DielsAlder Reaction u DielsAlder reaction A cycloaddition reaction

- Slides: 26

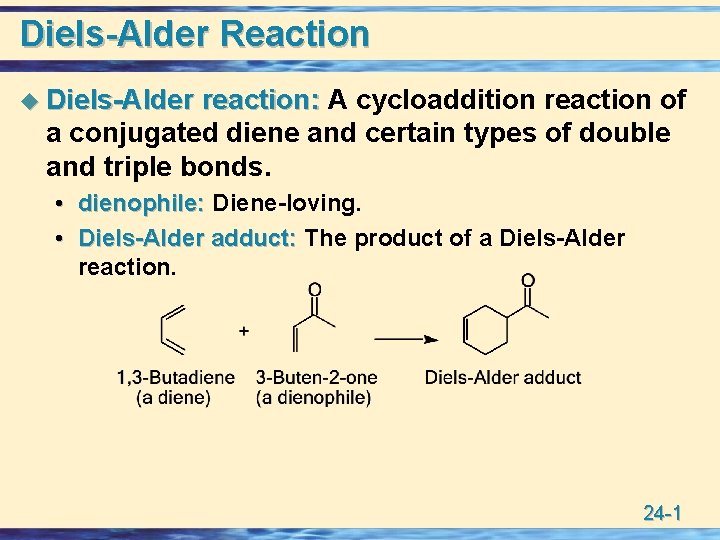
Diels-Alder Reaction u Diels-Alder reaction: A cycloaddition reaction of a conjugated diene and certain types of double and triple bonds. • dienophile: Diene-loving. • Diels-Alder adduct: The product of a Diels-Alder reaction. 24 -1

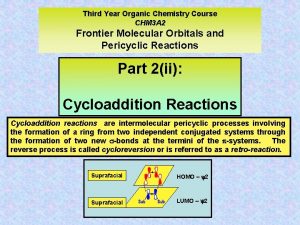
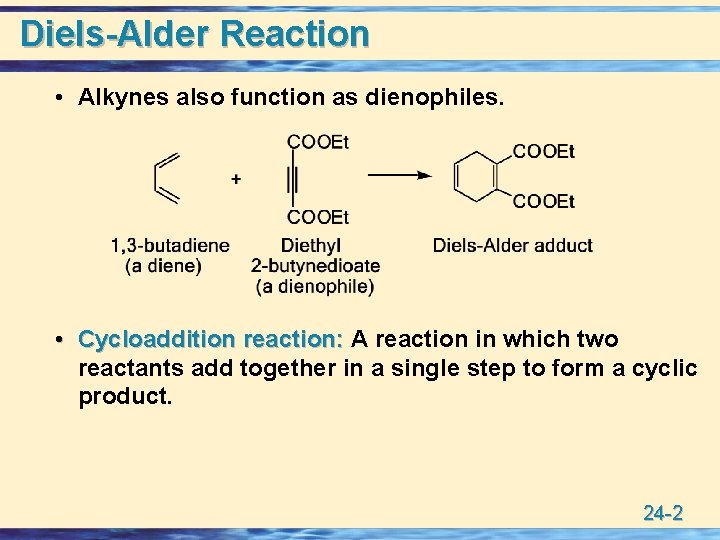
Diels-Alder Reaction • Alkynes also function as dienophiles. • Cycloaddition reaction: A reaction in which two reactants add together in a single step to form a cyclic product. 24 -2

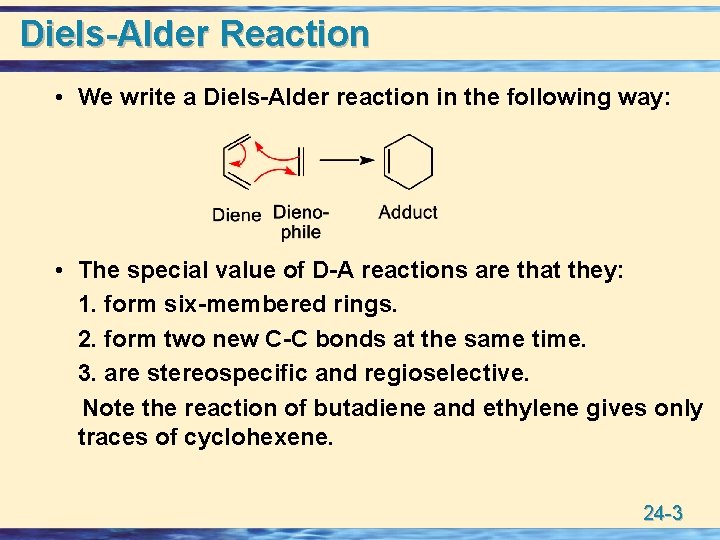
Diels-Alder Reaction • We write a Diels-Alder reaction in the following way: • The special value of D-A reactions are that they: 1. form six-membered rings. 2. form two new C-C bonds at the same time. 3. are stereospecific and regioselective. Note the reaction of butadiene and ethylene gives only traces of cyclohexene. 24 -3

