Kinetics Reaction Mechanism Chapter 14 part V Reaction








- Slides: 20

Kinetics Reaction Mechanism Chapter 14 part V

Reaction Mechanisms • The steps that add up to the overall balanced reaction equation. • May be simple with 2 or 3 steps or very complicated in reactions that may take many steps.

Consider the reaction A + B AB. The rate law for this reaction is k[A]2. A mechanism for this reaction that meets the criteria given might look like this: A + A A 2 SLOW A 2 + B AB + A These two steps reveal that when added they yield the balanced equation. The species A 2, which does not appear in the balanced equation, is considered an "unstable" intermediate. See Transition State.

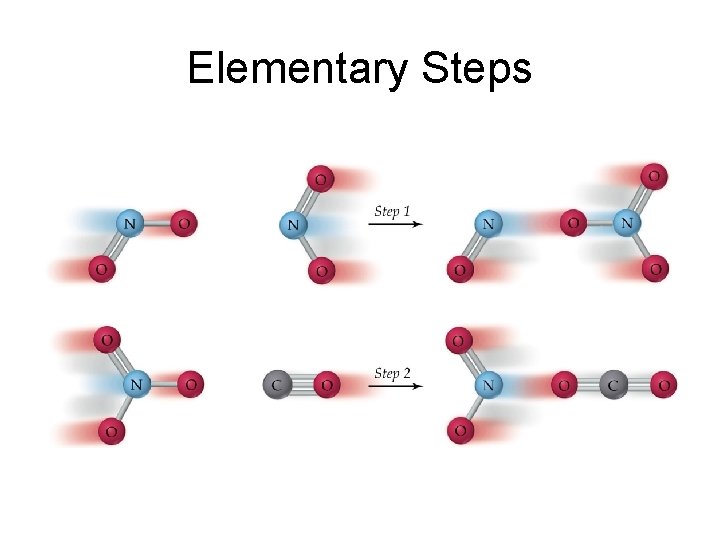
Elementary Steps

Elementary Steps • Each of the two steps is an elementary step. • Each elementary step is a reaction whose rate law may be written from is molecularity. • Molecularity is defined as the number of species that must collide to produce that reaction.

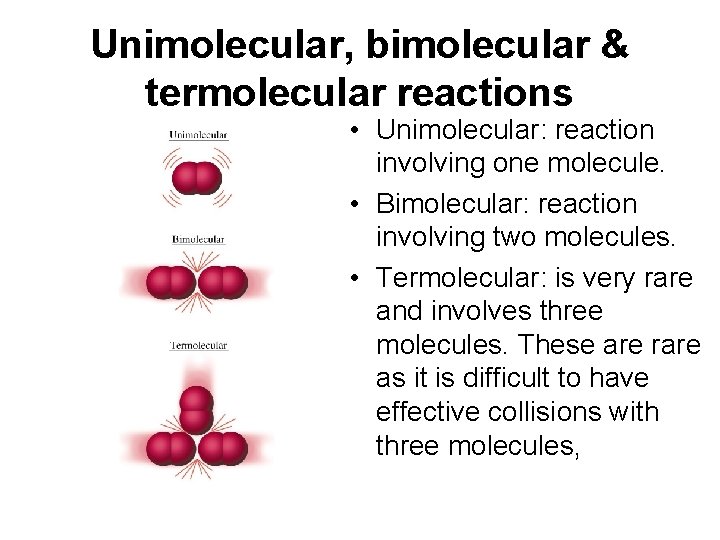
Unimolecular, bimolecular & termolecular reactions • Unimolecular: reaction involving one molecule. • Bimolecular: reaction involving two molecules. • Termolecular: is very rare and involves three molecules. These are rare as it is difficult to have effective collisions with three molecules,

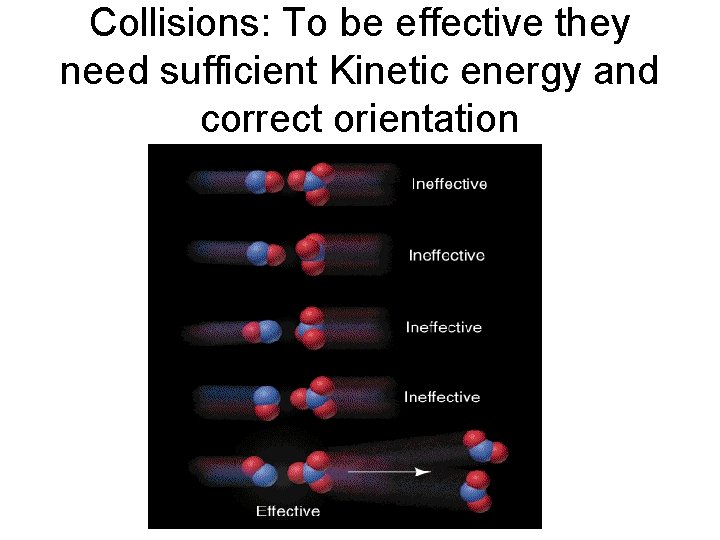
Collisions: To be effective they need sufficient Kinetic energy and correct orientation

Defining Reaction Mechanism • A Series of elementary steps that satisfy two requirements. 1. The sum of the elementary steps must give the overall balanced equation for the reaction. 2. The mechanism must agree with the experimentally determined rate law.

