Do Now 1 Write the general equation for







- Slides: 14

Do Now 1. Write the general equation for the following reactions: a) Acid + metal Metal oxide + acid Metal hydroxide + acid Metal carbonate + acid Metal hydrogencarbonate + acid b) c) d) e) 2. Complete the following equations and balance where necessary: a) b) c) d) e) f) Mg + HCl Cu. O + H 2 SO 4 Zn(OH)2 + H 2 SO 4 Na. OH + H 3 PO 4 Li 2 CO 3 + HCl Sodium hydrogencarbonate + citric acid

Calculation of Reacting Volumes of Gases Targets • Calculating reacting volumes of gases from chemical equations using the concept of molar volume of gases Homework: Read pages 144 – 149 and make notes. You will be tested on it next lesson. Due: Next Friday (17 th Nov) Test: Topic 1 and 5 – Soon!

Core Practical 2 and 3 Feedback

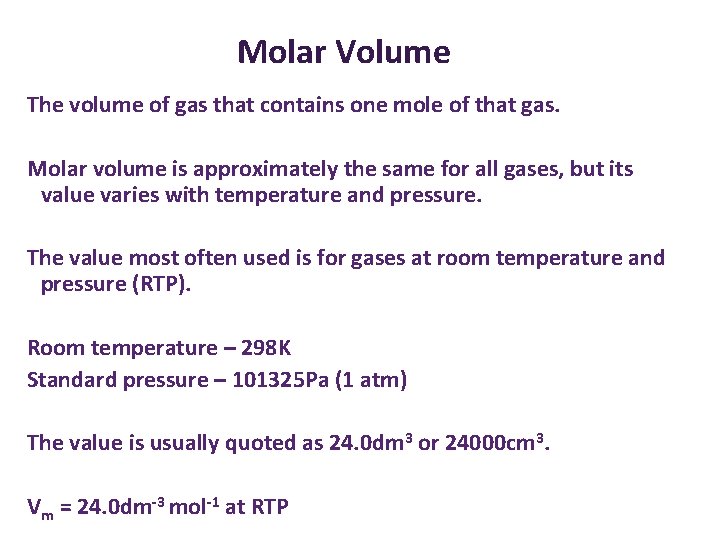
Molar Volume The volume of gas that contains one mole of that gas. Molar volume is approximately the same for all gases, but its value varies with temperature and pressure. The value most often used is for gases at room temperature and pressure (RTP). Room temperature – 298 K Standard pressure – 101325 Pa (1 atm) The value is usually quoted as 24. 0 dm 3 or 24000 cm 3. Vm = 24. 0 dm-3 mol-1 at RTP

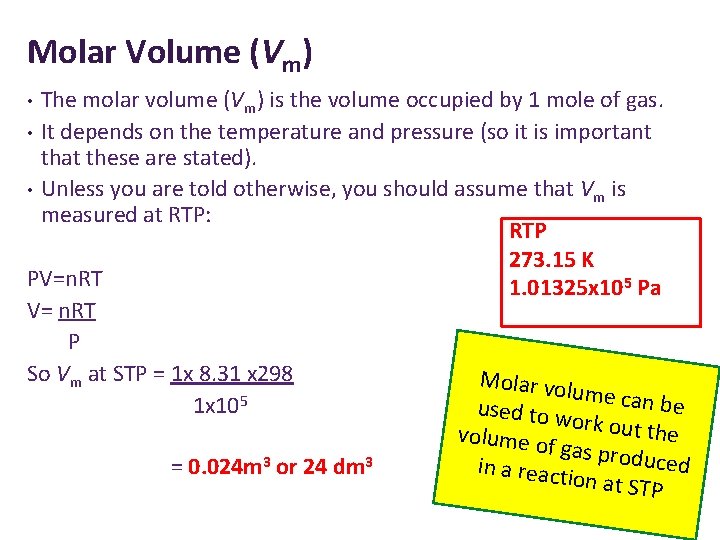
Molar Volume (Vm) The molar volume (Vm) is the volume occupied by 1 mole of gas. • It depends on the temperature and pressure (so it is important that these are stated). • Unless you are told otherwise, you should assume that Vm is measured at RTP: RTP 273. 15 K PV=n. RT 1. 01325 x 105 Pa V= n. RT P So Vm at STP = 1 x 8. 31 x 298 Molar vo lume can 5 be 1 x 10 used to w ork out th volume o e f gas prod uced in a react = 0. 024 m 3 or 24 dm 3 ion at STP •