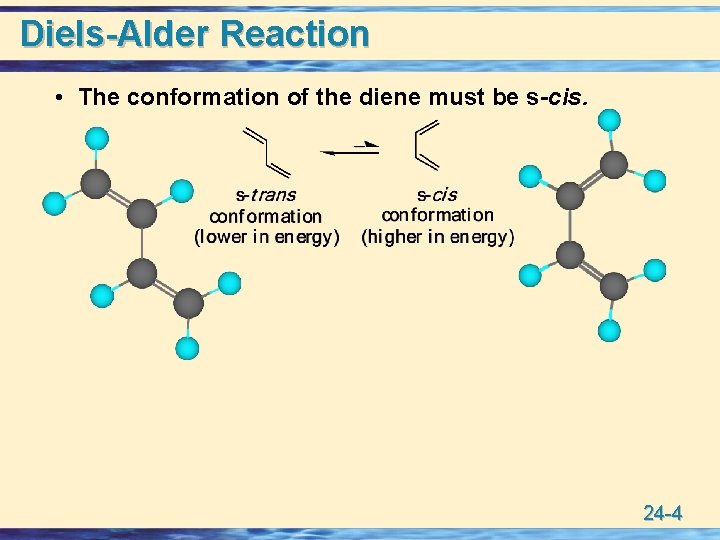
Diels-Alder Reaction • The conformation of the diene must be s-cis. 24 -4

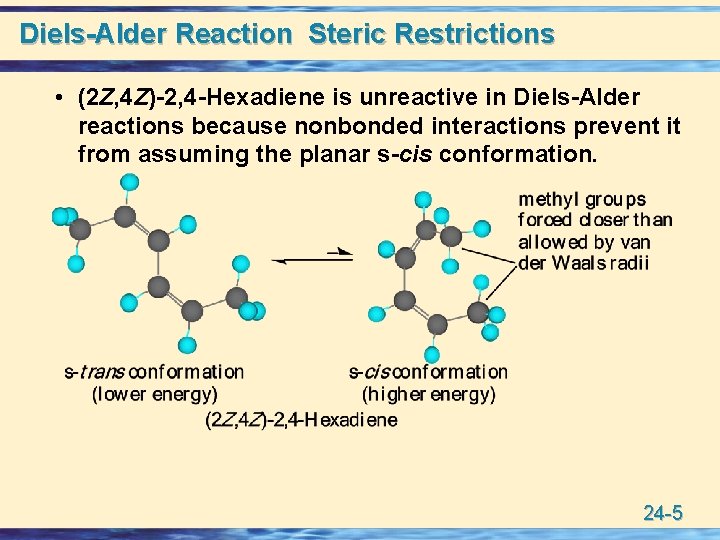
Diels-Alder Reaction Steric Restrictions • (2 Z, 4 Z)-2, 4 -Hexadiene is unreactive in Diels-Alder reactions because nonbonded interactions prevent it from assuming the planar s-cis conformation. 24 -5

