Name Reaction DielsAlder reaction The reaction in which


- Slides: 14

Name Reaction


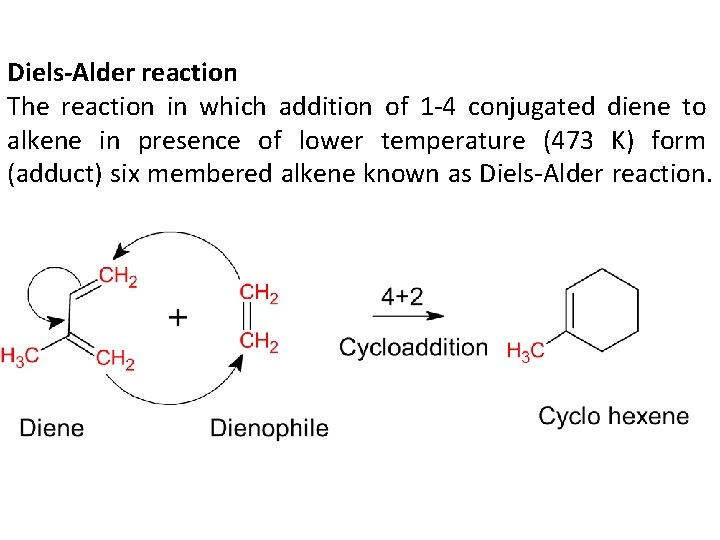
Diels-Alder reaction The reaction in which addition of 1 -4 conjugated diene to alkene in presence of lower temperature (473 K) form (adduct) six membered alkene known as Diels-Alder reaction.

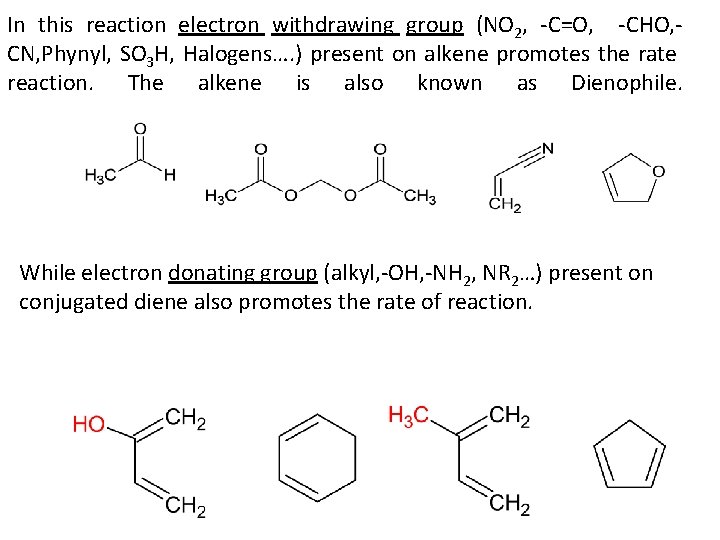
In this reaction electron withdrawing group (NO 2, -C=O, -CHO, CN, Phynyl, SO 3 H, Halogens…. ) present on alkene promotes the rate reaction. The alkene is also known as Dienophile. While electron donating group (alkyl, -OH, -NH 2, NR 2…) present on conjugated diene also promotes the rate of reaction.

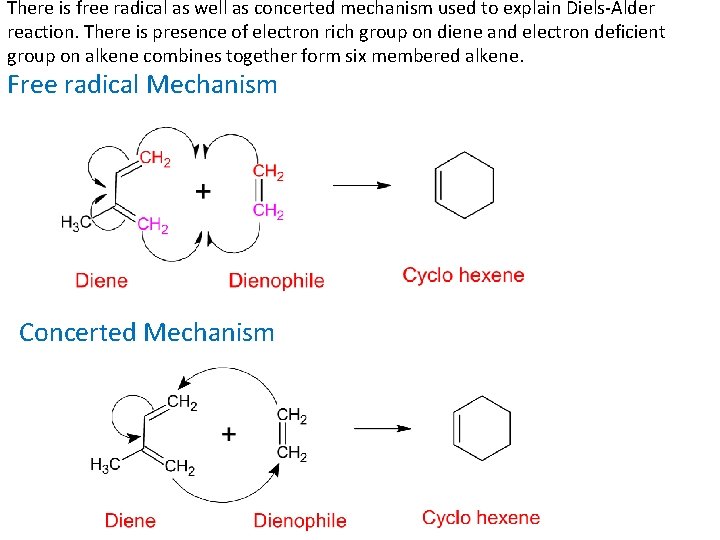
There is free radical as well as concerted mechanism used to explain Diels-Alder reaction. There is presence of electron rich group on diene and electron deficient group on alkene combines together form six membered alkene. Free radical Mechanism Concerted Mechanism