Rearrange the equation Vm = 24. 0 = ? Vm = 24000 = ? Volume in dm 3 = ? Volume in cm 3 = ? Amount in mol = ?
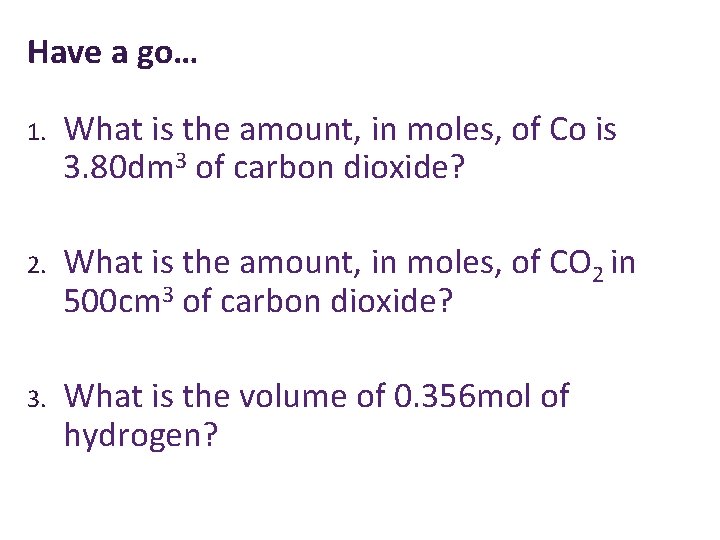
Have a go… 1. What is the amount, in moles, of Co is 3. 80 dm 3 of carbon dioxide? 2. What is the amount, in moles, of CO 2 in 500 cm 3 of carbon dioxide? 3. What is the volume of 0. 356 mol of hydrogen?

Have a go… 1. What is the amount, in moles, of Co is 3. 80 dm 3 of carbon dioxide? 0. 158 mol 2. What is the amount, in moles, of CO 2 in 500 cm 3 of carbon dioxide? 0. 0208 mol 3. What is the volume of 0. 356 mol of hydrogen? 8. 54 dm 3

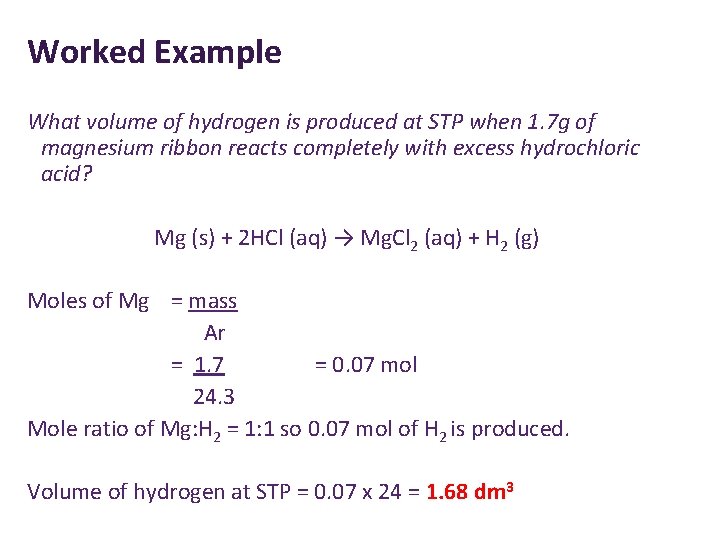
Worked Example What volume of hydrogen is produced at STP when 1. 7 g of magnesium ribbon reacts completely with excess hydrochloric acid? Mg (s) + 2 HCl (aq) → Mg. Cl 2 (aq) + H 2 (g) Moles of Mg = mass Ar = 1. 7 = 0. 07 mol 24. 3 Mole ratio of Mg: H 2 = 1: 1 so 0. 07 mol of H 2 is produced. Volume of hydrogen at STP = 0. 07 x 24 = 1. 68 dm 3