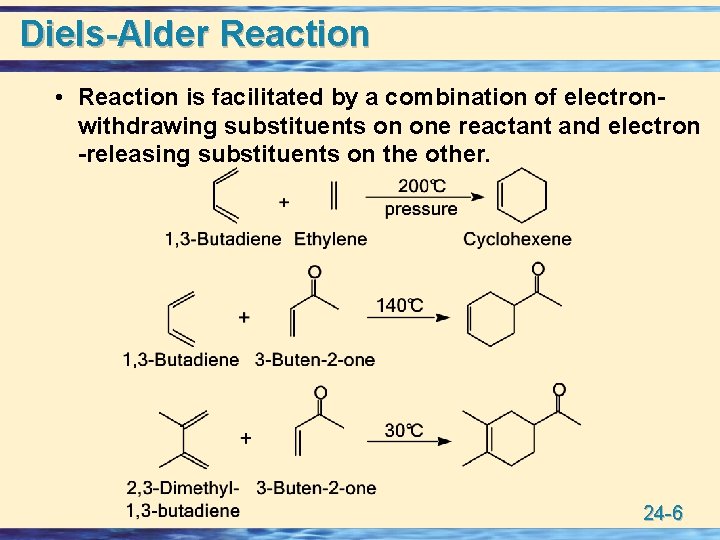
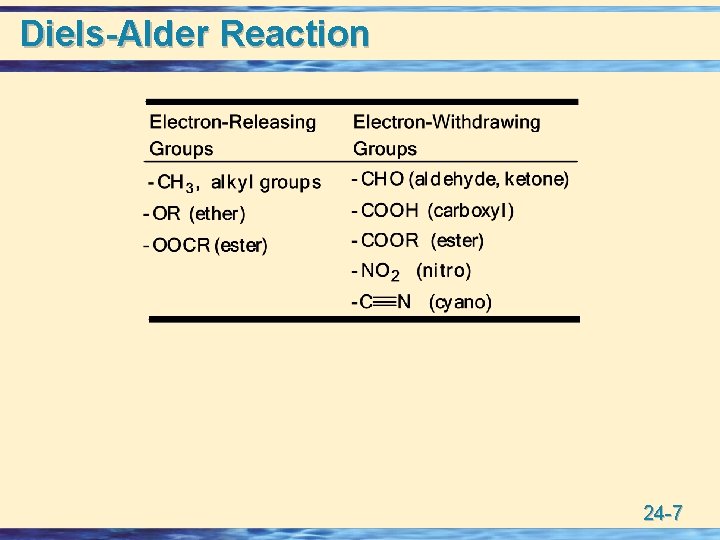
Diels-Alder Reaction • Reaction is facilitated by a combination of electronwithdrawing substituents on one reactant and electron -releasing substituents on the other. 24 -6

Diels-Alder Reaction 24 -7

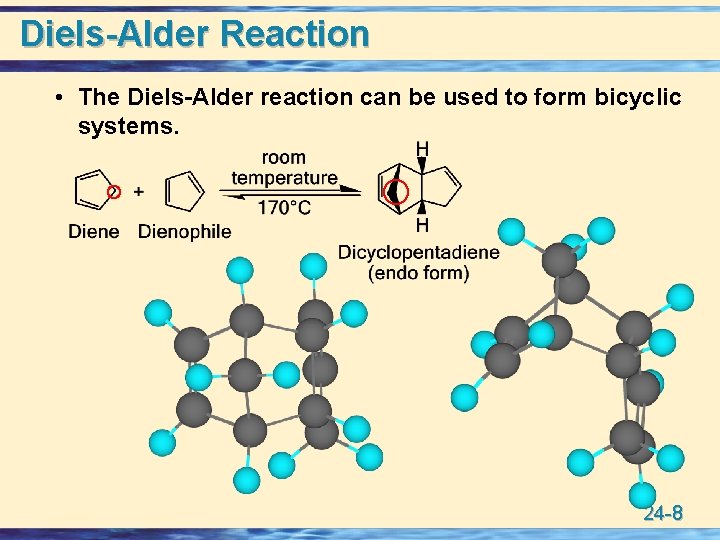
Diels-Alder Reaction • The Diels-Alder reaction can be used to form bicyclic systems. 24 -8

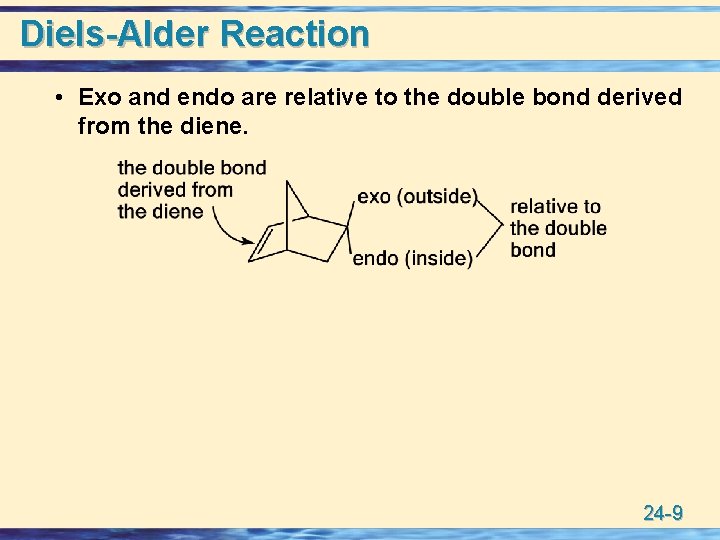
Diels-Alder Reaction • Exo and endo are relative to the double bond derived from the diene. 24 -9

