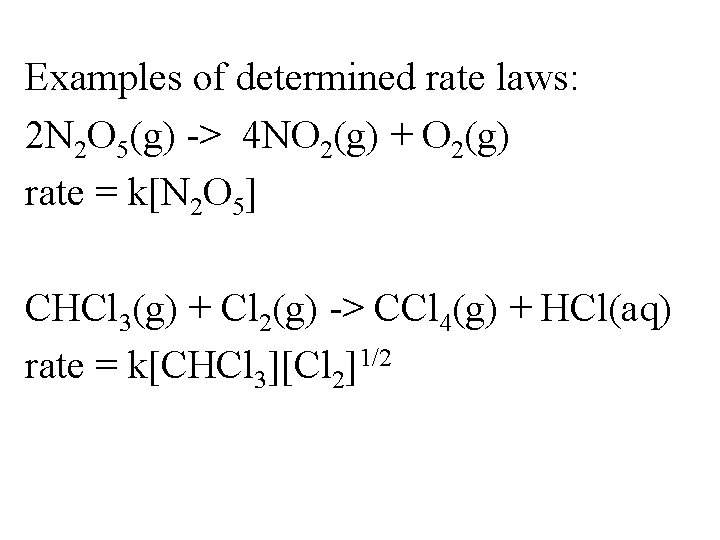
![H 2(g) + I 2(g) -> 2 HI(g) rate = k[H 2][I 2] exponents H 2(g) + I 2(g) -> 2 HI(g) rate = k[H 2][I 2] exponents](https://slidetodoc.com/presentation_image_h/099cdca299829da9ff7335301dd05d8b/image-11.jpg)









![The rate law for the reaction, therefore is: Rate = k[Br. O 3 -]1 The rate law for the reaction, therefore is: Rate = k[Br. O 3 -]1](https://slidetodoc.com/presentation_image_h/099cdca299829da9ff7335301dd05d8b/image-23.jpg)
- Slides: 23

Rate and Concentration

Determining the rate

A reaction rate is measured in molarity per second (or minutes/hours). As a reaction proceeds, its rate changes. Why?

The amount of reactants decreases. The reduction is from the decrease in the number of product forming collisions. Or the reactants have been forming products and they are disappearing.

To determine the rate of a reaction at any concentration, scientists discovered a constant (k). k is the rate law constant that is used with one side of the equation.
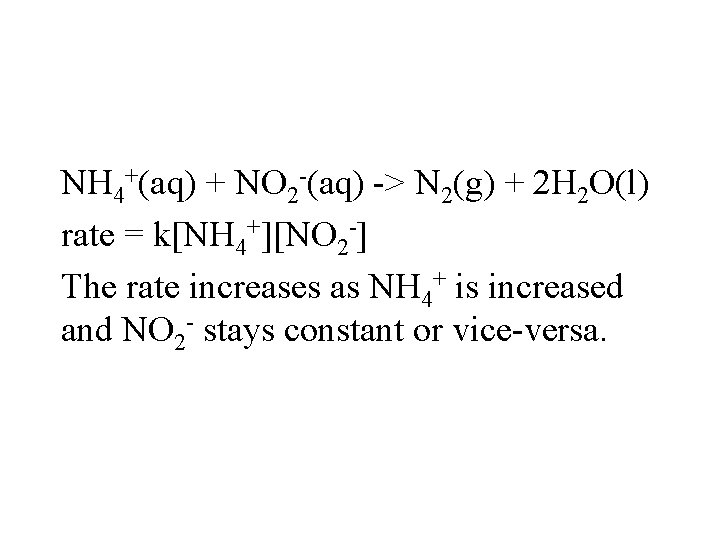
NH 4+(aq) + NO 2 -(aq) -> N 2(g) + 2 H 2 O(l) rate = k[NH 4+][NO 2 -] The rate increases as NH 4+ is increased and NO 2 - stays constant or vice-versa.

