Intermolecular Attractions Types of Intermolecular Attractions There are








- Slides: 17

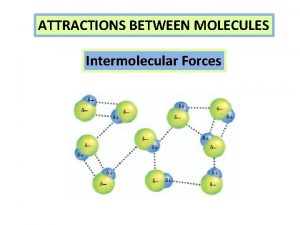
Intermolecular Attractions

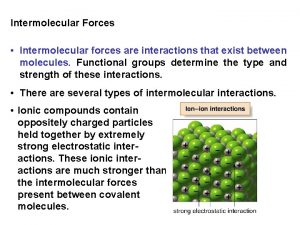
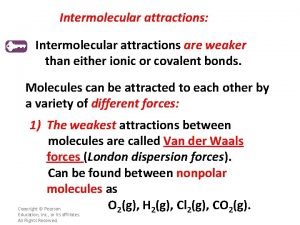
Types of Intermolecular Attractions There are three types of intermolecular attractions: • London Forces • Dipole-dipole • Hydrogen bonds

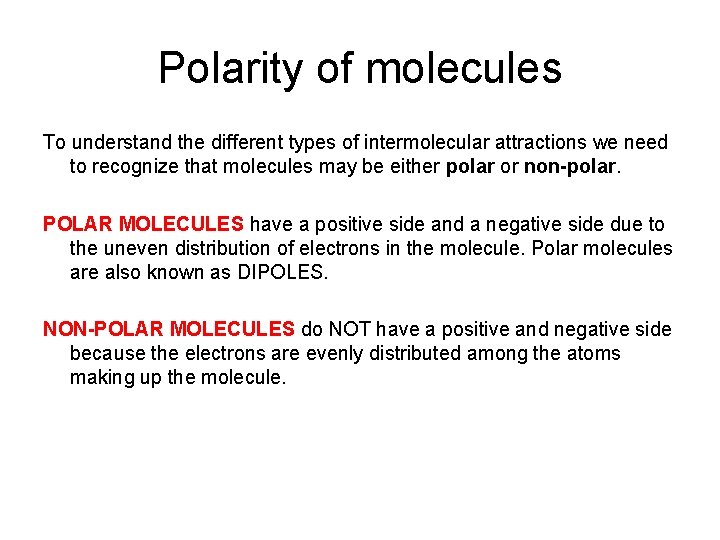
Polarity of molecules To understand the different types of intermolecular attractions we need to recognize that molecules may be either polar or non-polar. POLAR MOLECULES have a positive side and a negative side due to the uneven distribution of electrons in the molecule. Polar molecules are also known as DIPOLES. NON-POLAR MOLECULES do NOT have a positive and negative side because the electrons are evenly distributed among the atoms making up the molecule.

Polar molecules are molecules that have a positive end a negative end. These molecules consist of atoms that are bonded together by polar covalent bonds. The bonding electrons are pulled closer to the atoms in the molecule with the greatest electronegativity. This makes one end of the molecule slightly negative, while the other end of the molecule is slightly negative.

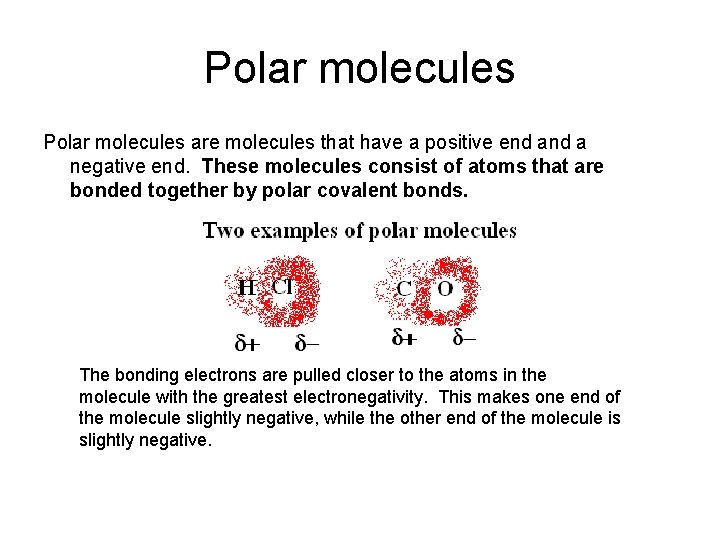
Non-polar molecules In a non-polar molecule, the electrons are distributed evenly throughout the molecule. If a molecule is made up only of non-polar bonds, then the molecule will be non-polar.