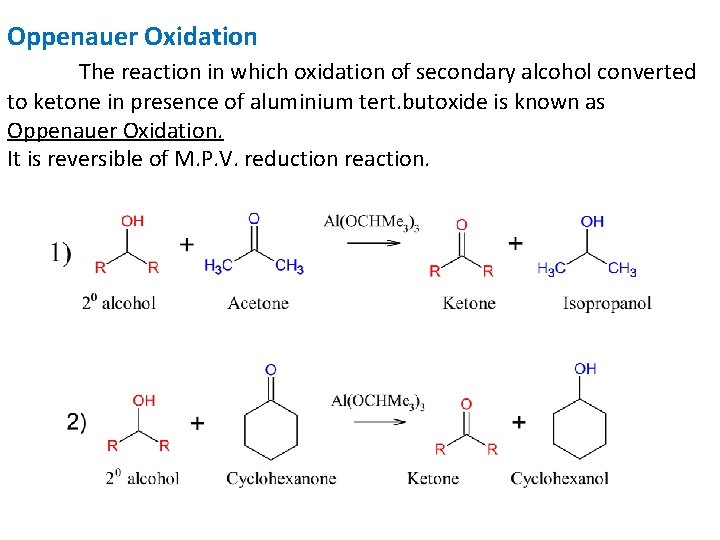
Oppenauer Oxidation The reaction in which oxidation of secondary alcohol converted to ketone in presence of aluminium tert. butoxide is known as Oppenauer Oxidation. It is reversible of M. P. V. reduction reaction.

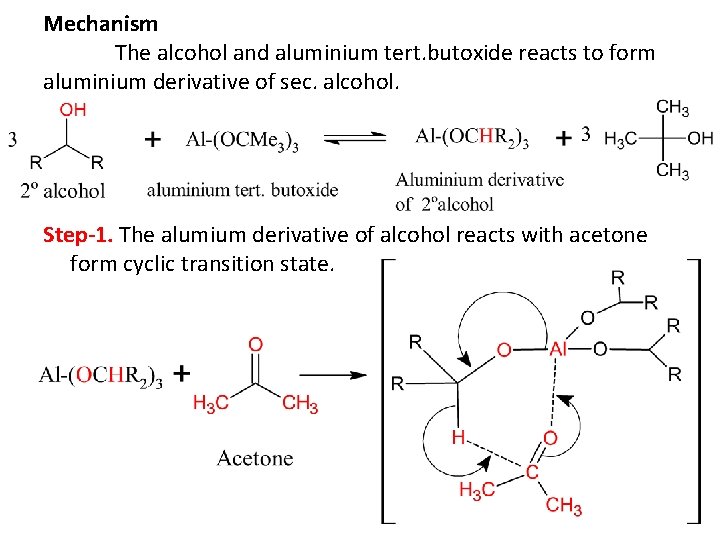
Mechanism The alcohol and aluminium tert. butoxide reacts to form aluminium derivative of sec. alcohol. Step-1. The alumium derivative of alcohol reacts with acetone form cyclic transition state.

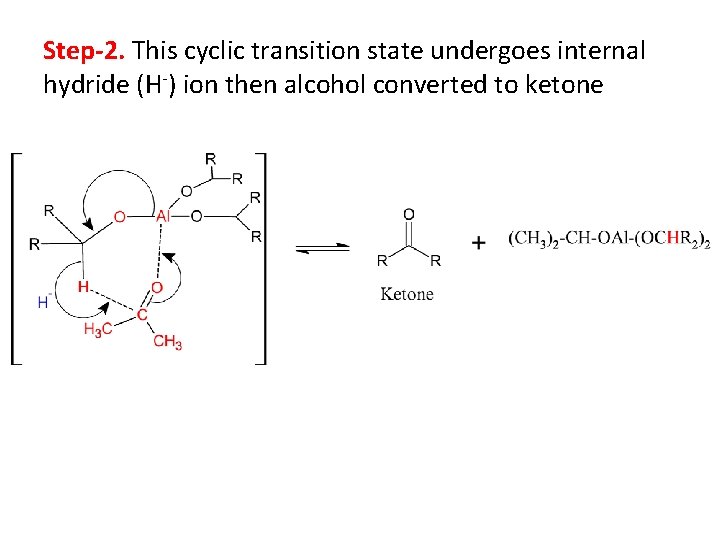
Step-2. This cyclic transition state undergoes internal hydride (H-) ion then alcohol converted to ketone