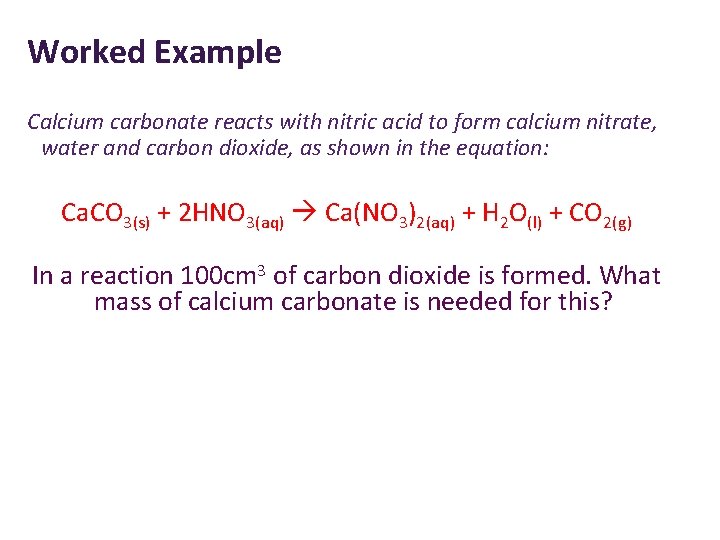
Worked Example Calcium carbonate reacts with nitric acid to form calcium nitrate, water and carbon dioxide, as shown in the equation: Ca. CO 3(s) + 2 HNO 3(aq) Ca(NO 3)2(aq) + H 2 O(l) + CO 2(g) In a reaction 100 cm 3 of carbon dioxide is formed. What mass of calcium carbonate is needed for this?

Have a go… Ammonium sulfate reacts with sodium hydroxide solution to form sodium sulfate, water and ammonia, as shown in the equation below. (NH 4)2 SO 4(s) + 2 Na. OH(aq) Na 2 SO 4(aq) + 2 H 2 O(l) + 2 NH 3(g) What volume of ammonia is formed by reacting 2. 16 g of ammonium sulfate with excess sodium hydroxide solution?

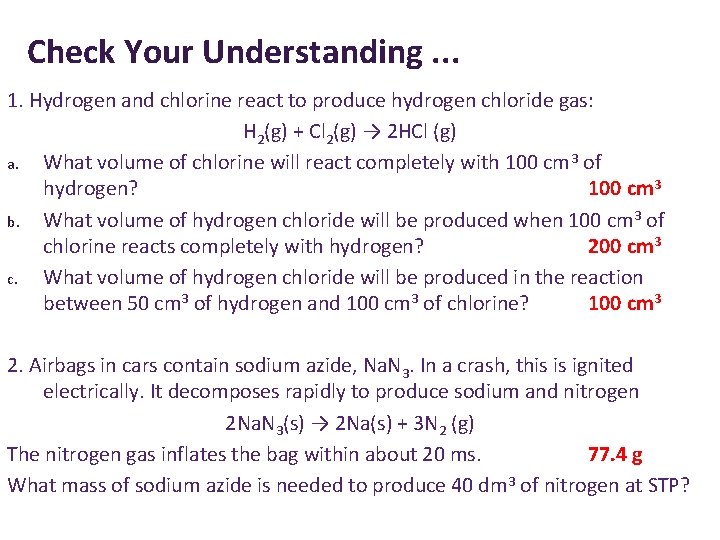
Check Your Understanding. . . 1. Hydrogen and chlorine react to produce hydrogen chloride gas: H 2(g) + Cl 2(g) → 2 HCl (g) a. What volume of chlorine will react completely with 100 cm 3 of hydrogen? b. What volume of hydrogen chloride will be produced when 100 cm 3 of chlorine reacts completely with hydrogen? c. What volume of hydrogen chloride will be produced in the reaction between 50 cm 3 of hydrogen and 100 cm 3 of chlorine? 2. Airbags in cars contain sodium azide, Na. N 3. In a crash, this is ignited electrically. It decomposes rapidly to produce sodium and nitrogen 2 Na. N 3(s) → 2 Na(s) + 3 N 2 (g) The nitrogen gas inflates the bag within about 20 ms. What mass of sodium azide is needed to produce 40 dm 3 of nitrogen at STP?

Check Your Understanding. . . 1. Hydrogen and chlorine react to produce hydrogen chloride gas: H 2(g) + Cl 2(g) → 2 HCl (g) a. What volume of chlorine will react completely with 100 cm 3 of hydrogen? 100 cm 3 b. What volume of hydrogen chloride will be produced when 100 cm 3 of chlorine reacts completely with hydrogen? 200 cm 3 c. What volume of hydrogen chloride will be produced in the reaction between 50 cm 3 of hydrogen and 100 cm 3 of chlorine? 100 cm 3 2. Airbags in cars contain sodium azide, Na. N 3. In a crash, this is ignited electrically. It decomposes rapidly to produce sodium and nitrogen 2 Na. N 3(s) → 2 Na(s) + 3 N 2 (g) The nitrogen gas inflates the bag within about 20 ms. 77. 4 g What mass of sodium azide is needed to produce 40 dm 3 of nitrogen at STP?

Task • Chemsheets Amount of Substance Booklet • Task 15