Non-polar molecules (2) However, some non-polar molecules may be made up of polar bonds, if the molecule is symmetrical. The symmetrical shape insures an even distribution of the electrons even when the bonds making up the molecule are polar covalent (have unequal sharing). For example, in carbon dioxide the oxygen and carbon form polar covalent bonds, however, due to the symmetry of the molecule the separations of charge cancel out making the molecule non-polar. A NON-POLAR MOLECULE DOES NOT HAVE A + OR – END.

Centers of Charge • In a non-polar molecule the center of positive and negative charge are at the same point in the molecule.

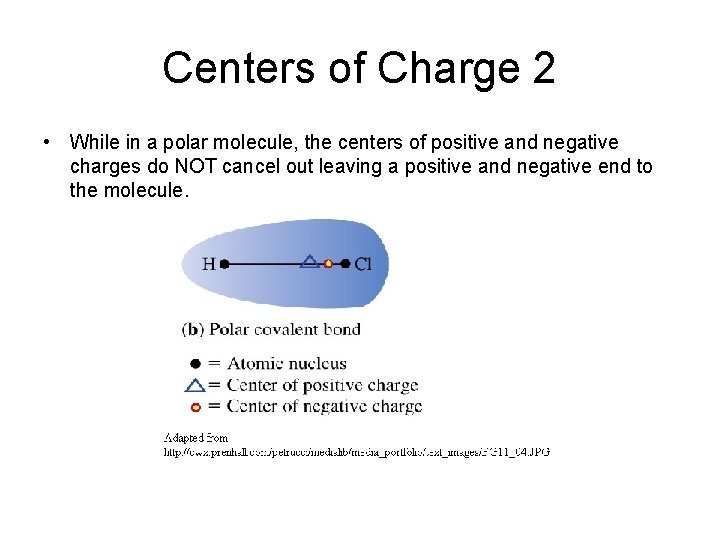
Centers of Charge 2 • While in a polar molecule, the centers of positive and negative charges do NOT cancel out leaving a positive and negative end to the molecule.

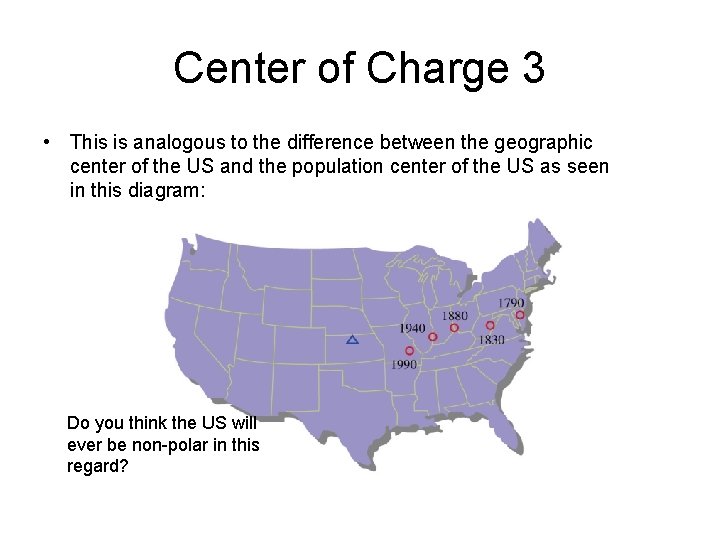
Center of Charge 3 • This is analogous to the difference between the geographic center of the US and the population center of the US as seen in this diagram: Do you think the US will ever be non-polar in this regard?