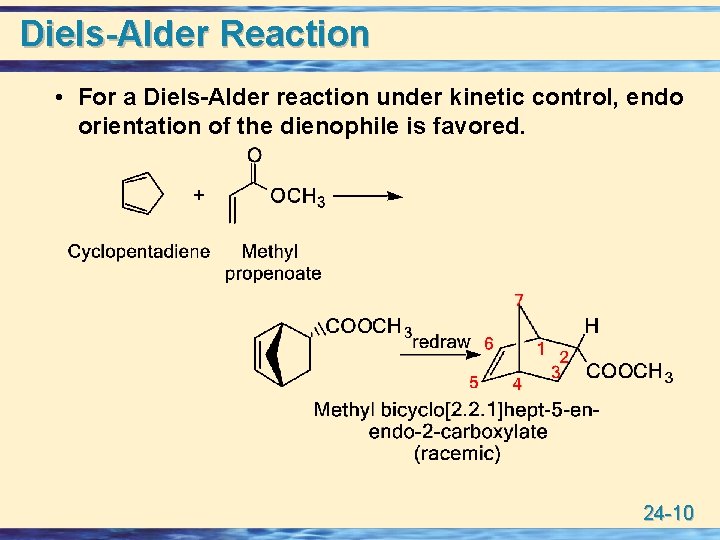
Diels-Alder Reaction • For a Diels-Alder reaction under kinetic control, endo orientation of the dienophile is favored. 24 -10

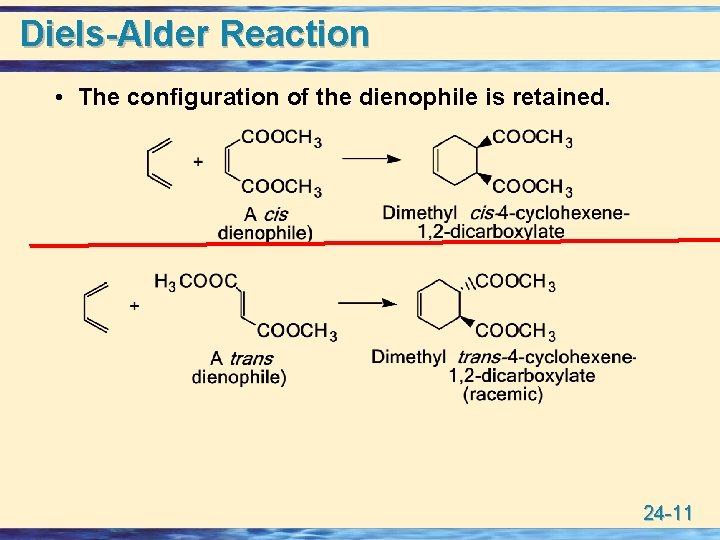
Diels-Alder Reaction • The configuration of the dienophile is retained. 24 -11

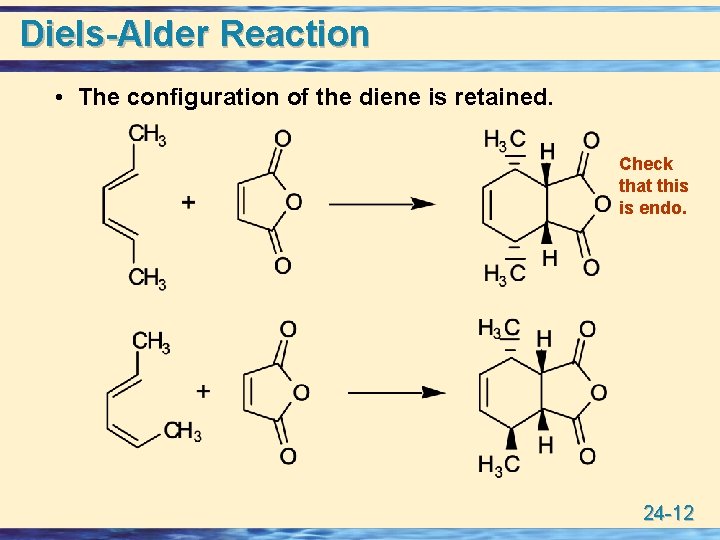
Diels-Alder Reaction • The configuration of the diene is retained. Check that this is endo. 24 -12

Diels-Alder Reaction u Mechanism • No evidence for the participation of either radical of ionic intermediates. • Chemists propose that the Diels-Alder reaction is a concerted pericyclic reaction. u Pericyclic reaction: reaction A reaction that takes place in a single step, without intermediates, and involves a cyclic redistribution of bonding electrons. u Concerted reaction: All bond making and bond breaking occurs simultaneously. 24 -13

