Starter Copy and complete We added Sodium Hydroxide




- Slides: 11

Starter Copy and complete; We added Sodium Hydroxide, which is an a______ drop by drop to hydrochloric acid, which is an a_____. We n_____ the solution which formed Sodium Chloride and water. Sodium Chloride is more commonly known as table s______.

Objectives • Know how to name compounds • Know how to write formulas • Know how to work out how many of each atom there are in a compound, based on a formula

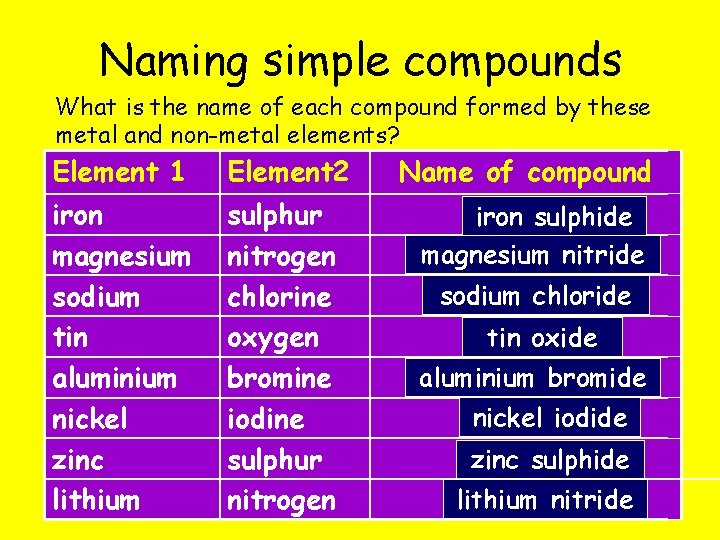
Naming simple compounds To name simple compounds of metals and non-metals: 1. Write down the name of the metal. 2. Write down the name of the non-metal, changing the ending of the word to “-ide”. What is the name of the compound made when the following elements combine? l magnesium and oxygen l sodium and chlorine l oxygen and iron magnesium oxide sodium chloride iron oxide

Naming simple compounds What is the name of each compound formed by these metal and non-metal elements? Element 1 Element 2 Name of compound iron magnesium sodium tin aluminium nickel zinc lithium sulphur nitrogen chlorine oxygen bromine iodine sulphur nitrogen iron sulphide magnesium nitride sodium chloride tin oxide aluminium bromide nickel iodide zinc sulphide lithium nitride

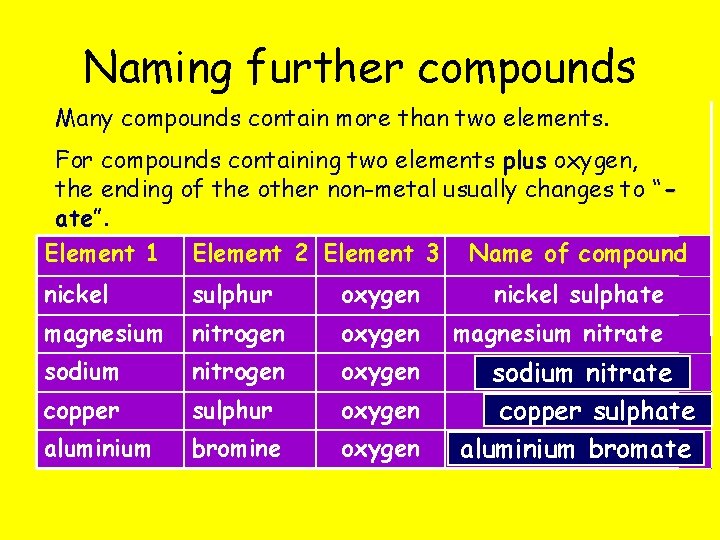
Naming further compounds Many compounds contain more than two elements. For compounds containing two elements plus oxygen, the ending of the other non-metal usually changes to “ ate”. Element 1 Element 2 Element 3 Name of compound nickel sulphur oxygen nickel sulphate magnesium nitrogen oxygen magnesium nitrate sodium nitrogen oxygen copper sulphur oxygen aluminium bromine oxygen sodium nitrate copper sulphate aluminium bromate

