Section 16 3 Reaction Rate Law Rate law




![Rate = k[A]m[B]n Reaction order = m + n If Rate = k [A]1[B]0 Rate = k[A]m[B]n Reaction order = m + n If Rate = k [A]1[B]0](https://slidetodoc.com/presentation_image_h2/ab0f0862d5093301cda26780ba3a280f/image-7.jpg)













- Slides: 26

Section 16. 3 Reaction Rate Law

Rate law Specific rate constant Reaction order Method of initial rates

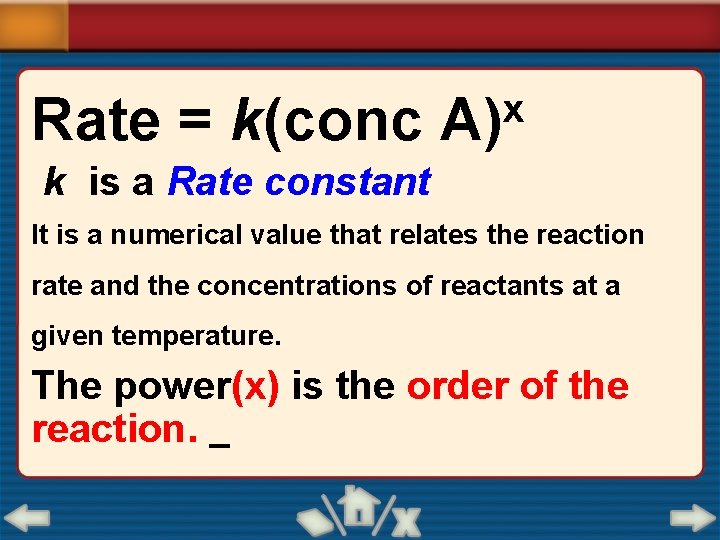
Rate = k(conc x A) k is a Rate constant It is a numerical value that relates the reaction rate and the concentrations of reactants at a given temperature. The power(x) is the order of the reaction.

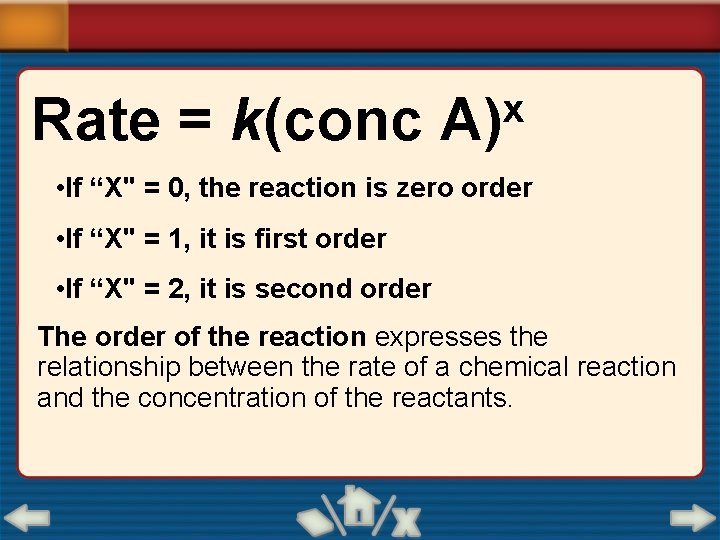
Rate = k(conc x A) • If “X" = 0, the reaction is zero order • If “X" = 1, it is first order • If “X" = 2, it is second order The order of the reaction expresses the relationship between the rate of a chemical reaction and the concentration of the reactants.

If( x) = 0 the reaction is zero order – rate is independent of the concentration. no effect on rate If x = 1: the reaction is first order - rate is directly proportional to the concentration of the reactant. Doubling the concentration increases the rate by a factor of 2. Doubling the concentration , doubles the rate of the reaction

1 6. 3 SECTION Reaction Rate Laws The specific rate constant( k ) is unique for every reaction. The unit for expressing a rate is mol /L ● s
![Rate kAmBn Reaction order m n If Rate k A1B0 Rate = k[A]m[B]n Reaction order = m + n If Rate = k [A]1[B]0](https://slidetodoc.com/presentation_image_h2/ab0f0862d5093301cda26780ba3a280f/image-7.jpg)
Rate = k[A]m[B]n Reaction order = m + n If Rate = k [A]1[B]0 = k [A] Then the reaction is 1 st order in [A] and 0 th order in [B] 1+0 = The reaction is 1 st order.