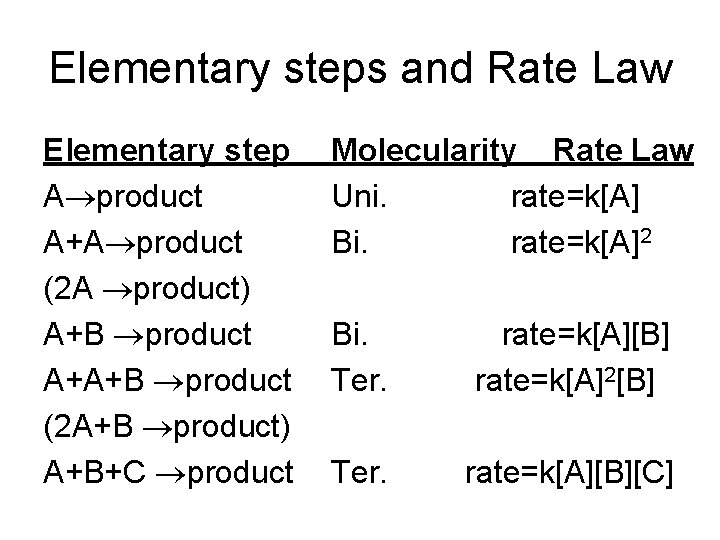
Elementary steps and Rate Law Elementary step A product A+A product (2 A product) A+B product A+A+B product (2 A+B product) A+B+C product Molecularity Rate Law Uni. rate=k[A] Bi. rate=k[A]2 Bi. Ter. rate=k[A][B] rate=k[A]2[B] Ter. rate=k[A][B][C]

The Slow Step: or the Rate Determining Step • The label SLOW on the first step in the mechanism produces the "agreement" with the rate law. Each of the steps in a mechanism has its own rate but there is thought to be one which is much slower than all the others. This step is known as the rate determining step because the reaction cannot proceed any faster than the slowest step in the mechanism.

The Slow Step • The general rule for assigning the SLOW step in a mechanism is that it must contain the same items (generally reactants) as the rate law (in the same amounts). • The slow step has the same rate law as the experimentally proven rate law.

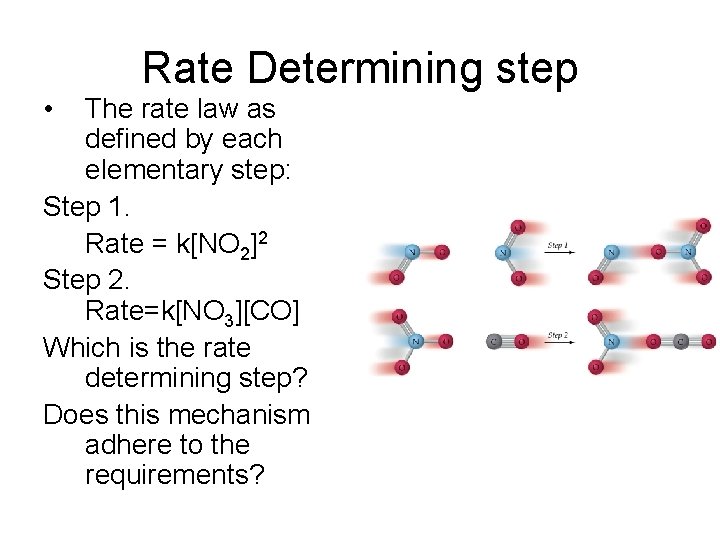
Rate Determining step • The rate law as defined by each elementary step: Step 1. Rate = k[NO 2]2 Step 2. Rate=k[NO 3][CO] Which is the rate determining step? Does this mechanism adhere to the requirements?

