A Cycloaddition Cascade Approach to the Total Synthesis




- Slides: 13

A Cycloaddition Cascade Approach to the Total Synthesis of (-)-FR 182877 David A. Evans and Jeremy. T. Starr Presented by Vijayarajan Devannah 2/19/2013

About Prof. David A. Evans Education and Professional: • 1941 - Born in Washington D. C • 1963 - A. B degree, Oberlin College • 1967 - Ph. D at Cal. Tech under Robert E. Ireland • 1973 -1983 Professor at Caltech. • 1983 -present- Professor at Harvard University Notable Awards: • 1999 - The prelog medal, ETH, Zurich switzerland • 2000 - Arthur C. Cope Award, ACS • 2007 - Herbert C. Brown Award for creative research in synthetic methods, ACS • 2008 -Elected to fellow of Royal Society of Chemistry, UK • 2010 -ACS Award for creativity in Molecular Design and synthesis • 2013 -ACS Roger Adams Award • >330 publications Research Focus: Target Oriented Synthesis and New reaction development 2

About (-)FR 182877 • In 1998, Sato and co-workers reported cytotoxic natural product (WS 9885 B), isolated from Streptomyces. • WS 9885 B renamed as FR 182877 • It is a potent microtubule-stabilizing agent and it exhibits potent antitumor activity • Its performance is similar to TAXOL in the untreated Baby Hamster Kidney (BHK) cells, and it holds forth promise as a new lead structure for the development of antitumor therapeutics. 3

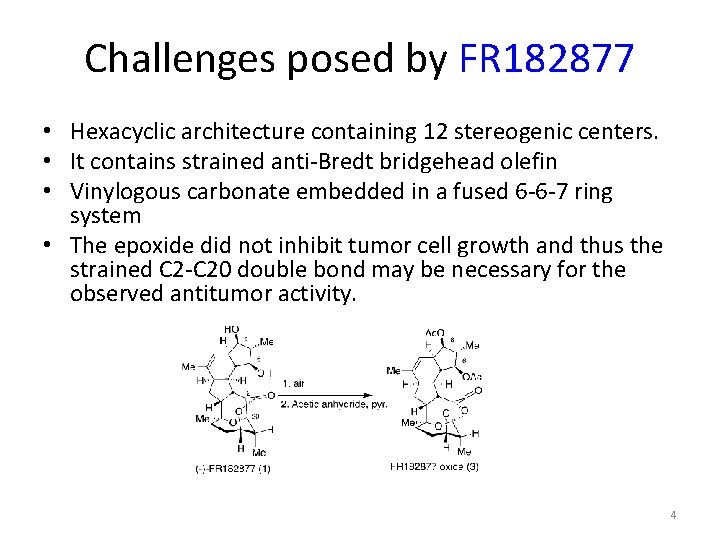
Challenges posed by FR 182877 • Hexacyclic architecture containing 12 stereogenic centers. • It contains strained anti-Bredt bridgehead olefin • Vinylogous carbonate embedded in a fused 6 -6 -7 ring system • The epoxide did not inhibit tumor cell growth and thus the strained C 2 -C 20 double bond may be necessary for the observed antitumor activity. 4