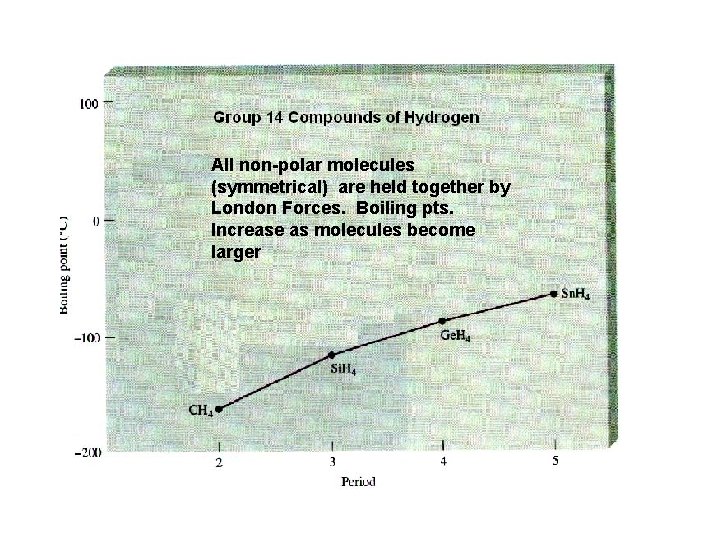
London Forces The London forces are the weakest inter-molecular attractions. They are the forces that are responsible for holding non-polar molecules together. Molecules held together by the London forces include all 7 diatomic elements, CO 2, and CH 4 (methane). Substances whose molecules are held together by the London forces tend to have very low boiling points (are gases at room temperature) because the London forces aren’t strong enough to hold the molecules together.

Dipole-Dipole Interactions The Dipole-dipole forces are stronger than the London forces. They are the forces that are responsible for holding polar molecules together. Molecules held together by the dipole-dipole attractions include all most polar molecules such as HCl and CO. Substances whose molecules are held together by dipole-dipole attractions tend to have higher boiling points than molecules held together by the weaker London forces.

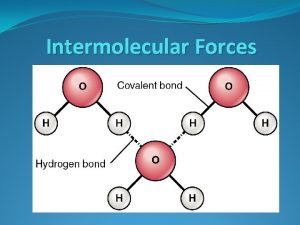
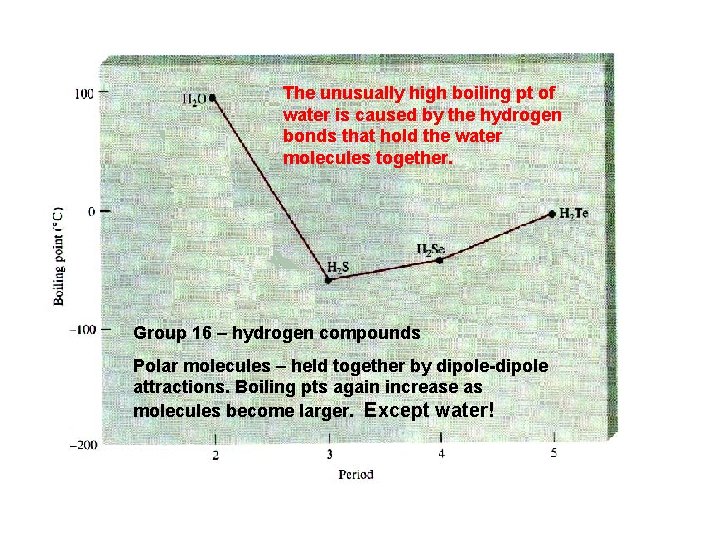
Hydrogen bonds The Hydrogen bonds are the strongest intermolecular attractions. They are the forces that are responsible for holding molecules that have hydrogen bonded to very electronegative elements such as nitrogen, oxygen, and fluorine. Hydrogen bonds are an unusually strong form of dipole-dipole attractions. Molecules held together by Hydrogen bonds include water (H 2 O), hydrogen fluoride (HF) and ammonia (NH 3). Substances whose molecules are held together by hydrogen bonds tend to have higher boiling points than molecules held together by the other intermolecular forces. .

All non-polar molecules (symmetrical) are held together by London Forces. Boiling pts. Increase as molecules become larger

The unusually high boiling pt of water is caused by the hydrogen bonds that hold the water molecules together. Group 16 – hydrogen compounds Polar molecules – held together by dipole-dipole attractions. Boiling pts again increase as molecules become larger. Except water!