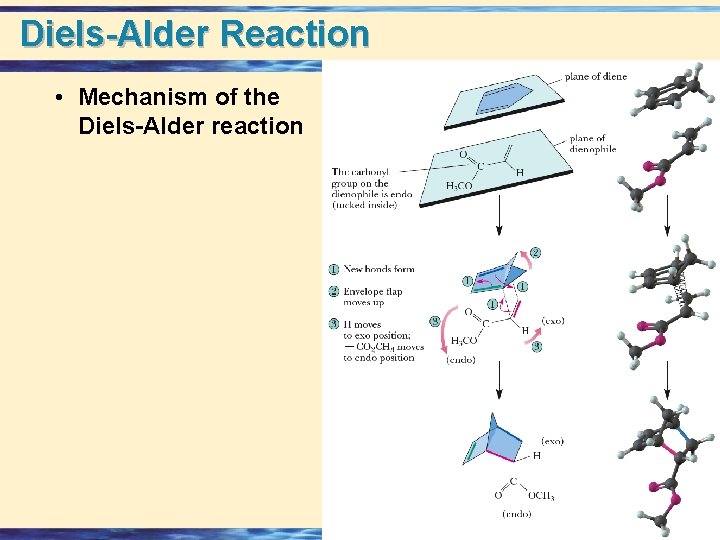
Diels-Alder Reaction • Mechanism of the Diels-Alder reaction 24 -14

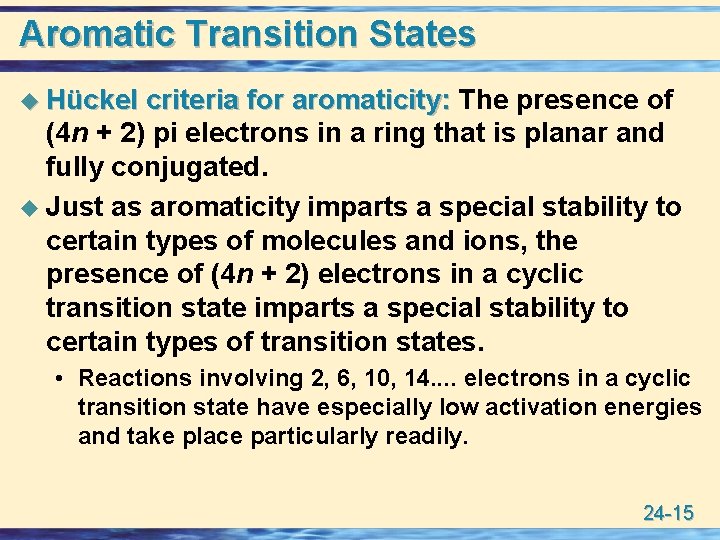
Aromatic Transition States u Hückel criteria for aromaticity: The presence of (4 n + 2) pi electrons in a ring that is planar and fully conjugated. u Just as aromaticity imparts a special stability to certain types of molecules and ions, the presence of (4 n + 2) electrons in a cyclic transition state imparts a special stability to certain types of transition states. • Reactions involving 2, 6, 10, 14. . electrons in a cyclic transition state have especially low activation energies and take place particularly readily. 24 -15

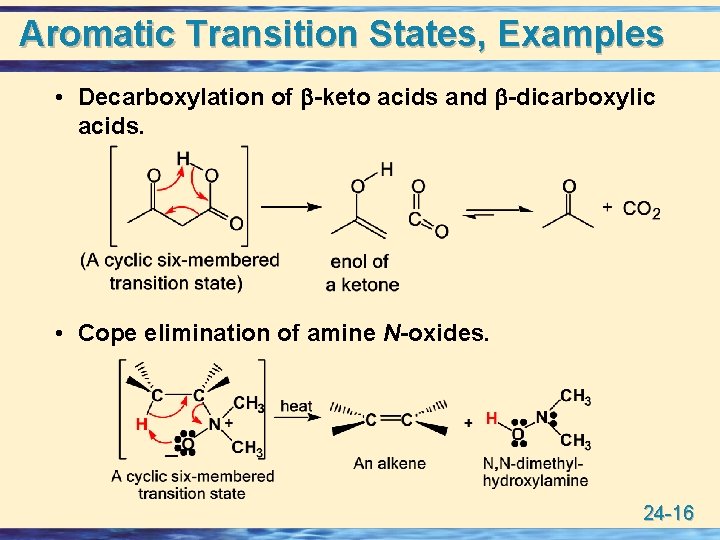
Aromatic Transition States, Examples • Decarboxylation of -keto acids and -dicarboxylic acids. • Cope elimination of amine N-oxides. 24 -16

