Saponification of Ethyl acetate by Sodium hydroxide in

Saponification of Ethyl acetate by Sodium hydroxide in a Plug Flow Reactor Lindsey Kato Shawna Togioka Luke Sugie February 2, 2005

Overview Ø Project Objectives Ø Project Planning and Execution Ø Background and Experimental Methods Ø Results and Conclusions Ø Recommendations and Future Work

Project Objectives Ø 1. 2. 3. Develop reaction kinetic data for the saponification of ethyl acetate by sodium hydroxide. Develop calibration curves for electric conductivity cell, using known concentrations of reactants and products Calibration of pump settings on Plug Flow Reactor (PFR). Ran Batch Reactor and PFR and gathered kinetic rate data

Project Planning Ø Roles & Responsibilities l Team Leader – Lindsey Kato • Planning agenda, Assigning tasks and goals, presentation l Operations Coordinator – Shawna Togioka • Knowledge of equipment, data collections and laboratory documentation l Safety Coordinator – Luke Sugie • Hazards of the Lab, chemical safety, MSDS l Group: Background data collection and analysis

Key Planning Elements 1. 2. 3. 4. 5. 6. 7. 8. Project Plan / Time Table Learn about the lab, equipment, safety, hazards Calibration Tests Batch Reactor Tests PFR Tests Analysis Oral Presentation Written Reports

Lessons Learned Ø Some activities take longer than expected Ø Experiments don’t always run smoothly. Must rethink the experimental design. Ø Overall – Lab time was utilized and original project plan didn’t need to be altered.

Background Information Reaction: Ethyl acetate+Sodium Hydroxide → Sodium acetate+Ethanol C 2 H 5 O 2 CCH 3 + Na-OH → CH 3 CO 2 Na + H 3 C-CH 2 -OH Theory: -r. OH = -d. COH/dt = -d. CEt-O-Ac/dt = k*COH*CEt-O-Ac A second order bimolecular reaction. Literature Value 1, 2: k. OH = 0. 111 L/mole-sec at 25°C Irreversible reaction

Equipment Conductivity Meter Uses: measured the conductivity in the batch reactions and PFR experiments Preparation: calibrated at beginning of every lab period. Calibration curves were constructed with different concentrations of reactants and products.

Equipment Constant Water Bath -Batch Reaction experiments done at 25°C -Reactants were submerged in the bath to reach temp. and then put together for the experiment.

Equipment Plug Flow Reactor -Packed with small spherical balls -Bed Void Fraction 3, ε, of ~0. 41 -Equimolar concentrations of Na. OH and Ethyl Acetate were pumped into PFR -Conductivity meter used to determine the composition of the product stream. -Experiment finished once reaction reached equilibrium.

Experiments Testing was done on the PFR pumps to determine the resonance time for each pump at different settings. 2. Calibration curves were generated for the conductivity meter for known concentrations of reactants and products. 3. Batch reactions were done using equimolar concentrations of reactants. 4. PFR experiments were done using equimolar concentrations and approx. equal molar flows. 1.
Batch Reactor Experiments • • Bath was set to 25°C Reactants were measured and put in bath separately to heat. Combined reactants and conductivity measurements taken at 5 and 10 second intervals. Batches were constantly stirred for the duration of the experiment.

PFR Experiments • • Large quantities of equimolar mixture of Ethyl acetate and Na. OH were prepared and placed at the inlet for each pump. The pumps were set so that the flow rates of each of the reactants would be equal. Conductivity Meter was connected to the PFR at the outlet and readings were taken during the experiment. Experiment was finished once the conductivity reached a steady state.

