Intermolecular attractions Intermolecular attractions are weaker than either
- Slides: 10

Intermolecular attractions: Intermolecular attractions are weaker than either ionic or covalent bonds. Molecules can be attracted to each other by a variety of different forces: 1) The weakest attractions between molecules are called Van der Waals forces (London dispersion forces). Can be found between nonpolar molecules as O 2(g), H 2(g), Cl 2(g), CO 2(g). Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

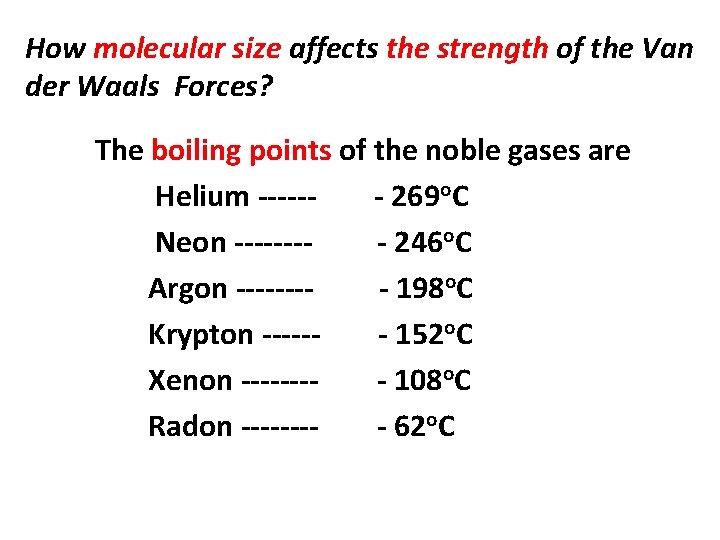
How molecular size affects the strength of the Van der Waals Forces? The boiling points of the noble gases are Helium ------ 269 o. C Neon ---- 246 o. C Argon ---- 198 o. C Krypton ------ 152 o. C Xenon ---- 108 o. C Radon ---- 62 o. C

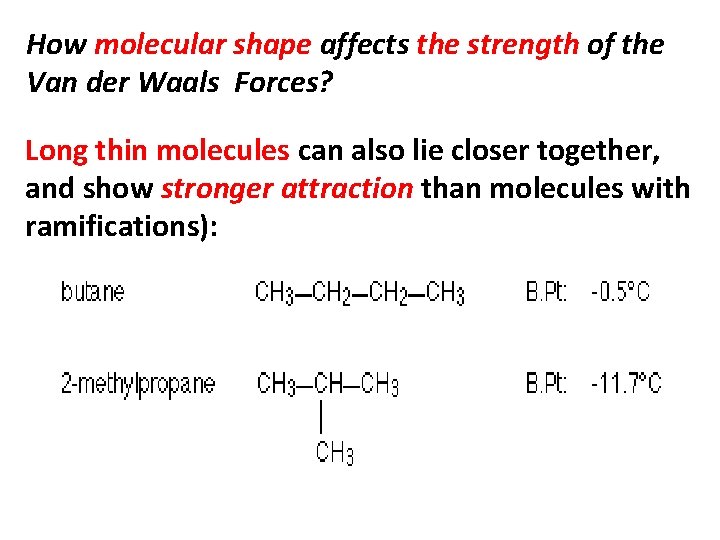
How molecular shape affects the strength of the Van der Waals Forces? Long thin molecules can also lie closer together, and show stronger attraction than molecules with ramifications):