Find the order of the reaction Example: CO + NO 2 -----> CO 2 + NO rate = k(conc CO)m(conc NO 2)n The coefficient of CO=1 The coefficient of NO 2 =1 First order with respect to CO, m = 1 First order with respect to NO 2, n = 1 second order overall, m + n = 2

1 6. 3 SECTION Reaction Rate Laws Writing Reaction Rate Laws • A rate law expresses the relationship between the rate of a chemical reaction and the concentration of the reactants.

1 6. 3 SECTION Reaction Rate Laws Writing Reaction Rate Laws (cont. ) • The symbol k is the specific rate constant, a numerical value that relates the reaction rate and the concentrations of reactants at a given temperature. • The specific rate constant is unique for every reaction.

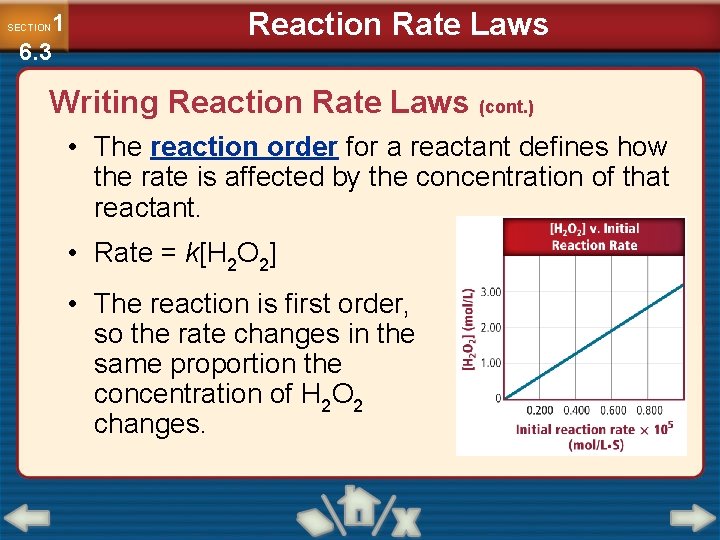
1 6. 3 SECTION Reaction Rate Laws Writing Reaction Rate Laws (cont. ) • The reaction order for a reactant defines how the rate is affected by the concentration of that reactant. • Rate = k[H 2 O 2] • The reaction is first order, so the rate changes in the same proportion the concentration of H 2 O 2 changes.

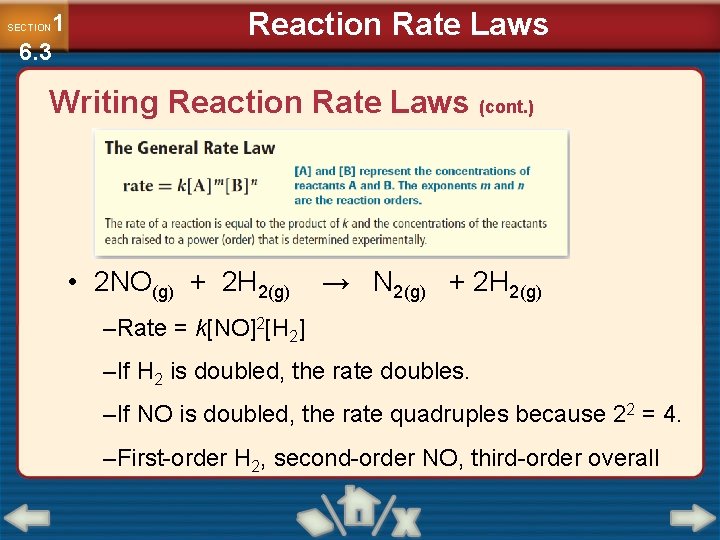
1 6. 3 SECTION Reaction Rate Laws Writing Reaction Rate Laws (cont. ) • 2 NO(g) + 2 H 2(g) → N 2(g) + 2 H 2(g) –Rate = k[NO]2[H 2] –If H 2 is doubled, the rate doubles. –If NO is doubled, the rate quadruples because 22 = 4. –First-order H 2, second-order NO, third-order overall

