Cycloaddition Reactions Cycloaddition reactions are intermolecular pericyclic processes
![Mechanistic features concerted mechanism [4+2] cycloaddition pericyclic reaction a concerted reaction that proceeds through Mechanistic features concerted mechanism [4+2] cycloaddition pericyclic reaction a concerted reaction that proceeds through](https://slidetodoc.com/presentation_image_h2/b2e44e07122a27136eba669d75b00889/image-12.jpg)

- Slides: 48

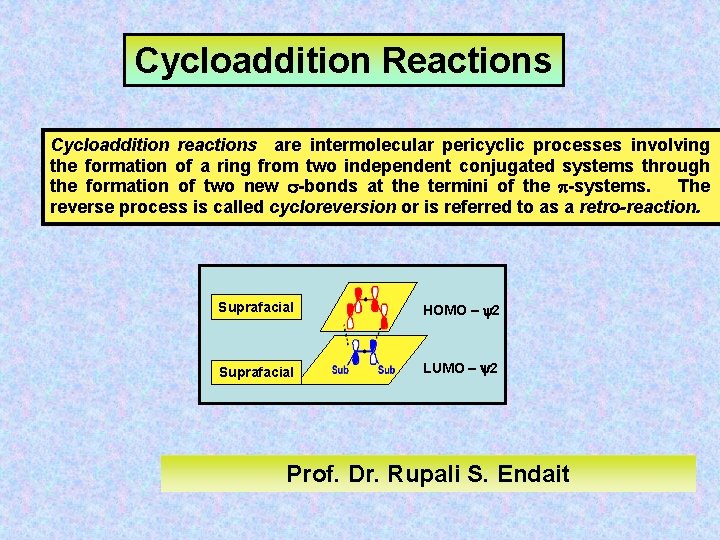
Cycloaddition Reactions Cycloaddition reactions are intermolecular pericyclic processes involving the formation of a ring from two independent conjugated systems through the formation of two new -bonds at the termini of the -systems. The reverse process is called cycloreversion or is referred to as a retro-reaction. Suprafacial HOMO – y 2 Suprafacial LUMO – y 2 Prof. Dr. Rupali S. Endait

(i) A cycloaddition reaction involves the formation of two bonds between the termini of two independent -systems, resulting in ring formation - or the reverse process. (ii) Cycloaddition reactions are stereospecific (e. g. cis/trans isomers). The stereospecificity being afforded by the suprafacial or antarafacial nature of the approach of the two -units in the transition state. (iii) The suprafacial or antarafacial process involved in the bond making process is controlled by the HOMO/LUMO interactions of the two -systems in the transition state.

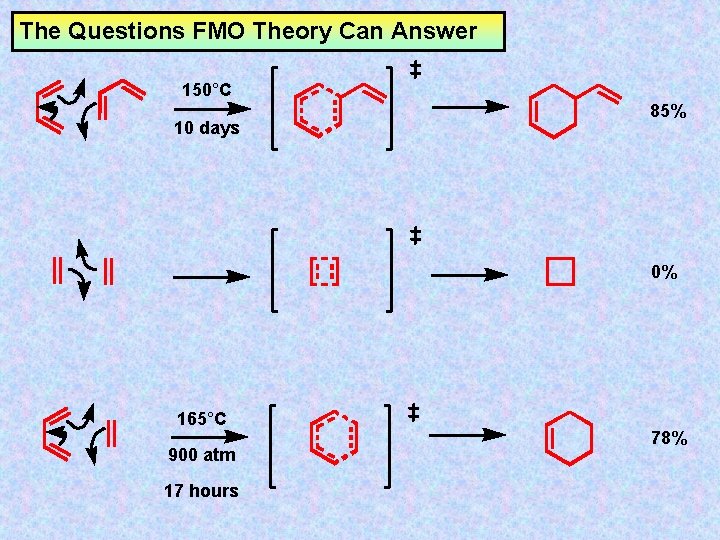
The Questions FMO Theory Can Answer 150°C 10 days 85% 0% 165°C 900 atm 17 hours 78%

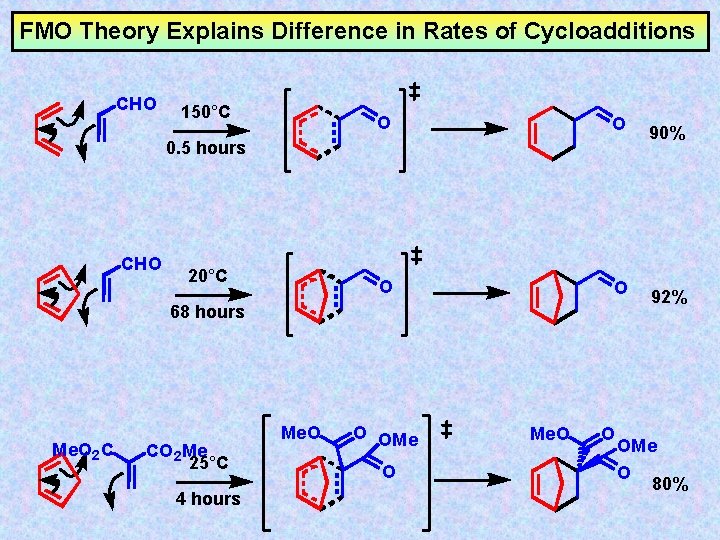
FMO Theory Explains Difference in Rates of Cycloadditions CHO 150°C O O 0. 5 hours CHO 20°C 68 hours Me. O 2 C CO 2 Me 25°C 4 hours Me. O O OMe O Me. O O 90% 92% OMe O 80%

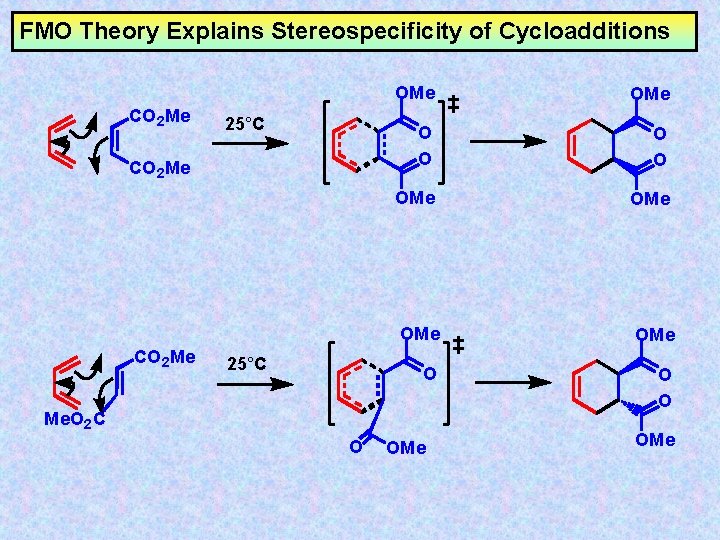
FMO Theory Explains Stereospecificity of Cycloadditions CO 2 Me 25°C OMe O O OMe OMe O O O Me. O 2 C O OMe

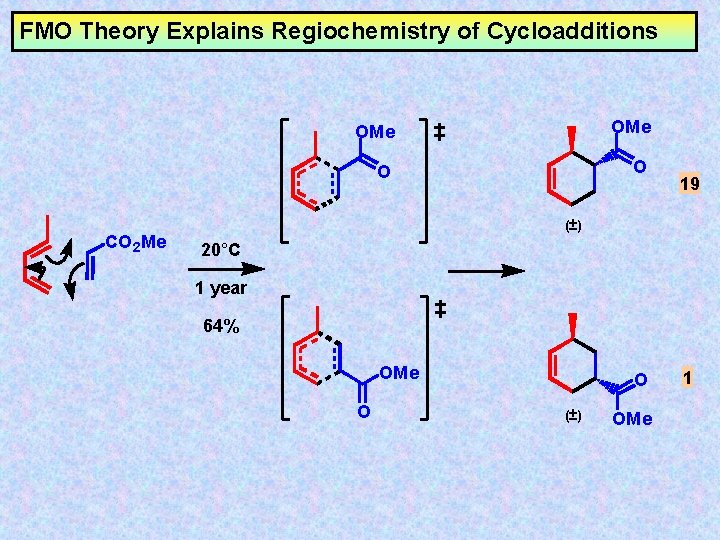
FMO Theory Explains Regiochemistry of Cycloadditions CO 2 Me OMe O O 19 (±) 20°C 1 year 64% OMe O O (±) OMe 1