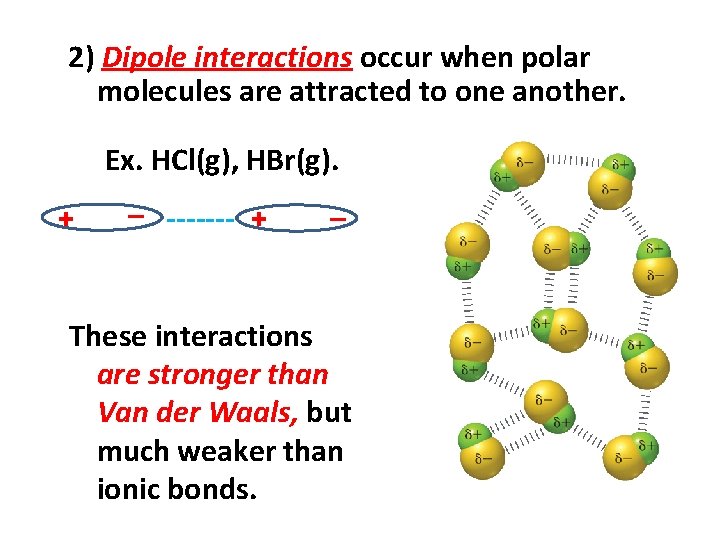
2) Dipole interactions occur when polar molecules are attracted to one another. Ex. HCl(g), HBr(g). _ ++= + ------- + ++= _ These interactions are stronger than Van der Waals, but much weaker than ionic bonds.

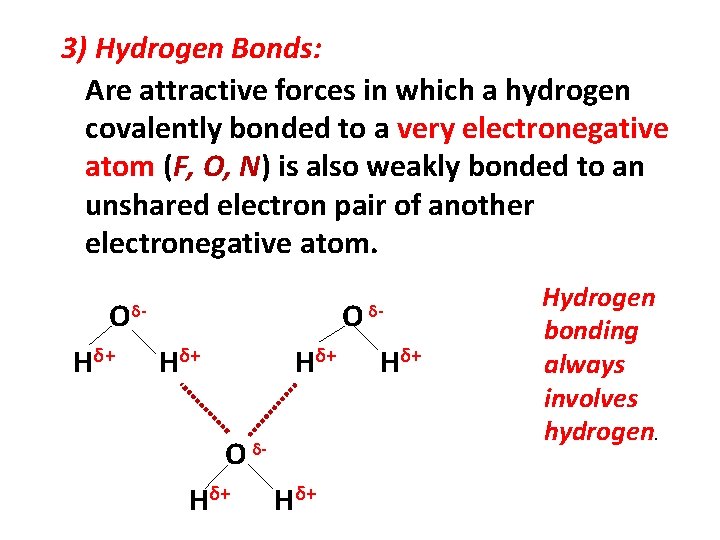
3) Hydrogen Bonds: Are attractive forces in which a hydrogen covalently bonded to a very electronegative atom (F, O, N) is also weakly bonded to an unshared electron pair of another electronegative atom. O Hδ+ O δ- Hδ+ Hδ+ δ- Hδ+ Hydrogen bonding always involves hydrogen.

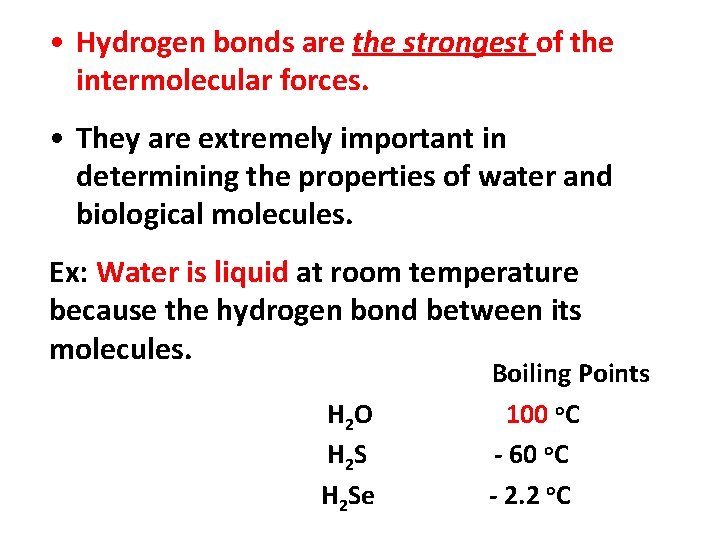
• Hydrogen bonds are the strongest of the intermolecular forces. • They are extremely important in determining the properties of water and biological molecules. Ex: Water is liquid at room temperature because the hydrogen bond between its molecules. H 2 O H 2 Se Boiling Points 100 o. C - 60 o. C - 2. 2 o. C