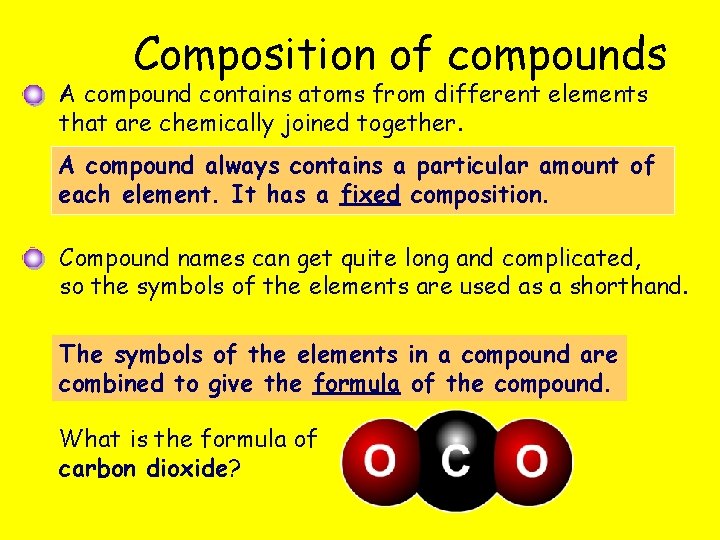
Composition of compounds A compound contains atoms from different elements that are chemically joined together. A compound always contains a particular amount of each element. It has a fixed composition. Compound names can get quite long and complicated, so the symbols of the elements are used as a shorthand. The symbols of the elements in a compound are combined to give the formula of the compound. What is the formula of carbon dioxide?

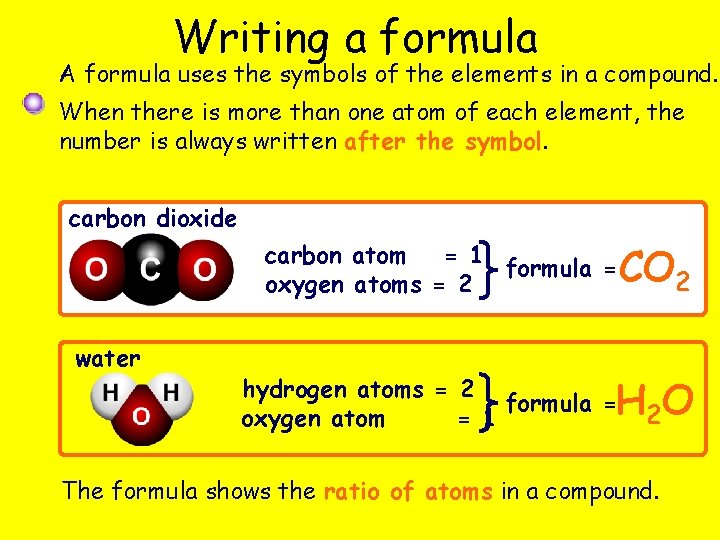
Writing a formula A formula uses the symbols of the elements in a compound. When there is more than one atom of each element, the number is always written after the symbol. carbon dioxide carbon atom = 1 formula = oxygen atoms = 2 water CO 2 H 2 O hydrogen atoms = 2 formula = oxygen atom = 1 The formula shows the ratio of atoms in a compound.

What is the formula? What is the formula of each of these compounds? (In a formula put the metal first as when naming a compound. ) 1. Titanium oxide For every titanium atom there are two oxygen atoms. Formula = Ti. O 2 2. Lithium oxide For every two lithium atoms there is one oxygen atom. Formula = Li O 2 3. Aluminium chloride For every aluminium atom there are three chlorine atoms Formula = Al. Cl 3