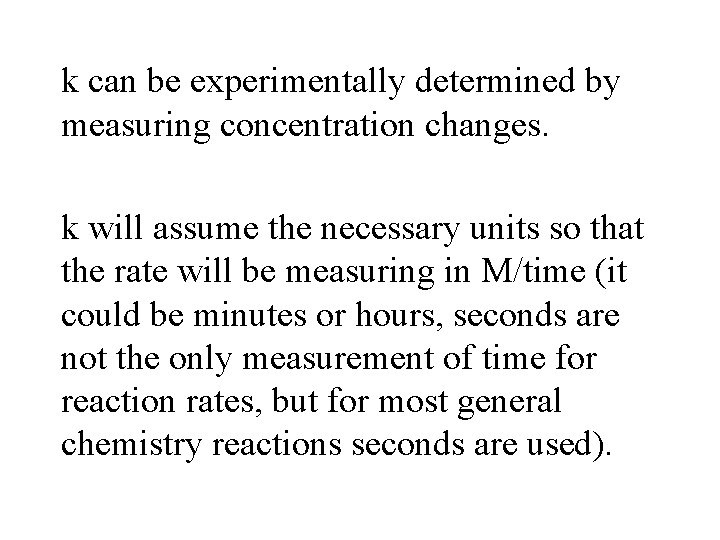
k can be experimentally determined by measuring concentration changes. k will assume the necessary units so that the rate will be measuring in M/time (it could be minutes or hours, seconds are not the only measurement of time for reaction rates, but for most general chemistry reactions seconds are used).
![The general rate law equation is rate k reactant 1m reactant 2n The general rate law equation is: rate = k [reactant 1]m [reactant 2]n. .](https://slidetodoc.com/presentation_image_h/099cdca299829da9ff7335301dd05d8b/image-8.jpg)
The general rate law equation is: rate = k [reactant 1]m [reactant 2]n. . .

m and n (the exponents) are determined experimentally. They may coincide with the coefficients, but often they are unrelated to them.
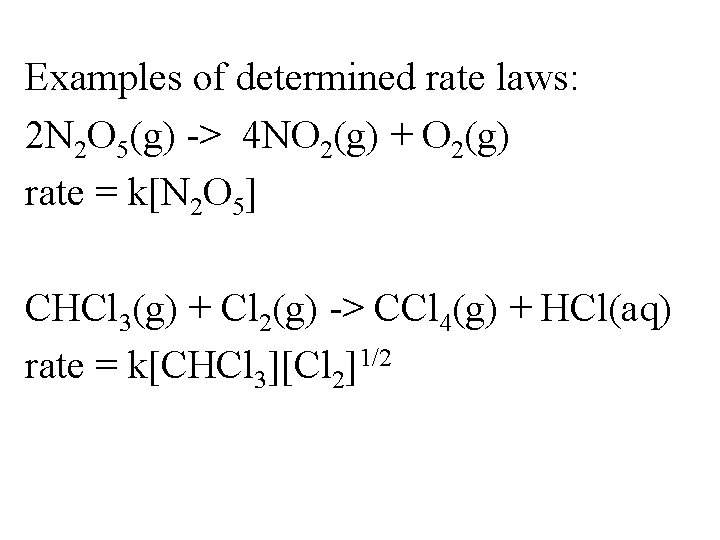
Examples of determined rate laws: 2 N 2 O 5(g) -> 4 NO 2(g) + O 2(g) rate = k[N 2 O 5] CHCl 3(g) + Cl 2(g) -> CCl 4(g) + HCl(aq) rate = k[CHCl 3][Cl 2]1/2
![H 2g I 2g 2 HIg rate kH 2I 2 exponents H 2(g) + I 2(g) -> 2 HI(g) rate = k[H 2][I 2] exponents](https://slidetodoc.com/presentation_image_h/099cdca299829da9ff7335301dd05d8b/image-11.jpg)
H 2(g) + I 2(g) -> 2 HI(g) rate = k[H 2][I 2] exponents are the rate order

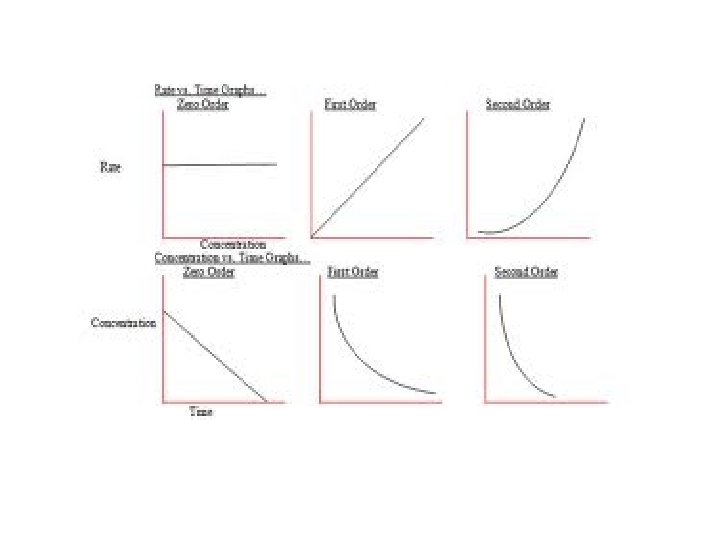
Rate order of 0, 1, and 2 are the most common. 0 means the concentration is not a factor. 1 means that the rate increases proportionally with the increase in concentration. 2 means the rate increases exponentially with increased concentration.