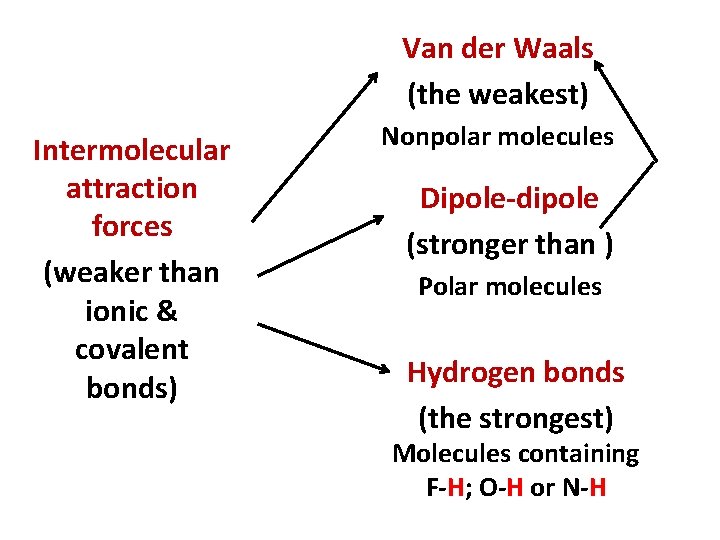
Van der Waals (the weakest) Intermolecular attraction forces (weaker than ionic & covalent bonds) Nonpolar molecules Dipole-dipole (stronger than ) Polar molecules Hydrogen bonds (the strongest) Molecules containing F-H; O-H or N-H

Properties of Molecular Compounds: 1) Weak attraction forces between molecules. 2) Low melting and boiling points. 3) Gases, liquids or solids at room temperature. 4) The solids are very soft. 5) Poor conductors of electricity. 6) Polar covalent: Show solubility in water. Ex. HCl(g), NH 3(g) No polar covalent: Show solubility in non polar solvents (CCl 4, oil, gasoline, acetone, etc. ) Examples: Cl 2(g), Br 2(l), CCl 4(l).

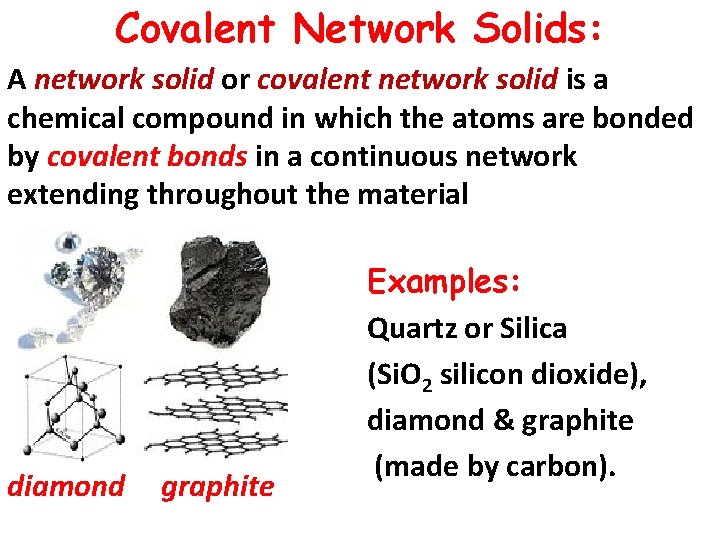
Covalent Network Solids: A network solid or covalent network solid is a chemical compound in which the atoms are bonded by covalent bonds in a continuous network extending throughout the material diamond graphite Examples: Quartz or Silica (Si. O 2 silicon dioxide), diamond & graphite (made by carbon).

Covalent Network Solids: Properties: 1) Brittle & extremely hard solids. 2) Very high melting points. 3) Non conductors (heat or electricity), except graphite (good conductor)