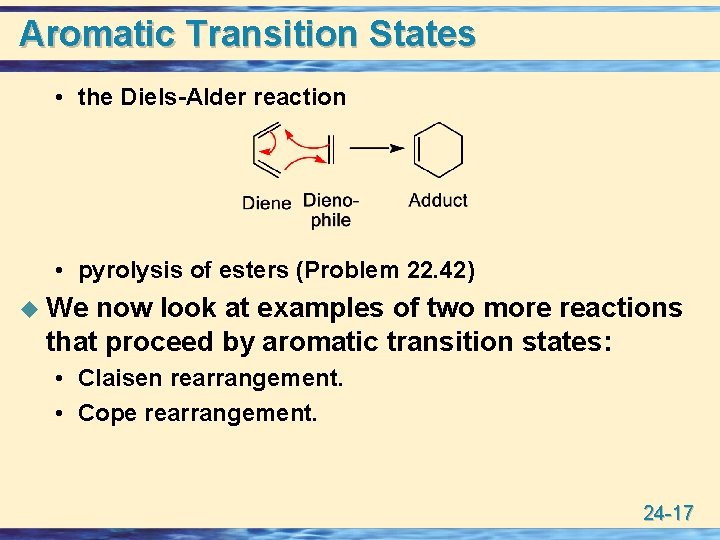
Aromatic Transition States • the Diels-Alder reaction • pyrolysis of esters (Problem 22. 42) u We now look at examples of two more reactions that proceed by aromatic transition states: • Claisen rearrangement. • Cope rearrangement. 24 -17

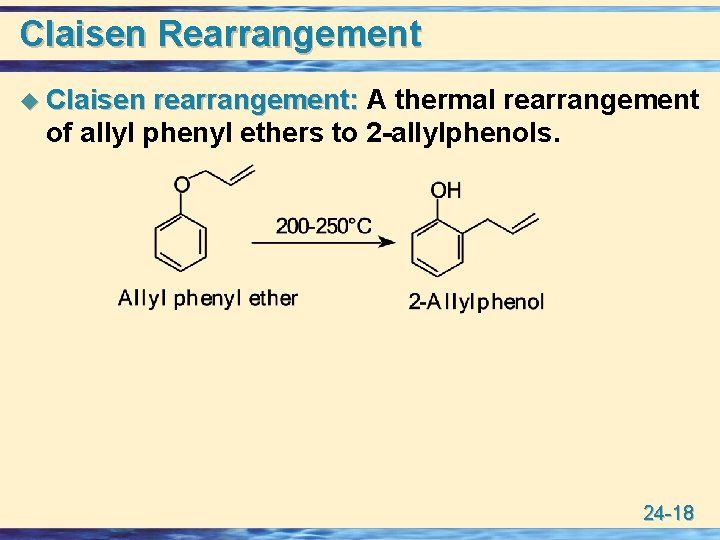
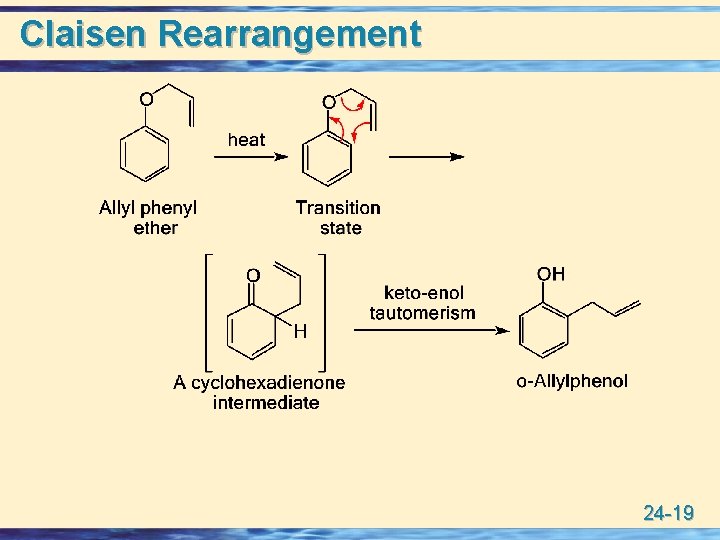
Claisen Rearrangement u Claisen rearrangement: A thermal rearrangement of allyl phenyl ethers to 2 -allylphenols. 24 -18

Claisen Rearrangement 24 -19

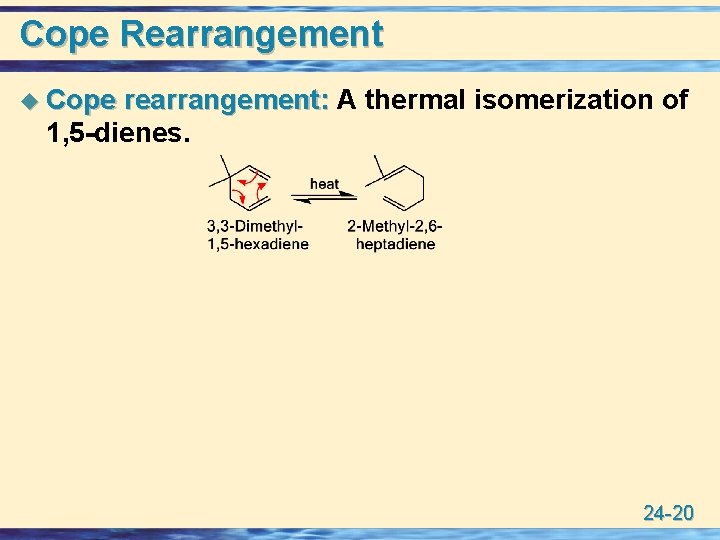
Cope Rearrangement u Cope rearrangement: A thermal isomerization of 1, 5 -dienes. 24 -20