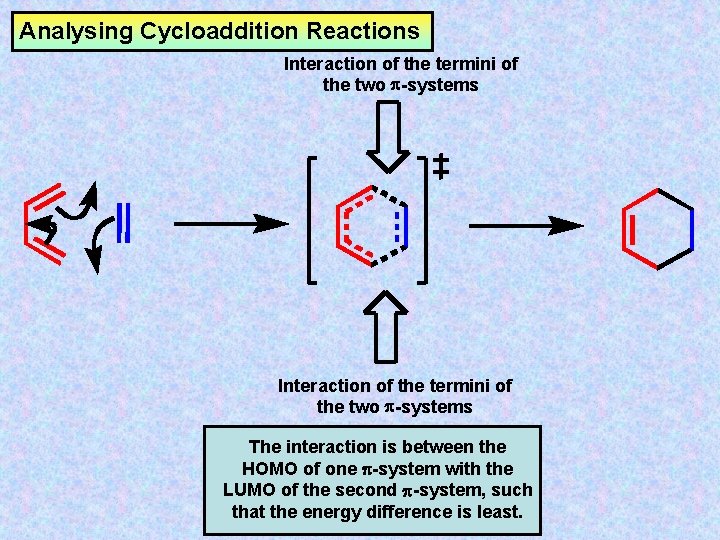
Analysing Cycloaddition Reactions Interaction of the termini of the two -systems The interaction is between the HOMO of one -system with the LUMO of the second -system, such that the energy difference is least.

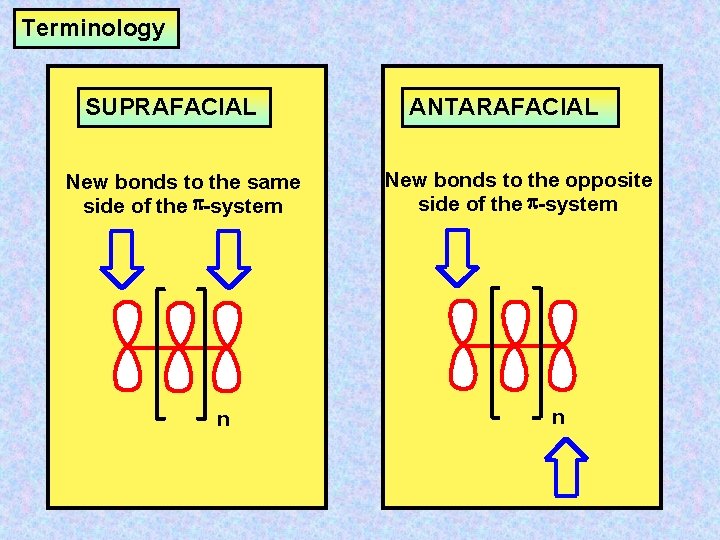
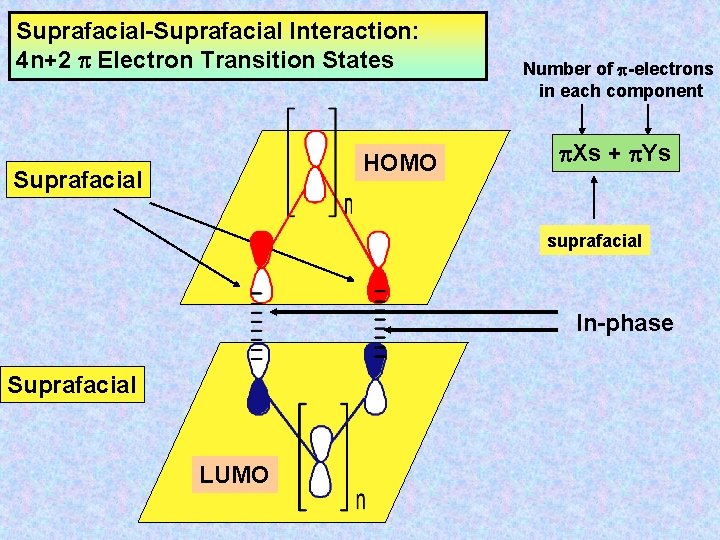
Terminology SUPRAFACIAL New bonds to the same side of the -system n ANTARAFACIAL New bonds to the opposite side of the -system n

4 n+2 Electron Cycloaddition Transition States

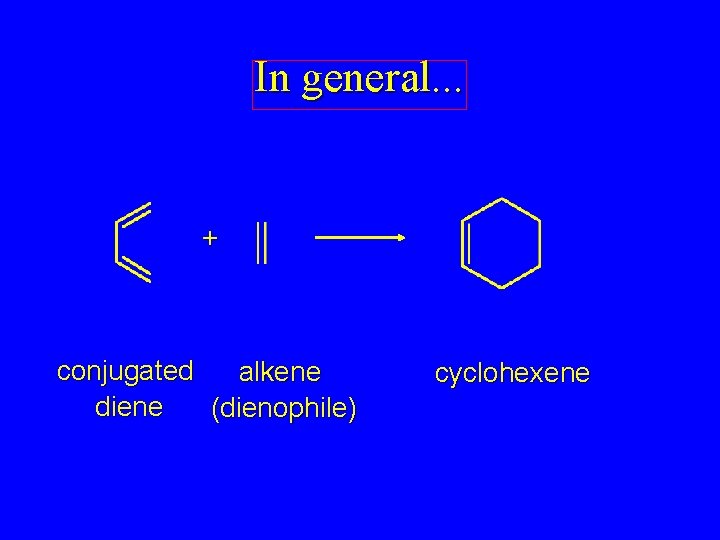
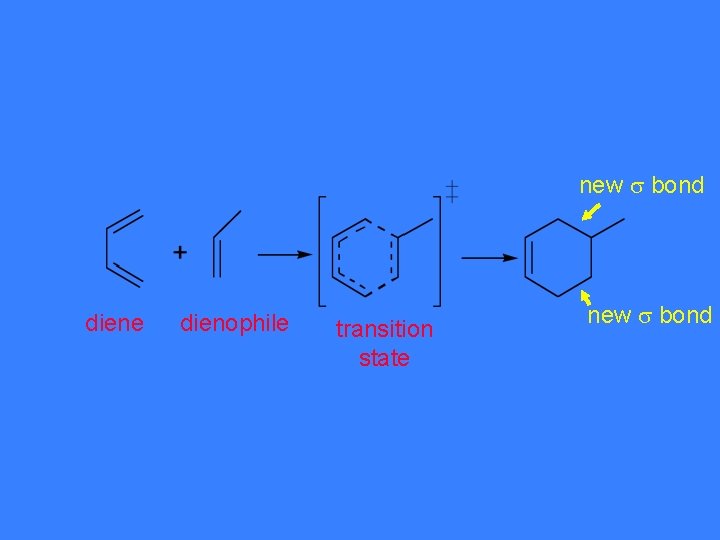
In general. . . + conjugated alkene diene (dienophile) cyclohexene