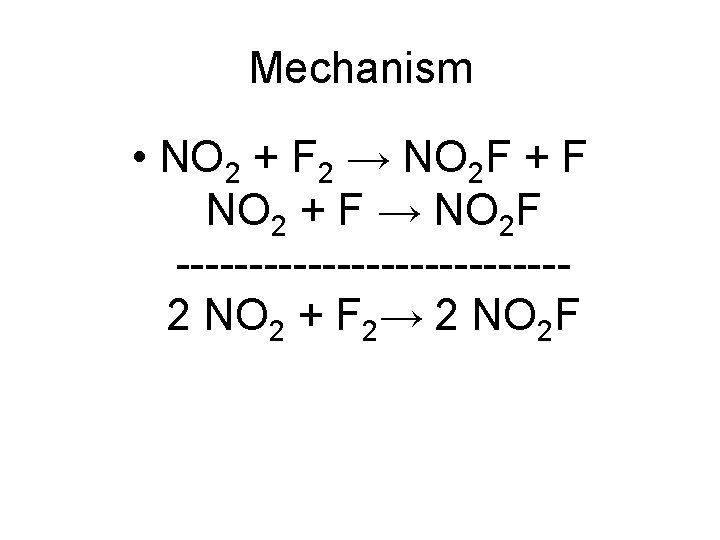
Consider the reaction: • 2 NO 2 + F 2→ 2 NO 2 F; the rate law for this reaction is k[NO 2][F 2]. • A proposed mechanism has two steps. The last step is shown below: • NO 2 + F → NO 2 F

To write the first step all we need to do is compare the balanced equation with the step we already have and fill in the difference:

Mechanism • NO 2 + F 2 → NO 2 F + F NO 2 + F → NO 2 F -------------2 NO 2 + F 2→ 2 NO 2 F

In order for this mechanism to "agree" with the given rate law the first step must be SLOW since it contains the two reactants found in the rate law. The species F does not appear in the balanced equation (and we know F is not a stable form of fluorine) so it must be an intermediate.

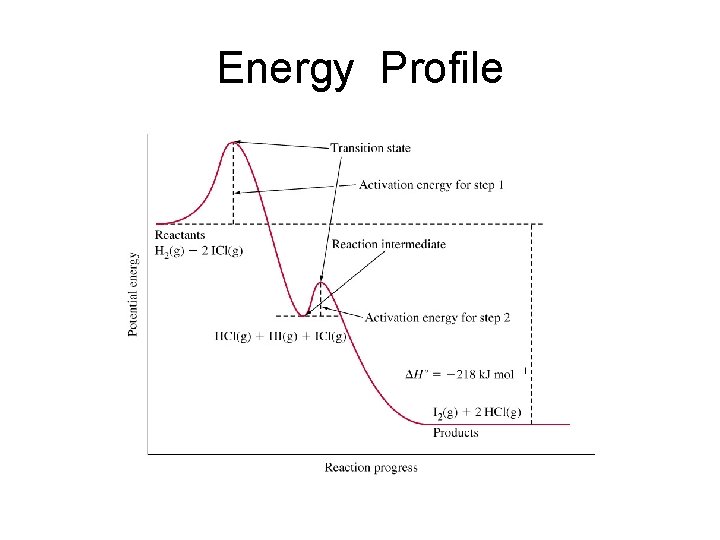
The reaction energy profile should show two "hills" with the first one being higher than the second. The activation energy of the overall reaction is thus determined by the SLOW step. Because the reaction is exothermic the diagram ends lower than it began

Energy Profile

Reaction Mechanisms • The SLOW steps are not necessarily the first steps in mechanisms. • There are many other possible scenarios for multi-step mechanisms. • And because proposing mechanisms for real reactions requires a lot of experience and background knowledge. • You should be able to do the sorts of things we have done here.

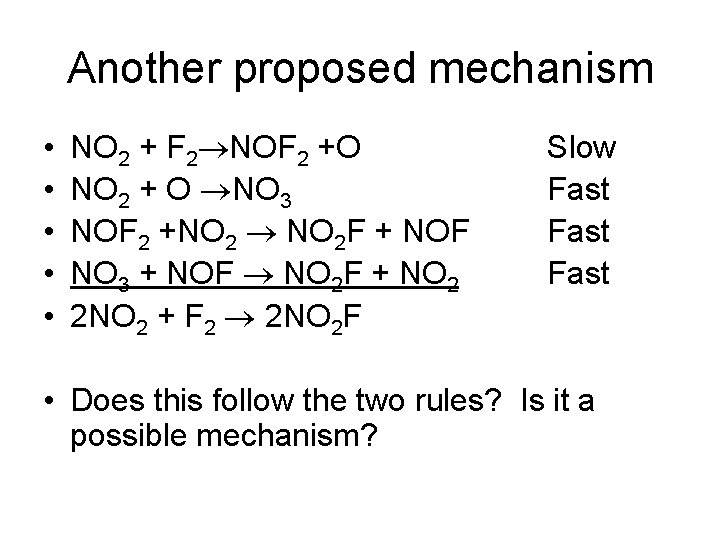
Another proposed mechanism • • • NO 2 + F 2 NOF 2 +O NO 2 + O NO 3 NOF 2 +NO 2 NO 2 F + NOF NO 3 + NOF NO 2 F + NO 2 2 NO 2 + F 2 2 NO 2 F Slow Fast • Does this follow the two rules? Is it a possible mechanism?