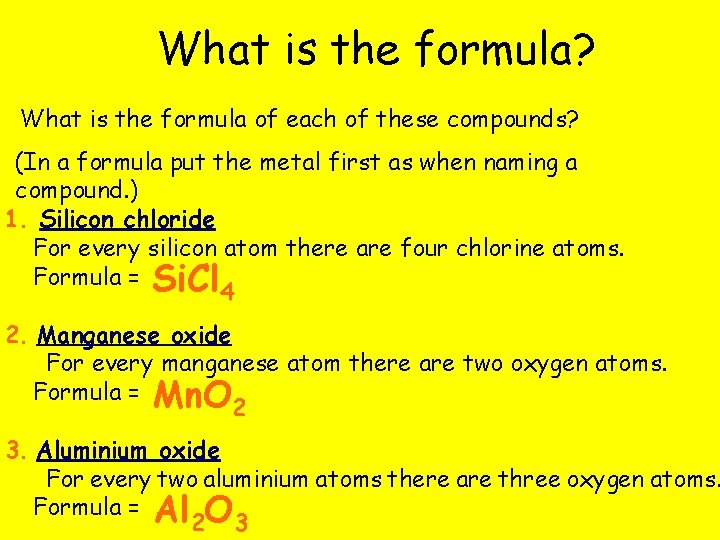
What is the formula? What is the formula of each of these compounds? (In a formula put the metal first as when naming a compound. ) 1. Silicon chloride For every silicon atom there are four chlorine atoms. Formula = Si. Cl 4 2. Manganese oxide For every manganese atom there are two oxygen atoms. Formula = Mn. O 2 3. Aluminium oxide For every two aluminium atoms there are three oxygen atoms. Formula = Al O 2 3

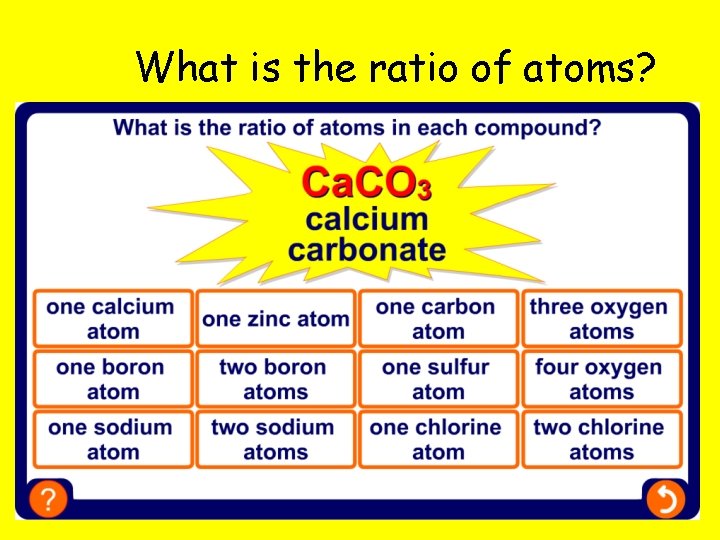
What is the ratio of atoms?

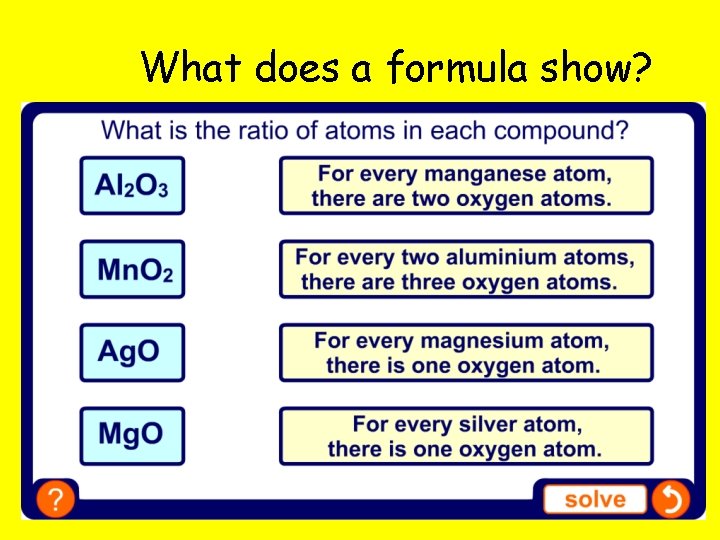
What does a formula show?