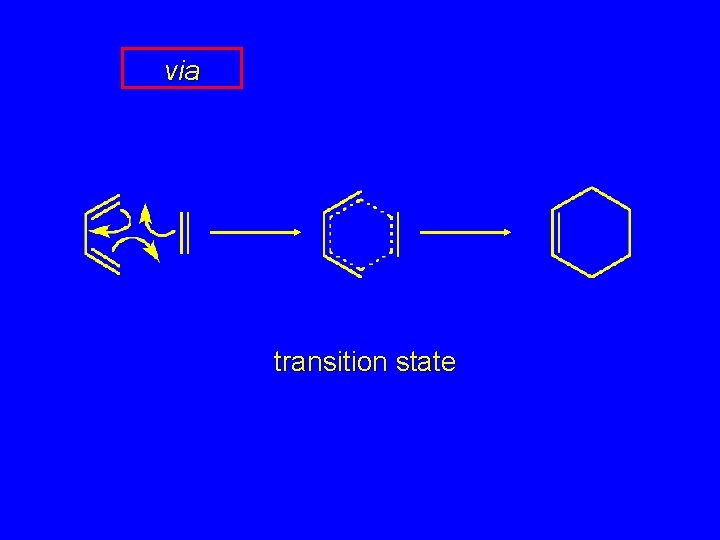
via transition state
![Mechanistic features concerted mechanism 42 cycloaddition pericyclic reaction a concerted reaction that proceeds through Mechanistic features concerted mechanism [4+2] cycloaddition pericyclic reaction a concerted reaction that proceeds through](https://slidetodoc.com/presentation_image_h2/b2e44e07122a27136eba669d75b00889/image-12.jpg)
Mechanistic features concerted mechanism [4+2] cycloaddition pericyclic reaction a concerted reaction that proceeds through a cyclic transition state Only the s-cis conformation of the diene can react

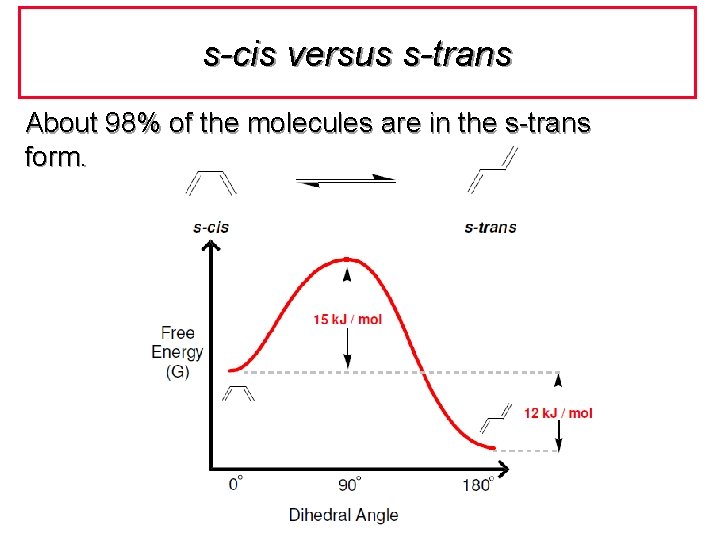
s-cis versus s-trans About 98% of the molecules are in the s-trans form.

new bond diene dienophile transition state new bond

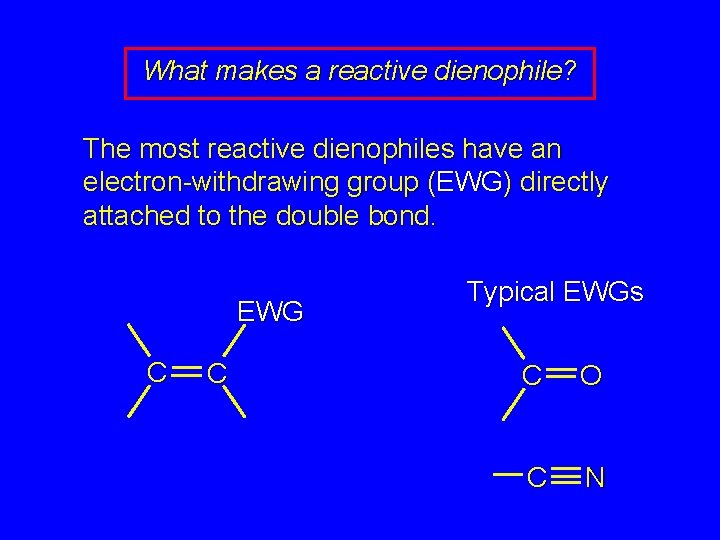
What makes a reactive dienophile? The most reactive dienophiles have an electron-withdrawing group (EWG) directly attached to the double bond. EWG C C Typical EWGs C O C N

Suprafacial-Suprafacial Interaction: 4 n+2 Electron Transition States HOMO Suprafacial Number of -electrons in each component Xs + Ys suprafacial In-phase Suprafacial LUMO

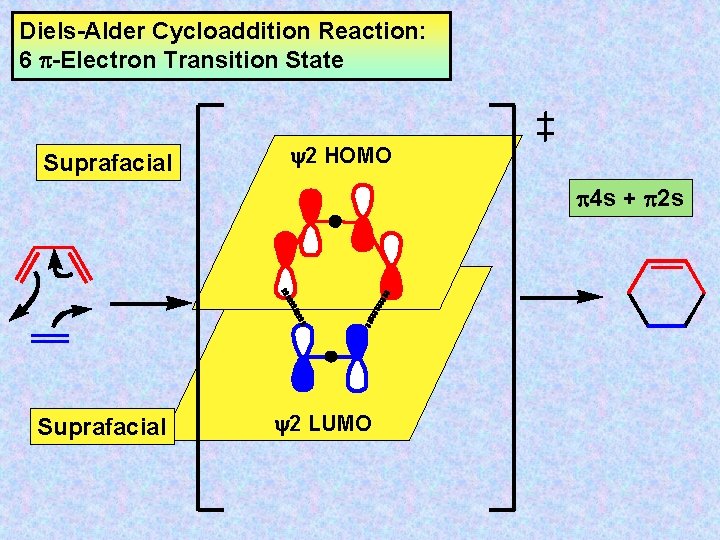
Diels-Alder Cycloaddition Reaction: 6 -Electron Transition State Suprafacial y 2 HOMO 4 s + 2 s Suprafacial y 2 LUMO

The Diels-Alder Reaction: In Detail The Diels-Alder reaction is an extremely well studied cycloaddition reaction, The reason for this is that careful design of the diene component and the ene component (the dienophile) has led to a great insight into the reaction mechanism.

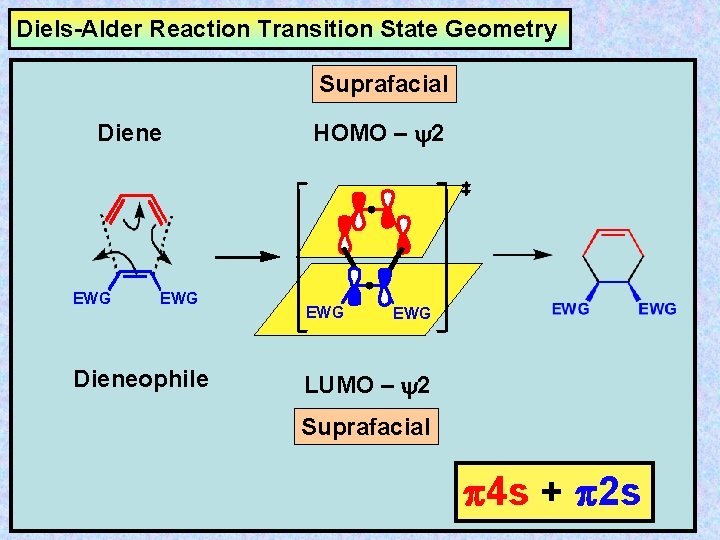
Diels-Alder Reaction Transition State Geometry Suprafacial Diene EWG Dieneophile HOMO – y 2 EWG LUMO – y 2 Suprafacial 4 s + 2 s