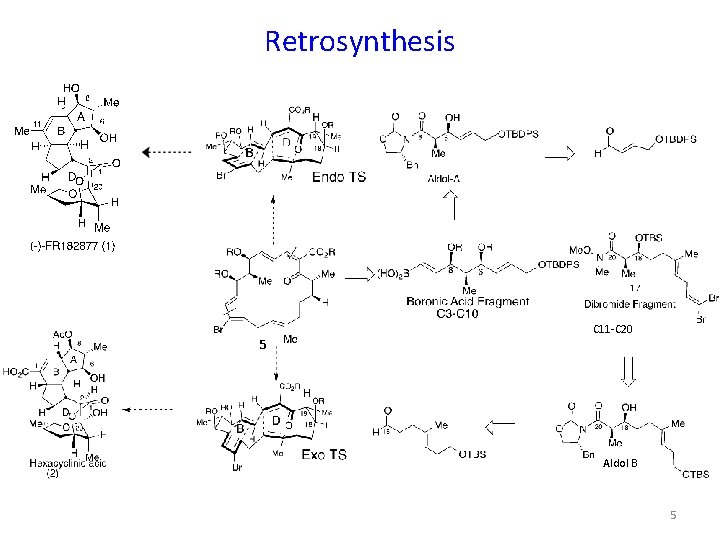
Retrosynthesis 5 C 11 -C 20 Aldol B 5

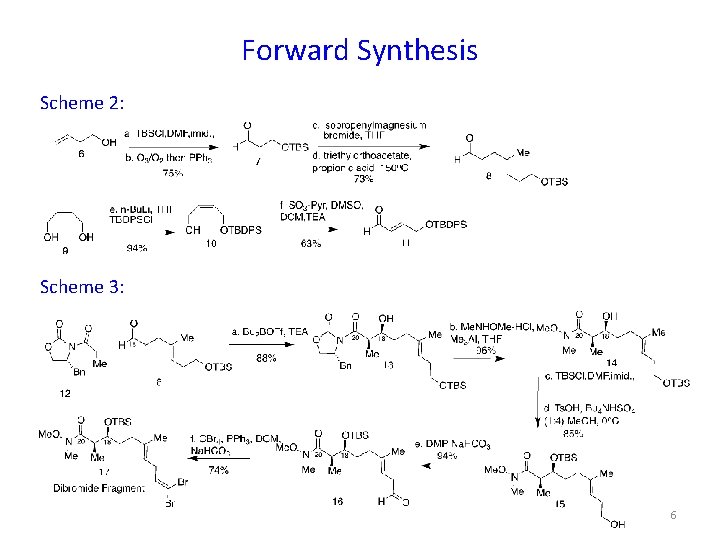
Forward Synthesis Scheme 2: Scheme 3: 6

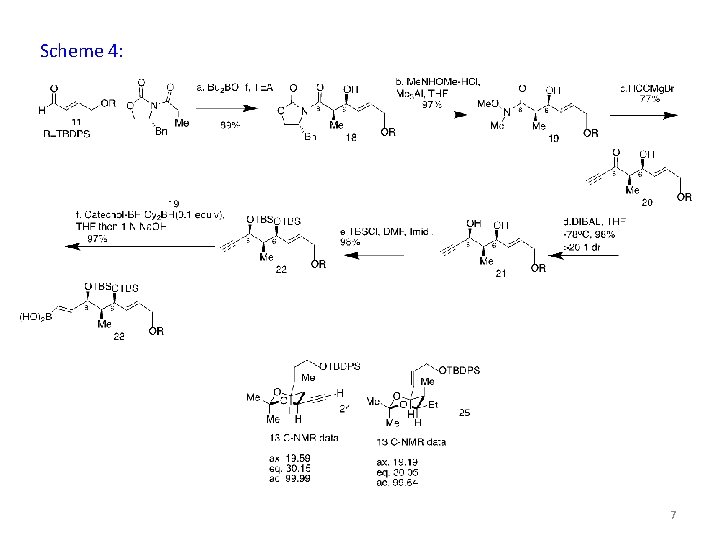
Scheme 4: 7

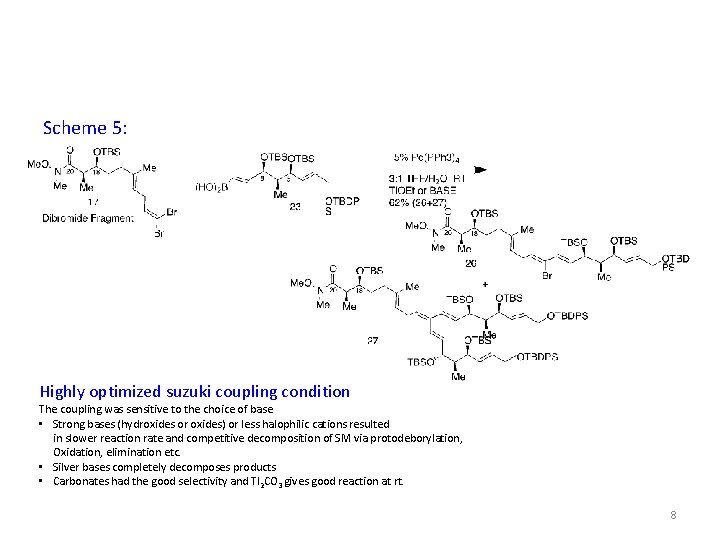
Scheme 5: Highly optimized suzuki coupling condition The coupling was sensitive to the choice of base • Strong bases (hydroxides or oxides) or less halophilic cations resulted in slower reaction rate and competitive decomposition of SM via protodeborylation, Oxidation, elimination etc. • Silver bases completely decomposes products • Carbonates had the good selectivity and Tl 2 CO 3 gives good reaction at rt. 8