To determine reaction orders a series of experiments are done collecting the concentration of the reacting species and the initial rates.

Steps to determine the rate of each species 1. Compare just two experiments. 2. Make sure that the concentration of one species remains constant (or only one changes). 3. Determine what happens to the concentration. Does it double, does it triple? 4. Look at the rate, does it double, triple, stay the same?

If the concentration of the species changes, but the rate remains constant, it is zero order. If the concentration of the species doubles and the rate doubles, the order is first.

If the concentration doubles and the rate is the concentration squared, the order is second.

Units on K If the overall order is 0, units are M/s 1, /s 2, M-1 s-1 3, M-2 s-1

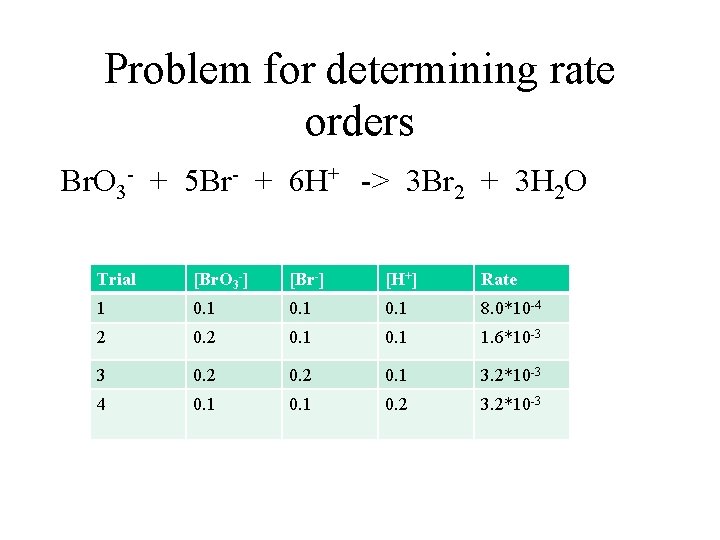
Problem for determining rate orders Br. O 3 - + 5 Br- + 6 H+ -> 3 Br 2 + 3 H 2 O Trial [Br. O 3 -] [Br-] [H+] Rate 1 0. 1 8. 0*10 -4 2 0. 1 1. 6*10 -3 3 0. 2 0. 1 3. 2*10 -3 4 0. 1 0. 2 3. 2*10 -3

In experiment 1 and experiment 2, Br- has a a concentration of 0. 10 M and H+ has a concentration of 0. 10 M. Now look at the concentrations of Br. O 3 - and decide how it was changed. The Br. O 3 - concentration is doubled. Then you have to compare it to the quotient of the initial rates. So: 1. 6 x 10 -3 / 8. 0 x 10 -4 = 2 times faster [2 Br. O 3 -]x = 2 times rate, so x = 1; the order for Br. O 3 - is first order

In both experiment 2 and experiment 3, Br. O 3 - has a concentration of 0. 20 M and H+ has a concentration of 0. 10 M. Now look at the concentration of Br- and decide how it was changed and what effect it had on the reaction rate. The concentration 1 was doubled, and the rate doubled. This is found by taking the quotient of the initial rates: 3. 2 x 10 -3 / 1. 6 x 10 -3 = 2 times faster [2 Br-]y = 2 times rate, so y = 1; the order for Br- is first.

Finally, In both experiment 1 and experiment 4, Br. O 3 - has a concentration of 0. 10 M and Br- has a concentration of 0. 10 M. Now look at the concentrations of H+ and decide how it was changed and what effect it had on the reaction rate. The concentration 1 was doubled, and the rate goes up by four times. This is found by taking the quotient of the initial rates: 3. 2 x 10 -3 / 8. 0 x 10 -4 = 4 times faster [2 H+]z = 4 times rate, so z = 2; the order for H+ is second.
![The rate law for the reaction therefore is Rate kBr O 3 1 The rate law for the reaction, therefore is: Rate = k[Br. O 3 -]1](https://slidetodoc.com/presentation_image_h/099cdca299829da9ff7335301dd05d8b/image-23.jpg)
The rate law for the reaction, therefore is: Rate = k[Br. O 3 -]1 [Br-]1 [H+]2 or Rate = k[Br. O 3 -] [Br-] [H+]2