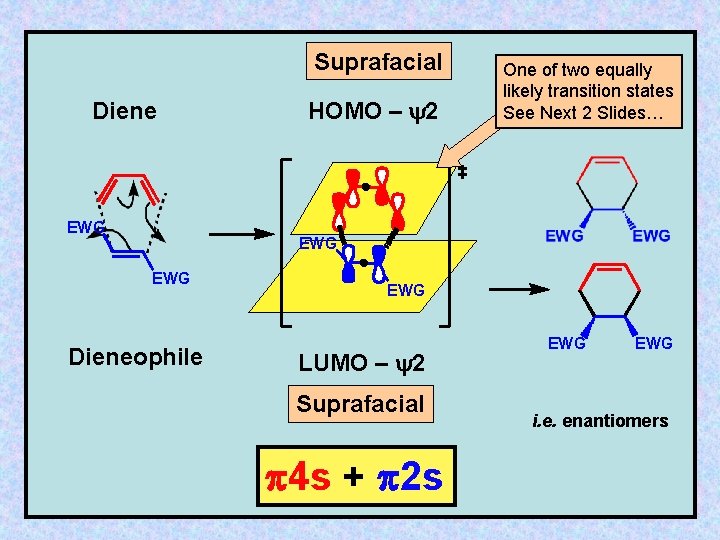
Suprafacial Diene EWG HOMO – y 2 One of two equally likely transition states See Next 2 Slides… EWG Dieneophile EWG LUMO – y 2 Suprafacial 4 s + 2 s EWG i. e. enantiomers

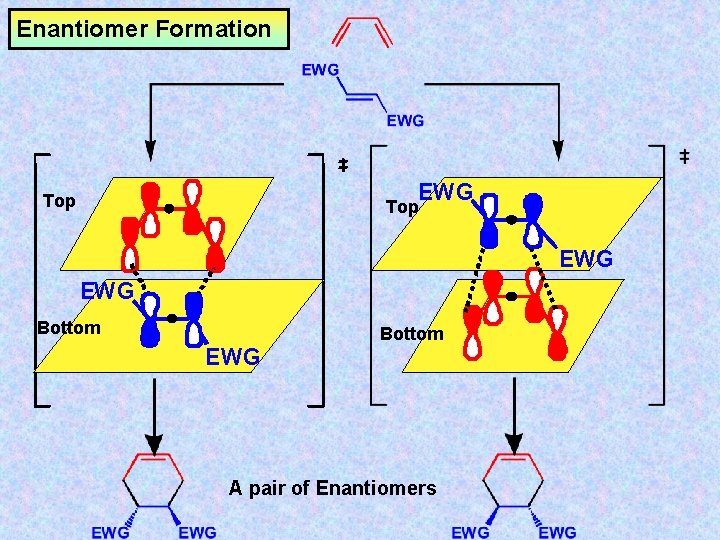
Enantiomer Formation EWG Top EWG Bottom EWG A pair of Enantiomers

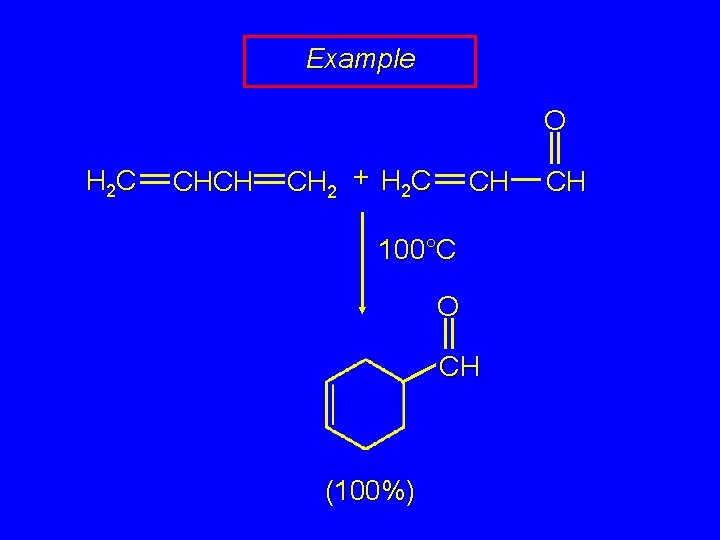
Example O H 2 C CHCH CH 2 + H 2 C CH 100°C O CH (100%) CH

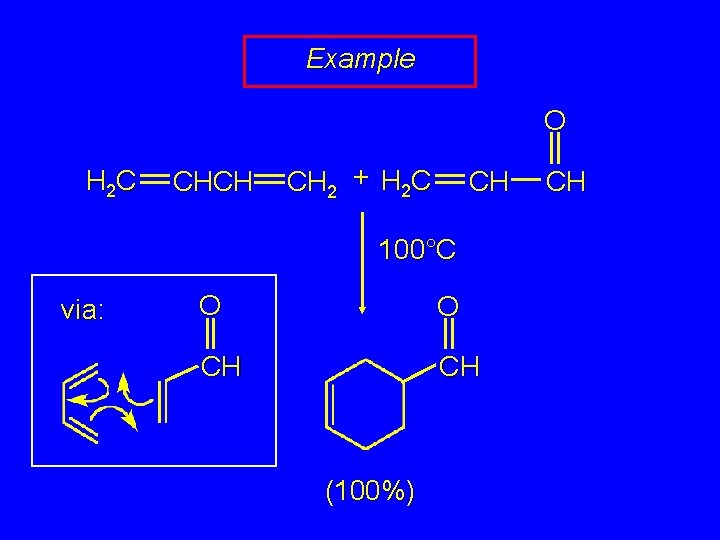
Example O H 2 C CHCH CH 2 + H 2 C CH 100°C via: O O CH CH (100%) CH

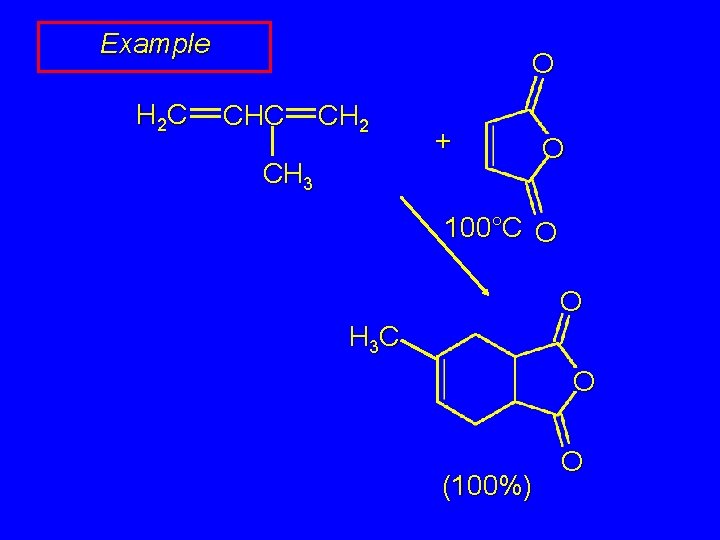
Example H 2 C O CHC CH 2 + CH 3 O 100°C O O H 3 C O (100%) O

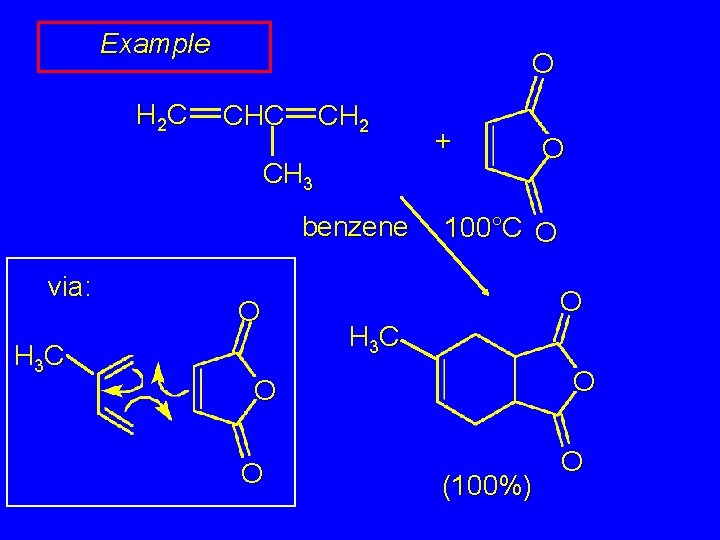
Example H 2 C O CHC CH 2 + CH 3 benzene via: H 3 C O 100°C O O H 3 C O O (100%) O