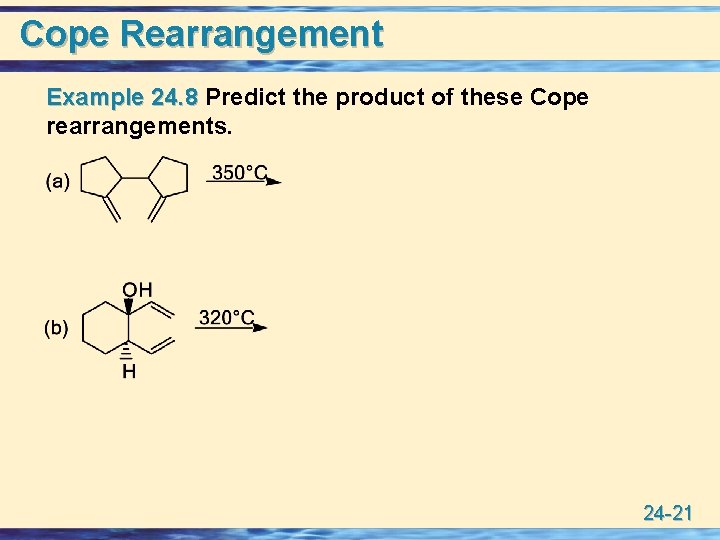
Cope Rearrangement Example 24. 8 Predict the product of these Cope rearrangements. 24 -21

Synthesis of Single Enantiomers • We have stressed throughout the text that the synthesis of chiral products from achiral starting materials and under achiral reaction conditions of necessity gives enantiomers as a racemic mixture. • Nature achieves the synthesis of single enantiomers by using enzymes, which create a chiral environment in which reaction takes place. • Enzymes show high enantiomeric and diastereomeric selectivity with the result that enzyme-catalyzed reactions invariably give only one of all possible stereoisomers. 24 -22

Synthesis of Single Enantiomers u How do chemists achieve the synthesis of single enantiomers? u The most common method is to produce a racemic mixture and then resolve it. How? • the different physical properties of diastereomeric salts. • the use of enzymes as resolving agents. • chromatographic on a chiral substrate. 24 -23

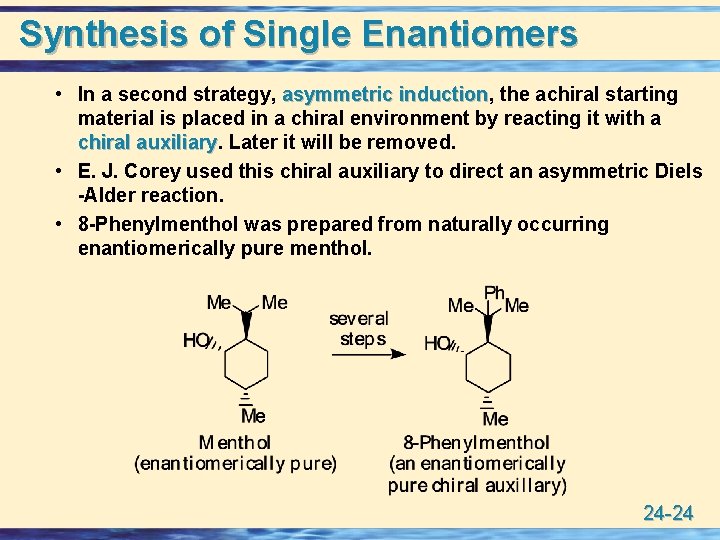
Synthesis of Single Enantiomers • In a second strategy, asymmetric induction, induction the achiral starting material is placed in a chiral environment by reacting it with a chiral auxiliary Later it will be removed. • E. J. Corey used this chiral auxiliary to direct an asymmetric Diels -Alder reaction. • 8 -Phenylmenthol was prepared from naturally occurring enantiomerically pure menthol. 24 -24