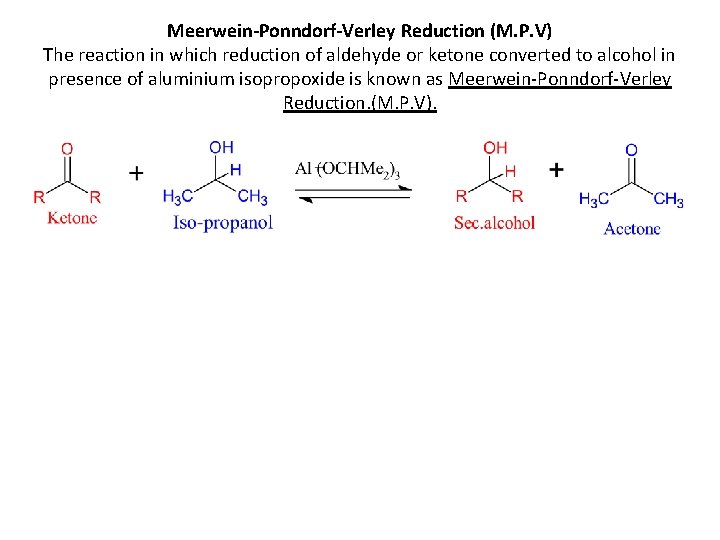
Meerwein-Ponndorf-Verley Reduction (M. P. V) The reaction in which reduction of aldehyde or ketone converted to alcohol in presence of aluminium isopropoxide is known as Meerwein-Ponndorf-Verley Reduction. (M. P. V).

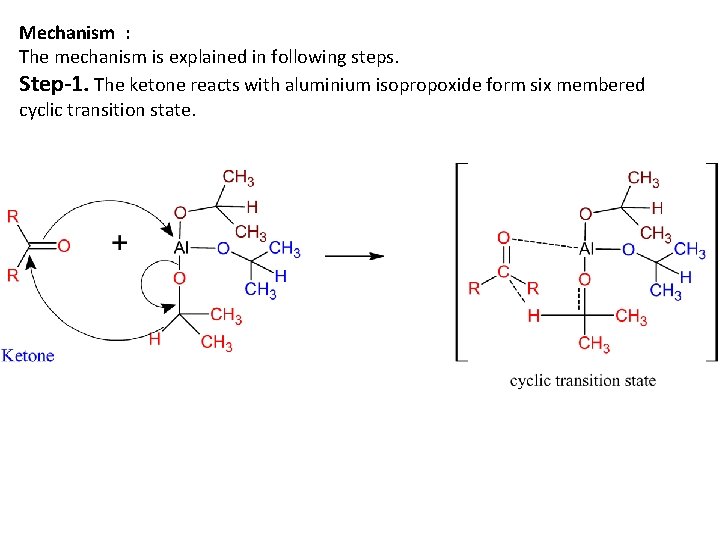
Mechanism : The mechanism is explained in following steps. Step-1. The ketone reacts with aluminium isopropoxide form six membered cyclic transition state.

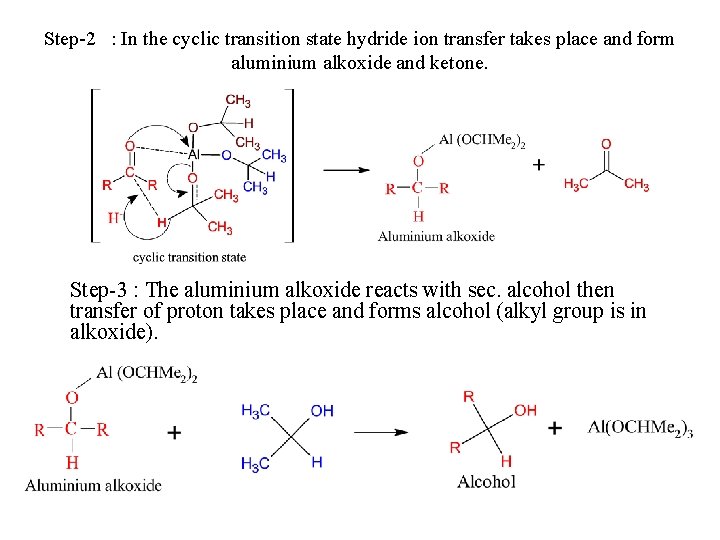
Step-2 : In the cyclic transition state hydride ion transfer takes place and form aluminium alkoxide and ketone. Step-3 : The aluminium alkoxide reacts with sec. alcohol then transfer of proton takes place and forms alcohol (alkyl group is in alkoxide).