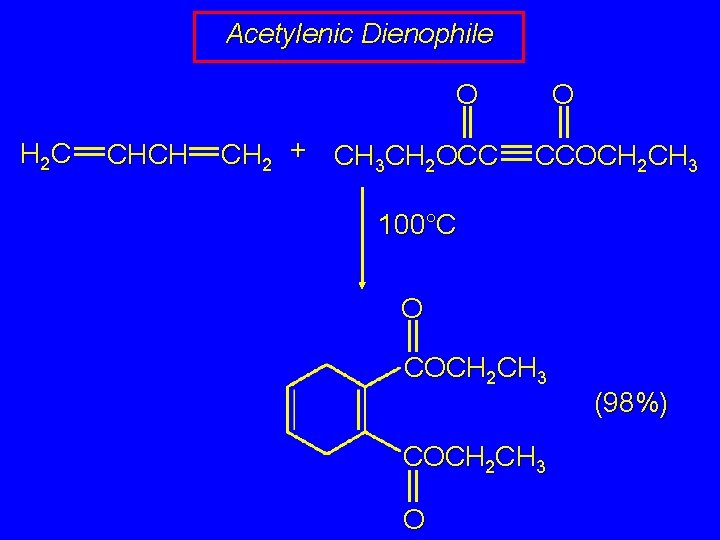
Acetylenic Dienophile O O H 2 C CHCH CH 2 + CH 3 CH 2 OCC CCOCH 2 CH 3 100°C O COCH 2 CH 3 O (98%)

Diels-Alder Reaction Stereospecific syn addition cis-trans relationship of substituents of alkene is retained in the product The most reactive dienophiles have an electron-withdrawing group (EWG) directly attached to the double bond. The most reactive dienes have an electrondonating (releasing) group (ERG) directly attached to the double bond. Eg. -OR (ether)

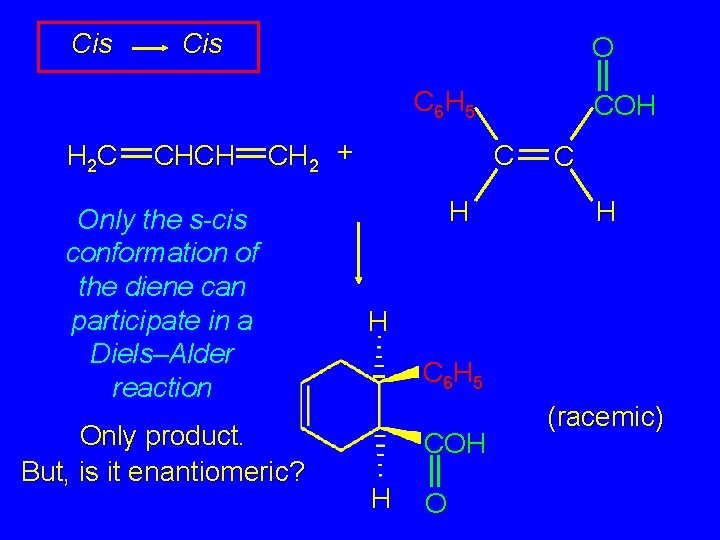
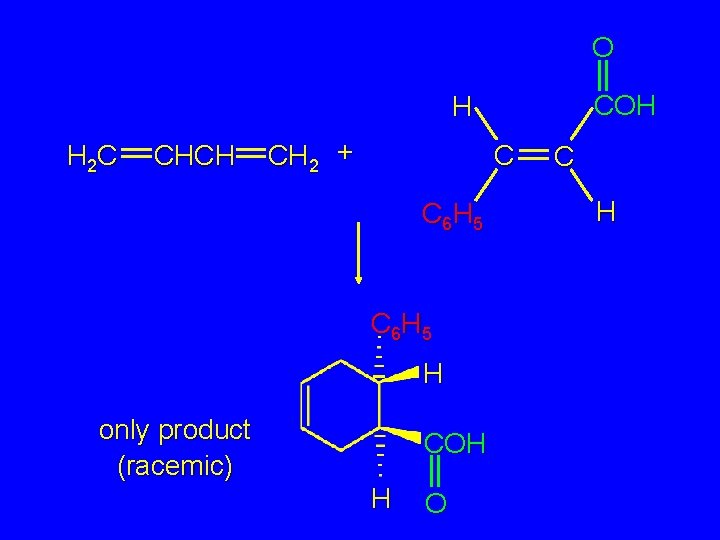
Cis O C 6 H 5 H 2 C CHCH CH 2 + Only the s-cis conformation of the diene can participate in a Diels–Alder reaction Only product. But, is it enantiomeric? COH C H H C 6 H 5 COH H O (racemic)

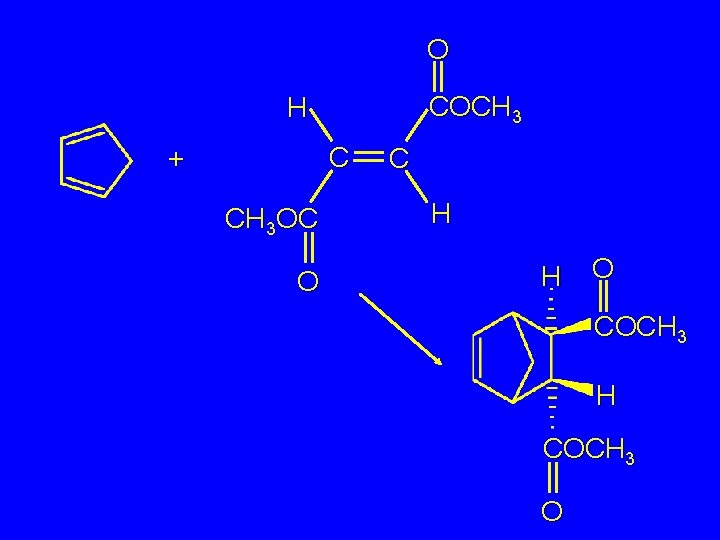
O COCH 3 H C + CH 3 OC O C H H O COCH 3 H COCH 3 O

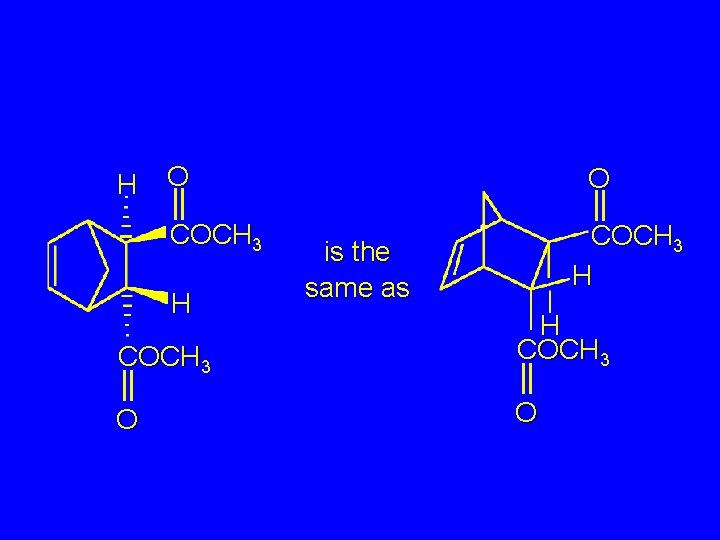
H O COCH 3 H is the same as COCH 3 H COCH 3 O O

O COH H H 2 C CHCH CH 2 + C C 6 H 5 H only product (racemic) COH H O C H

Orbitals and Chemical Reactions A deeper understanding of chemical reactivity can be modeled using frontier orbitals of the reactants. Electrons flow from the highest occupied molecular orbital (HOMO) of one reactant to the lowest unoccupied molecular orbital (LUMO) of the other.