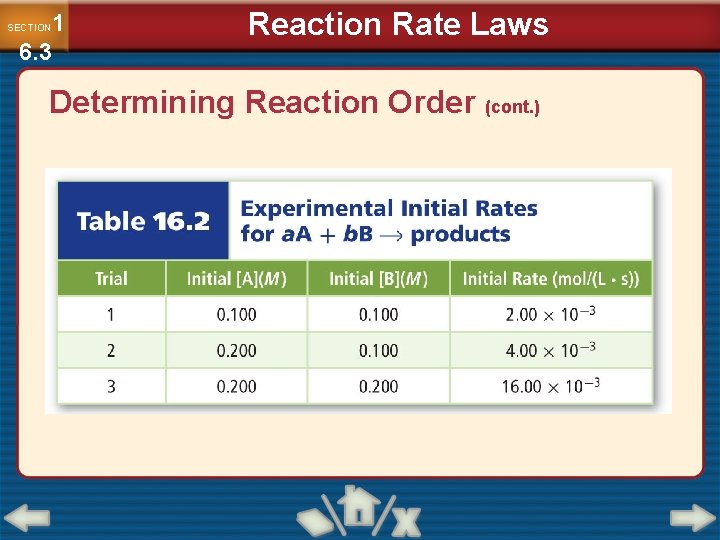
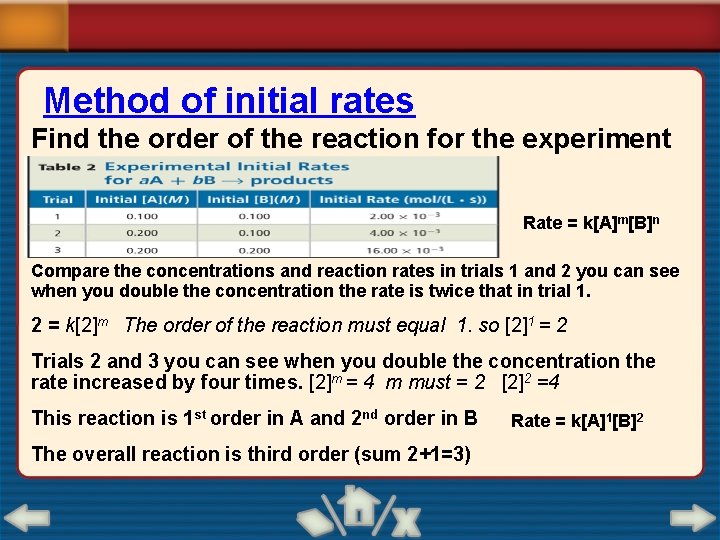
1 6. 3 SECTION Reaction Rate Laws Determining Reaction Order • The method of initial rates determines reaction order by comparing the initial rates of a reaction carried out with varying reactant concentrations. • Initial rate measures how fast the reaction proceeds at the moment when reactants are mixed.

1 6. 3 SECTION Reaction Rate Laws Determining Reaction Order (cont. )

Method of initial rates Find the order of the reaction for the experiment Rate = k[A]m[B]n Compare the concentrations and reaction rates in trials 1 and 2 you can see when you double the concentration the rate is twice that in trial 1. 2 = k[2]m The order of the reaction must equal 1. so [2]1 = 2 Trials 2 and 3 you can see when you double the concentration the rate increased by four times. [2]m = 4 m must = 2 [2]2 =4 This reaction is 1 st order in A and 2 nd order in B The overall reaction is third order (sum 2+1=3) Rate = k[A]1[B]2

1 6. 3 SECTION Reaction Rate Laws Determining Reaction Order (cont. ) • Doubling [A] doubles the reaction rate, so [A] is first order. • Doubling [B] quadruples the reaction rate, so [B] is second order. • Rate = k[A][B]2

See your notebook for the answer.

1 6. 3 SECTION Section Check What is the overall reaction order of the following reaction? Rate = k[A]2[B]2 A. 1 st B. 2 nd C. 3 rd D. 4 th

1 6. 3 SECTION Section Check In the following reaction, what is the overall reaction order if doubling [A] results in quadrupling the reaction rate and doubling [B] results in a reaction rate eight times faster? Rate = k[A]m[B]n A. 12 B. 5 C. 6 D. 10

See your notebook for the answer.