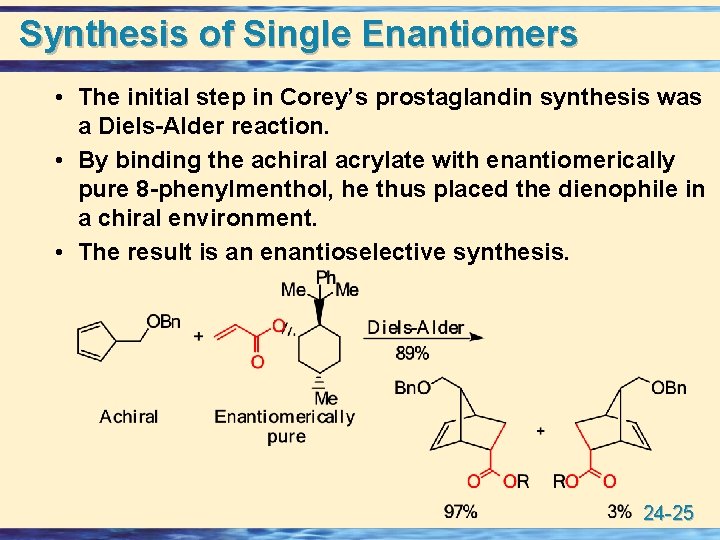
Synthesis of Single Enantiomers • The initial step in Corey’s prostaglandin synthesis was a Diels-Alder reaction. • By binding the achiral acrylate with enantiomerically pure 8 -phenylmenthol, he thus placed the dienophile in a chiral environment. • The result is an enantioselective synthesis. 24 -25

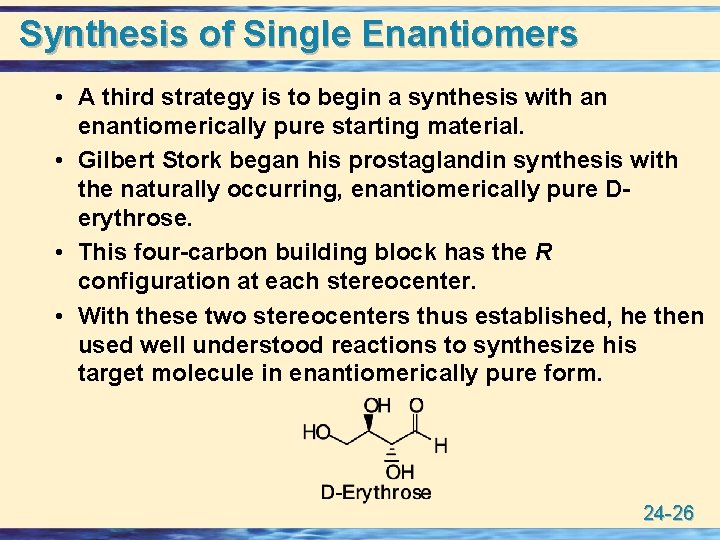
Synthesis of Single Enantiomers • A third strategy is to begin a synthesis with an enantiomerically pure starting material. • Gilbert Stork began his prostaglandin synthesis with the naturally occurring, enantiomerically pure Derythrose. • This four-carbon building block has the R configuration at each stereocenter. • With these two stereocenters thus established, he then used well understood reactions to synthesize his target molecule in enantiomerically pure form. 24 -26
 Antarafacial
Antarafacial Supra antara cycloaddition
Supra antara cycloaddition Suprafacial and antarafacial cycloaddition
Suprafacial and antarafacial cycloaddition Half-life formula
Half-life formula Equation for rate of reaction
Equation for rate of reaction E1cb elimination reaction
E1cb elimination reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Anionation
Anionation Electrophilic substitution reaction of isoquinoline
Electrophilic substitution reaction of isoquinoline Nuclephiles
Nuclephiles Oxidation gcse
Oxidation gcse Redox reaction tutorial
Redox reaction tutorial Endergonic and exergonic reaction
Endergonic and exergonic reaction Diels alder reaction
Diels alder reaction Oxidation half reaction
Oxidation half reaction Elements in group 1
Elements in group 1 Ethanol with iodoform test
Ethanol with iodoform test Example of a double replacement reaction
Example of a double replacement reaction Ethyl acetate reaction with sodium hydroxide
Ethyl acetate reaction with sodium hydroxide Law of conservation of mass examples
Law of conservation of mass examples Multiphase reactor
Multiphase reactor Difference between reaction turbine and impulse turbine
Difference between reaction turbine and impulse turbine Organic chemistry reaction pathways
Organic chemistry reaction pathways Reaction of alkyne
Reaction of alkyne Bond enthalpy definition
Bond enthalpy definition Lady capulet reaction to juliet's death
Lady capulet reaction to juliet's death Reactant
Reactant