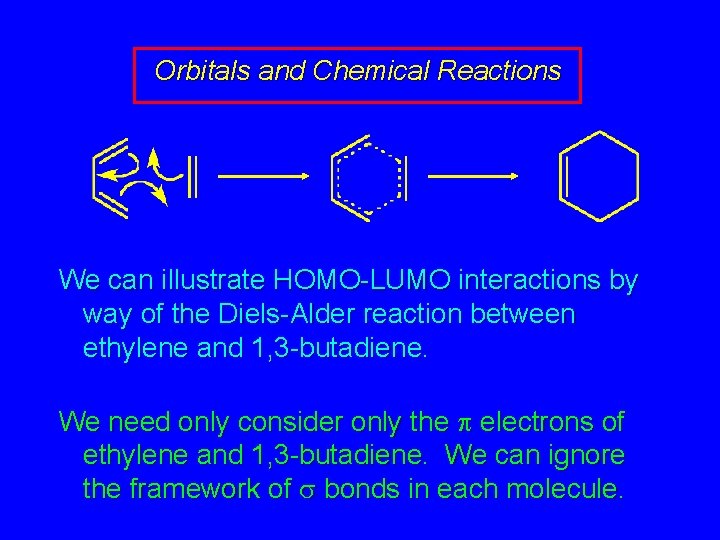
Orbitals and Chemical Reactions We can illustrate HOMO-LUMO interactions by way of the Diels-Alder reaction between ethylene and 1, 3 -butadiene. We need only consider only the electrons of ethylene and 1, 3 -butadiene. We can ignore the framework of bonds in each molecule.

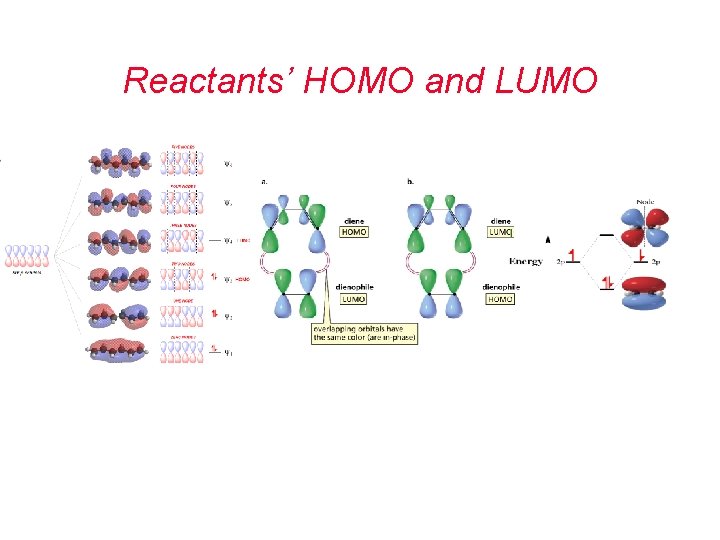
Reactants’ HOMO and LUMO

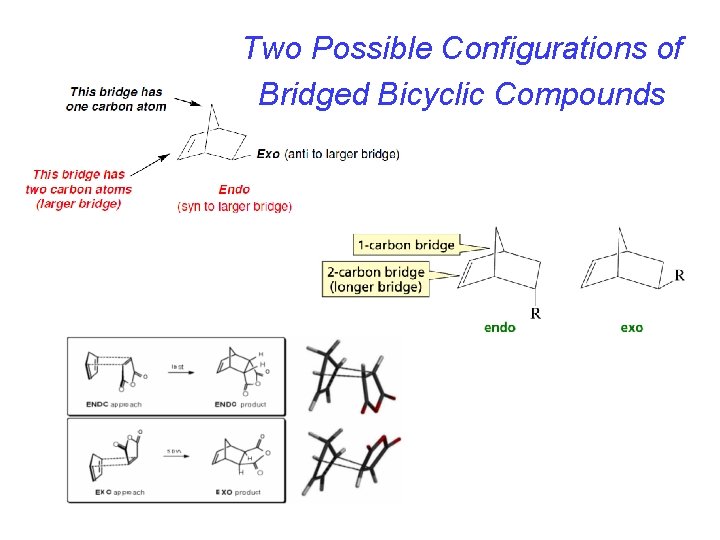
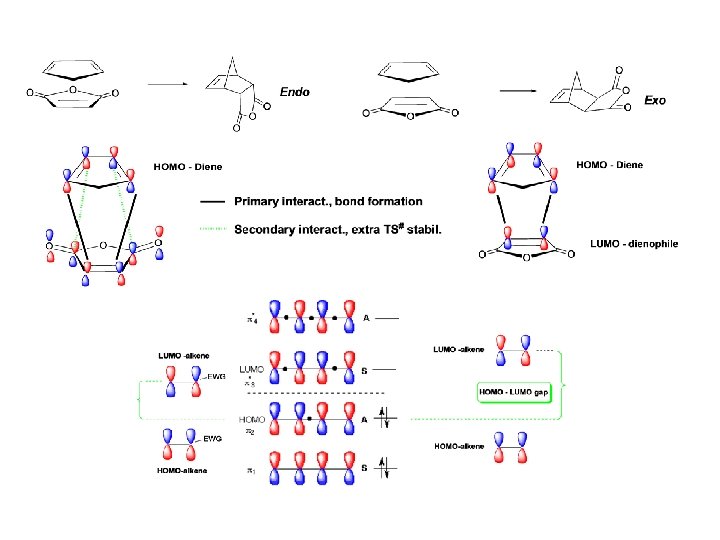
Two Possible Configurations of Bridged Bicyclic Compounds

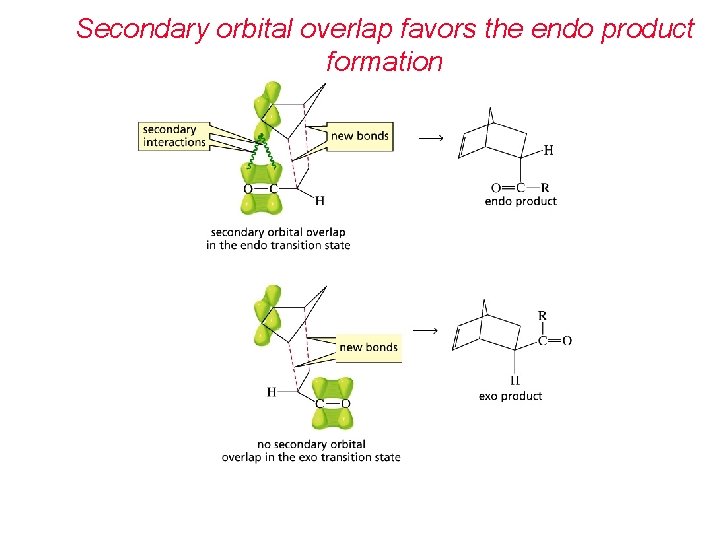
Secondary orbital overlap favors the endo product formation


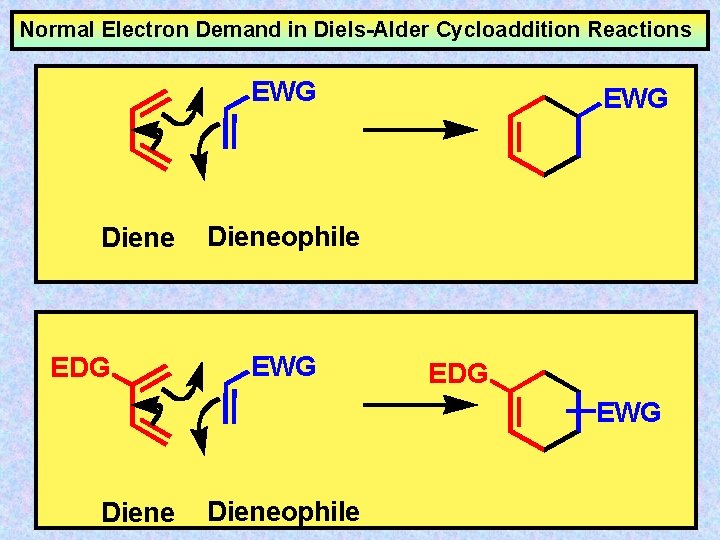
Normal Electron Demand in Diels-Alder Cycloaddition Reactions EWG Diene EDG EWG Dieneophile EWG EDG EWG Dieneophile