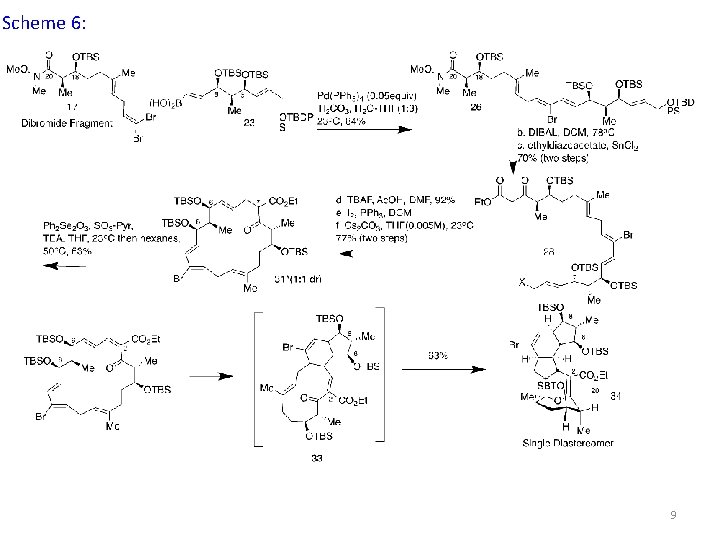
Scheme 6: 9

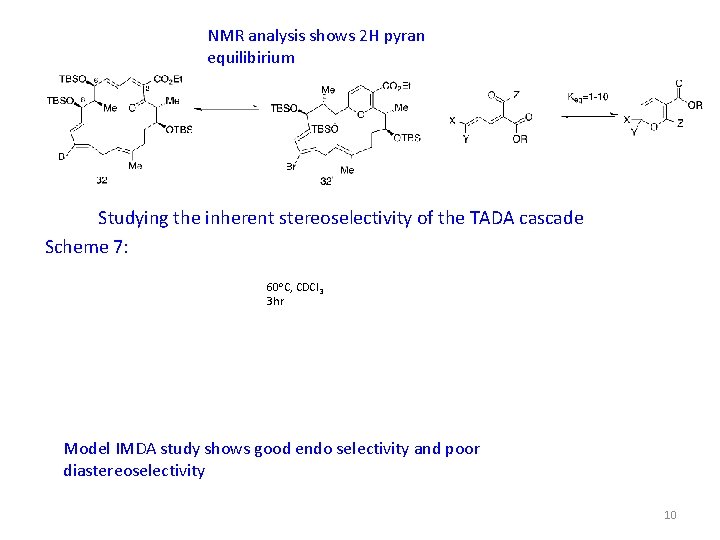
NMR analysis shows 2 H pyran equilibirium Studying the inherent stereoselectivity of the TADA cascade Scheme 7: 60 o. C, CDCl 3 3 hr Model IMDA study shows good endo selectivity and poor diastereoselectivity 10

Scheme 8: 11

Conclusion • 1 H NMR and Mass spectral characteristics were identical to those published for the natural product 1. • 13 C NMR spectral data agreed within 2% margin of error. • Synthetic 1 exhibited an optical rotation of [αD 23]= -5 o as compared to [αD 23]= 3. 5 o reported for the natural sample, and it lead to conclude that synthetic 1 was of the same absolute stereochemistry as natural (-)-FR 182877. • Semiempirical calculations of the transannular Diels-Alder cycloaddition cascade were carried out to determine the origins of asymmetric induction. 12

Thank You 13