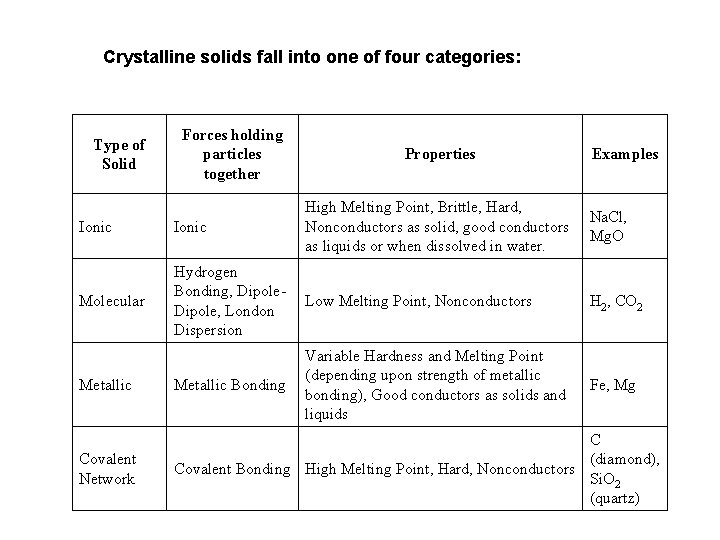
Crystalline solids fall into one of four categories: Type of Solid Forces holding particles together Properties Examples Ionic High Melting Point, Brittle, Hard, Nonconductors as solid, good conductors as liquids or when dissolved in water. Molecular Hydrogen Bonding, Dipole, London Dispersion Low Melting Point, Nonconductors H 2, CO 2 Metallic Bonding Variable Hardness and Melting Point (depending upon strength of metallic bonding), Good conductors as solids and liquids Fe, Mg Covalent Network C (diamond), Covalent Bonding High Melting Point, Hard, Nonconductors Si. O 2 (quartz) Na. Cl, Mg. O

Network solids

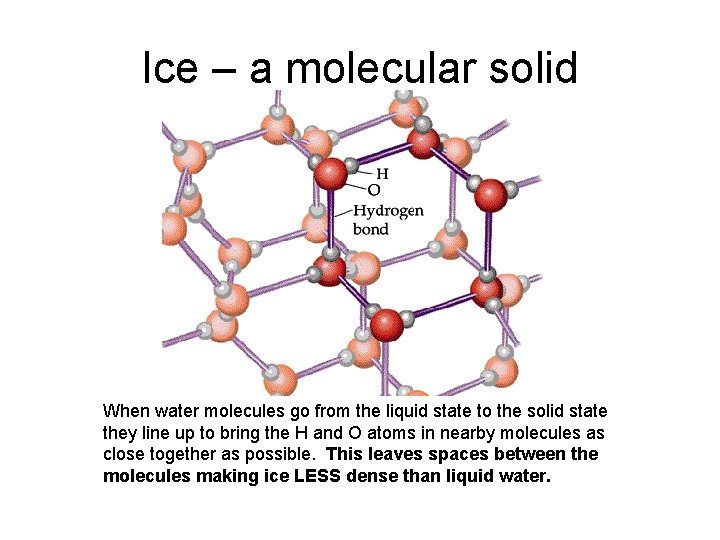
Ice – a molecular solid When water molecules go from the liquid state to the solid state they line up to bring the H and O atoms in nearby molecules as close together as possible. This leaves spaces between the molecules making ice LESS dense than liquid water.
 Insidan region jh
Insidan region jh Intermolecular forces and boiling point
Intermolecular forces and boiling point 3 types of intermolecular forces
3 types of intermolecular forces Types of intermolecular forces
Types of intermolecular forces Butanal intermolecular forces
Butanal intermolecular forces Differentiate the four main types of intermolecular forces
Differentiate the four main types of intermolecular forces In managing attractions, a programmed decision is
In managing attractions, a programmed decision is Significant in the success of attractions
Significant in the success of attractions Attractions in the amazon rainforest
Attractions in the amazon rainforest Polar attractions are
Polar attractions are Melecule
Melecule Which diagram best illustrates the ion molecule attractions
Which diagram best illustrates the ion molecule attractions Tourist attractions in the coastal plain region of georgia
Tourist attractions in the coastal plain region of georgia What are the weakest attractions between molecules
What are the weakest attractions between molecules Kanchanaburi toursist attractions
Kanchanaburi toursist attractions Hy attractions manager
Hy attractions manager Coastal plain region of georgia
Coastal plain region of georgia The physical attractions
The physical attractions