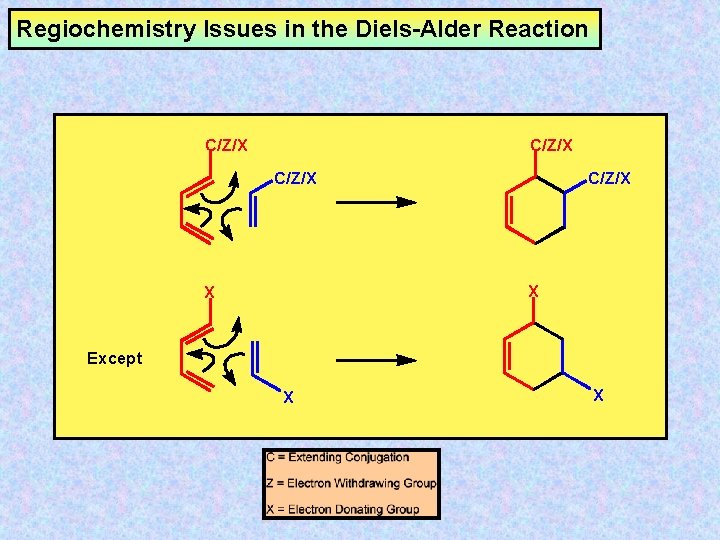
Regiochemistry Issues in the Diels-Alder Reaction C/Z/X X X Except X X

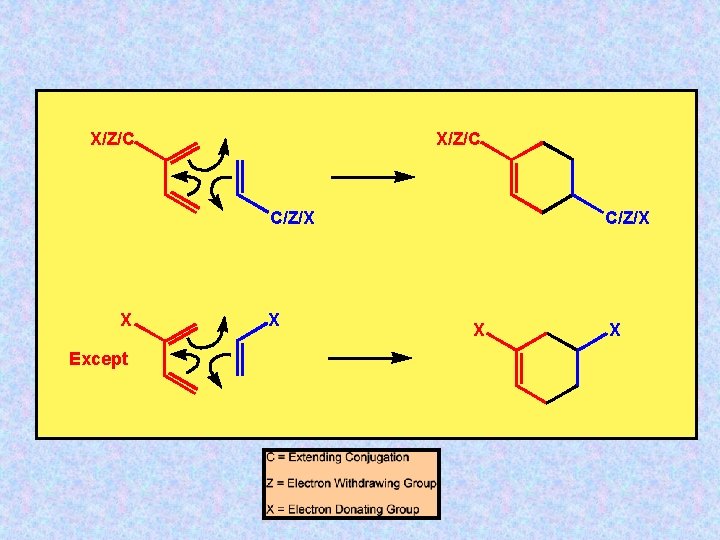
X/Z/C C/Z/X X Except X C/Z/X X X

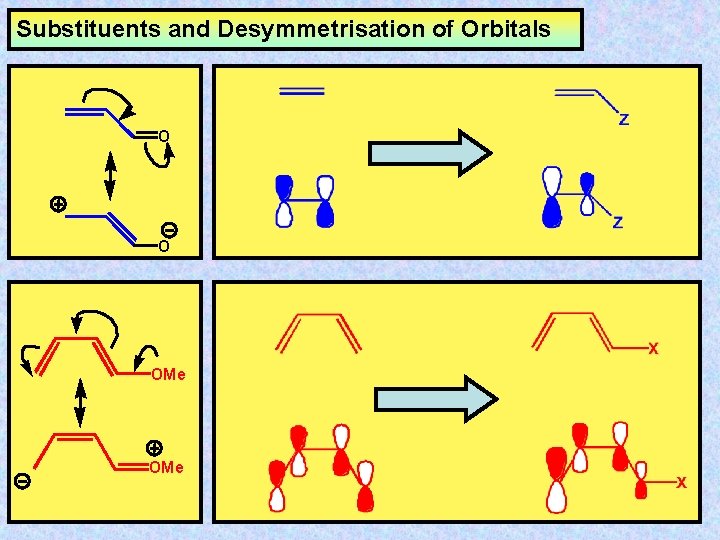
Substituents and Desymmetrisation of Orbitals O O OMe

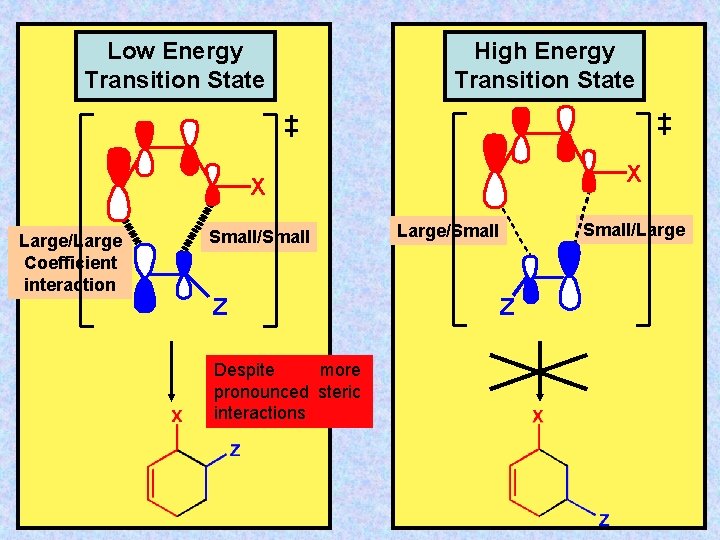
Low Energy Transition State High Energy Transition State X X Large/Large Coefficient interaction Small/Small Z Despite more pronounced steric interactions Small/Large/Small Z

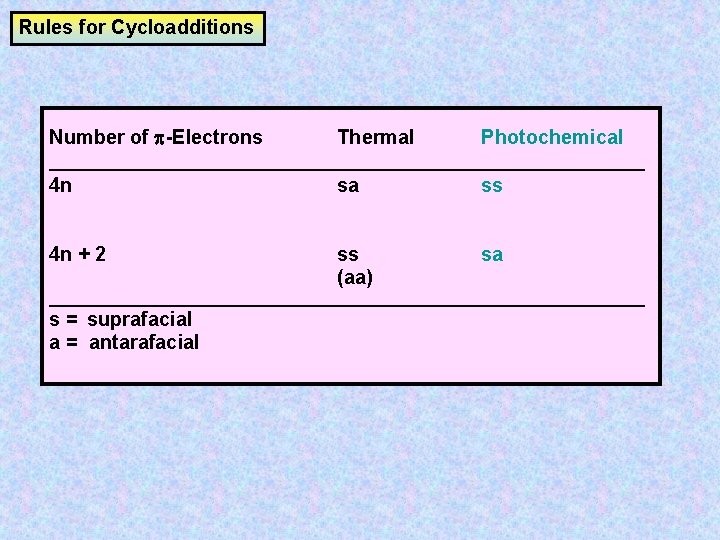
Rules for Cycloadditions Number of -Electrons Thermal Photochemical __________________________________ 4 n sa ss 4 n + 2 ss (aa) sa __________________________________ s = suprafacial a = antarafacial