Key Equations Batch Reactor COH=CEt-O-Ac Relationship: 1 = k*t + 1 COHo PFR COH=CEt-O-Ac Relationship: 1 * XOH = k*τ COH 1 - XOH τ = V/vo (Space-time)
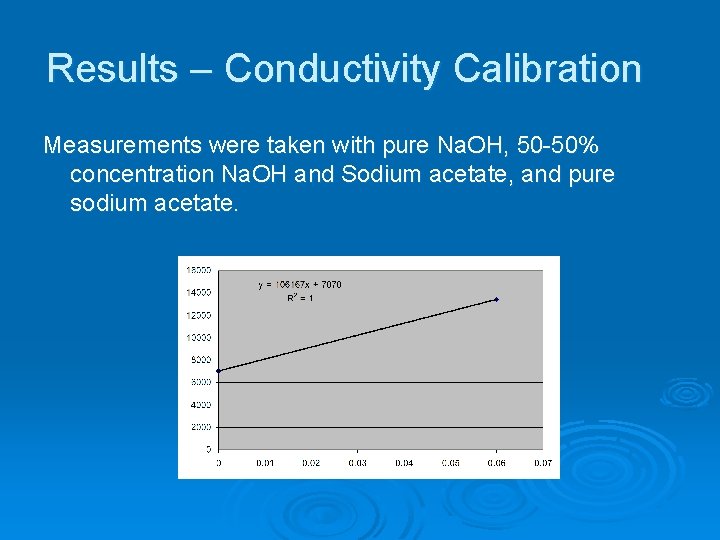
Results – Conductivity Calibration Measurements were taken with pure Na. OH, 50 -50% concentration Na. OH and Sodium acetate, and pure sodium acetate.
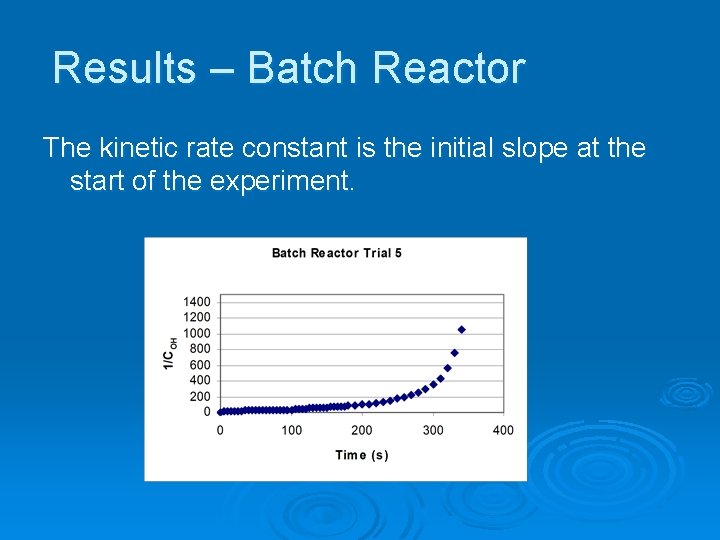
Results – Batch Reactor The kinetic rate constant is the initial slope at the start of the experiment.

Results – Batch Reactor

Results - PFR The flow rate of the pumps was varied to five different settings for data collection.

Results – Batch and Plug Flow Reactor • Batch Reactor showed a kinetic rate constant of ~0. 19 L/mole-sec • • Tests showed the rate constant to be 2 times higher than literary value, but was consistent for all trials. Plug Flow Reactor showed the kinetic rate constant to be ~0. 24 L/mole-sec • The experimental value was 2. 5 times higher than the literary value.
Major Conclusions 1. 2. 3. 4. 5. The kinetic rate constant for batch is 0. 19 L/mole -sec The kinetic rate constant for a PFR is 0. 24 L/mole -sec. The literary value was 0. 111 L/mole-sec Discrepancies in the experiment and literature could be caused from slightly unequal concentrations, incorrect molar flow rates, or conductivity calibration problems. Reaction data showed characteristics of being second order as theory predicted.

Lessons Learned Ø Some activities take longer than expected Ø Experiments don’t always run smoothly. Must rethink the experimental design. Ø Overall – Lab time was utilized and original project plan didn’t need to be altered.

Future Recommendations More careful research done early on, so work in the lab could go more smoothly. • Run more trials on the PFR and batch to confirm data. • • Plan out your lab times carefully and set reasonable goals and be safe.

References 1. 2. 3. Bamford, C. H. and C. F. H. Tipper. 1970. Comprehensive Chemical Kinetics v. 10. Elsevier Publishing Company. New York. p. 169. Batch Reactor Kinetic Analysis. Jan 15, 2005. www. csupomona. edu/~tknguyen/che 435/Notes/P 5 -kinetic. pdf Levenspiel, Octave. 1998. Engineering Flow and Heat Exchange. Plenum Press. New York. p. 128.

Questions?
- Slides: 24