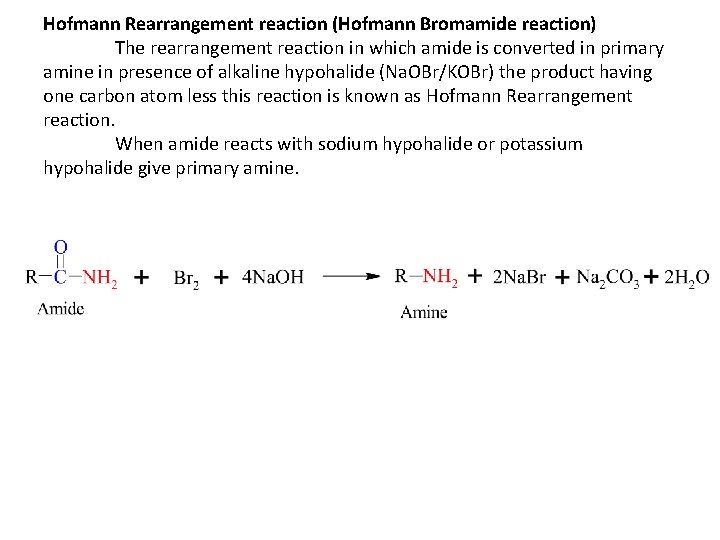
Hofmann Rearrangement reaction (Hofmann Bromamide reaction) The rearrangement reaction in which amide is converted in primary amine in presence of alkaline hypohalide (Na. OBr/KOBr) the product having one carbon atom less this reaction is known as Hofmann Rearrangement reaction. When amide reacts with sodium hypohalide or potassium hypohalide give primary amine.

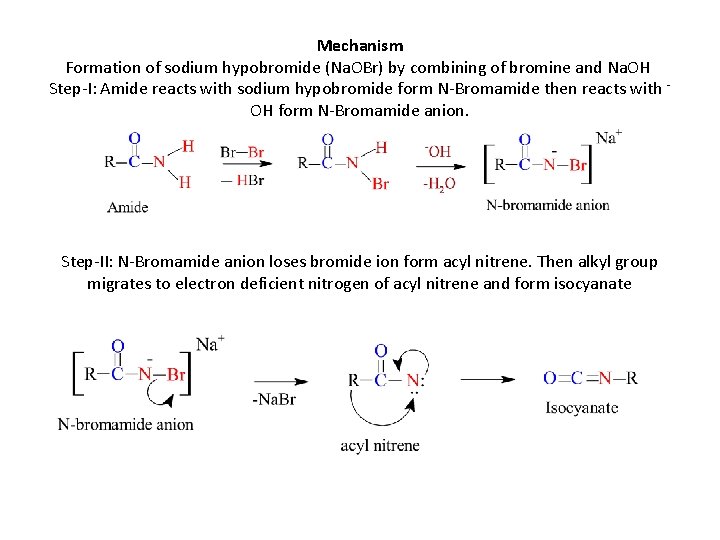
Mechanism Formation of sodium hypobromide (Na. OBr) by combining of bromine and Na. OH Step-I: Amide reacts with sodium hypobromide form N-Bromamide then reacts with - OH form N-Bromamide anion. Step-II: N-Bromamide anion loses bromide ion form acyl nitrene. Then alkyl group migrates to electron deficient nitrogen of acyl nitrene and form isocyanate

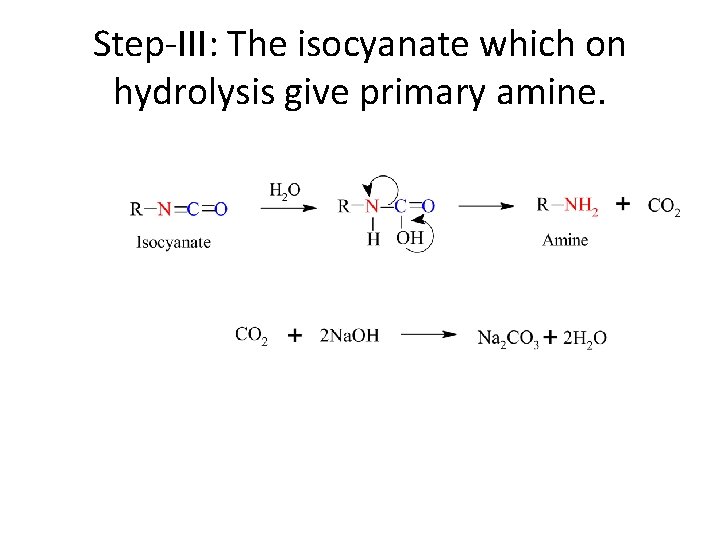
Step-III: The isocyanate which on hydrolysis give primary amine.