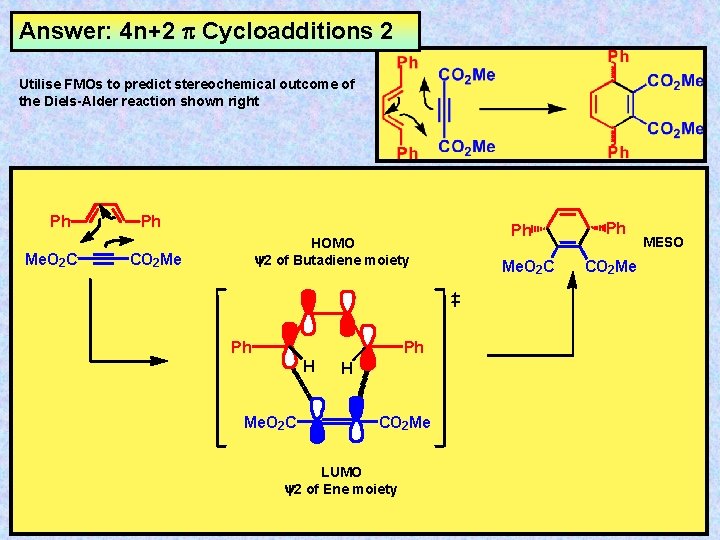
Answer: 4 n+2 Cycloadditions 2 Utilise FMOs to predict stereochemical outcome of the Diels-Alder reaction shown right Ph Me. O 2 C Ph HOMO y 2 of Butadiene moiety CO 2 Me Ph Ph H Me. O 2 C H CO 2 Me LUMO y 2 of Ene moiety Ph Me. O 2 C Ph CO 2 Me MESO

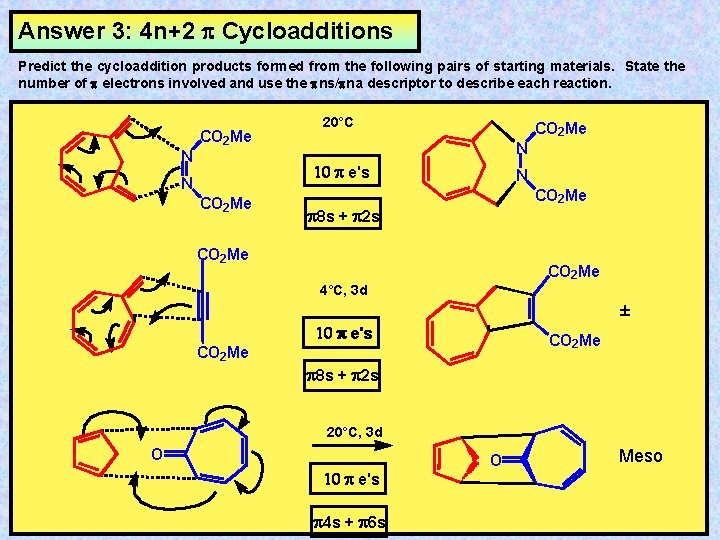
Answer 3: 4 n+2 Cycloadditions Predict the cycloaddition products formed from the following pairs of starting materials. State the number of electrons involved and use the ns/ na descriptor to describe each reaction. CO 2 Me N 20°C CO 2 Me N 10 e's N CO 2 Me 8 s + 2 s CO 2 Me 4°C, 3 d ± 10 e's CO 2 Me 8 s + 2 s 20°C, 3 d O 10 e's 4 s + 6 s O Meso

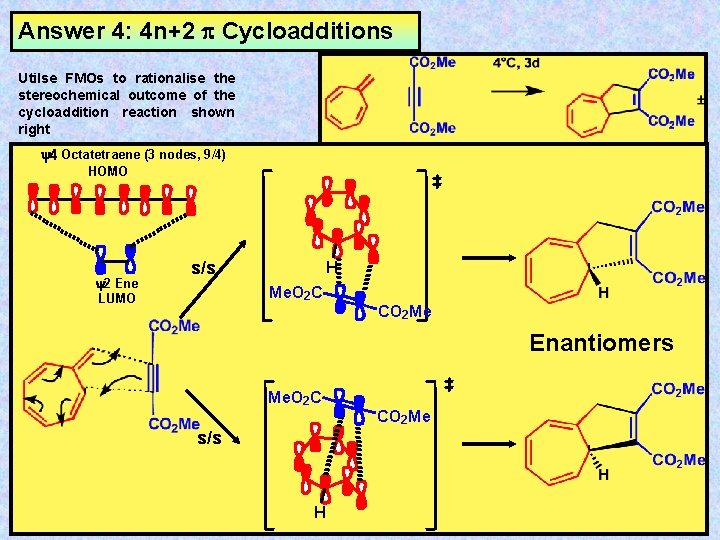
Answer 4: 4 n+2 Cycloadditions Utilse FMOs to rationalise the stereochemical outcome of the cycloaddition reaction shown right y 4 Octatetraene (3 nodes, 9/4) HOMO y 2 Ene LUMO s/s H Me. O 2 C CO 2 Me Enantiomers Me. O 2 C s/s H CO 2 Me

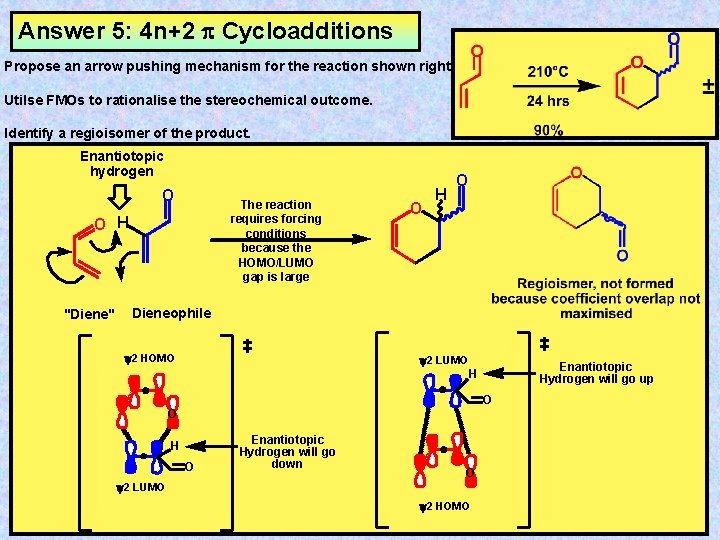
Answer 5: 4 n+2 Cycloadditions Propose an arrow pushing mechanism for the reaction shown right Utilse FMOs to rationalise the stereochemical outcome. Identify a regioisomer of the product. Enantiotopic hydrogen O The reaction requires forcing conditions because the HOMO/LUMO gap is large O H "Diene" O H O Dieneophile y 2 HOMO y 2 LUMO Enantiotopic Hydrogen will go up H O O H O Enantiotopic Hydrogen will go down O y 2 LUMO y 2 HOMO

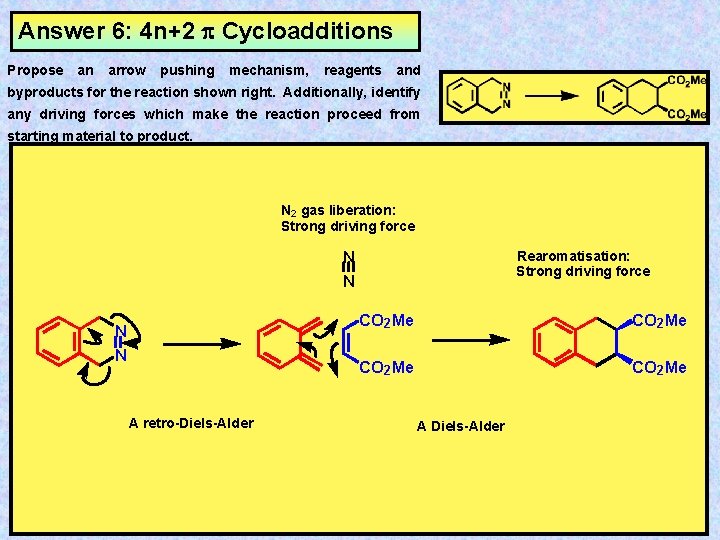
Answer 6: 4 n+2 Cycloadditions Propose an arrow pushing mechanism, reagents and byproducts for the reaction shown right. Additionally, identify any driving forces which make the reaction proceed from starting material to product. N 2 gas liberation: Strong driving force N N A retro-Diels-Alder Rearomatisation: Strong driving force CO 2 Me A Diels-Alder