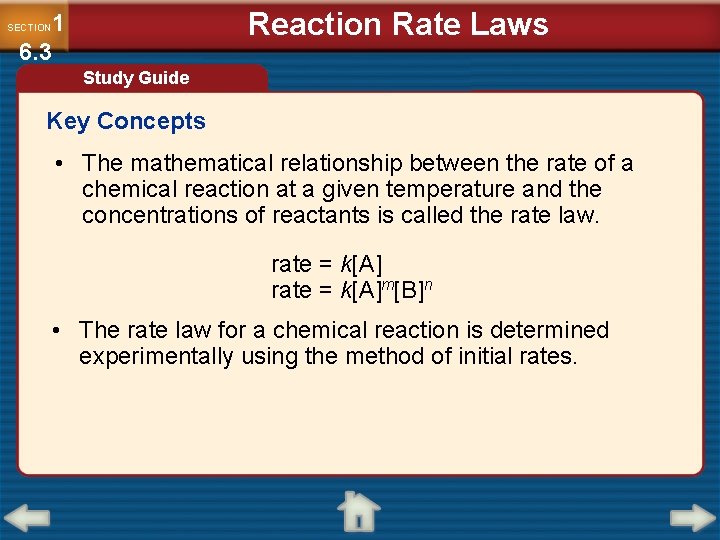
Reaction Rate Laws 1 6. 3 SECTION Study Guide Key Concepts • The mathematical relationship between the rate of a chemical reaction at a given temperature and the concentrations of reactants is called the rate law. rate = k[A]m[B]n • The rate law for a chemical reaction is determined experimentally using the method of initial rates.

1 6. 3 SECTION Section Check What is the overall reaction order of the following reaction? Rate = k[A]2[B]2 A. 1 st B. 2 nd C. 3 rd D. 4 th

1 6. 3 SECTION Section Check In the following reaction, what is the overall reaction order if doubling [A] results in quadrupling the reaction rate and doubling [B] results in a reaction rate eight times faster? Rate = k[A]m[B]n A. 12 B. 5 C. 6 D. 10

CHAPTER 16 Reaction Rates Chapter Assessment What is the overall reaction order of the following reaction? Rate = k[A][B]2[C] A. 1 st order B. 2 nd order C. 3 rd order D. 4 th order

CHAPTER 16 Reaction Rates Standardized Test Practice Doubling the concentration of one reactant in a reaction causes the reaction rate to double. What is the order of that reactant? A. 1 st B. 2 nd C. unable to determine D. none of the above

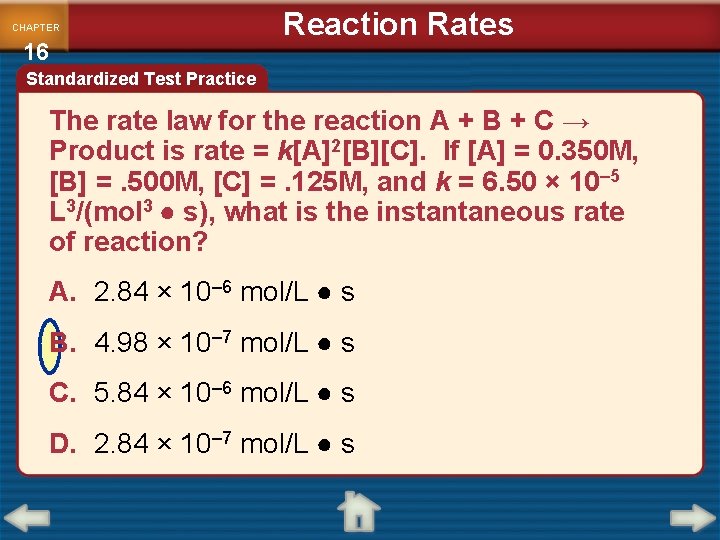
CHAPTER 16 Reaction Rates Standardized Test Practice The rate law for the reaction A + B + C → Product is rate = k[A]2[B][C]. If [A] = 0. 350 M, [B] =. 500 M, [C] =. 125 M, and k = 6. 50 × 10– 5 L 3/(mol 3 ● s), what is the instantaneous rate of reaction? A. 2. 84 × 10– 6 mol/L ● s B. 4. 98 × 10– 7 mol/L ● s C. 5. 84 × 10– 6 mol/L ● s D. 2. 84 × 10– 7 mol/L ● s