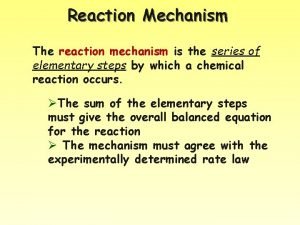
Reaction Mechanism The reaction mechanism is the series






![Fast Initial Step � Because Ratef = Rater , k 1 [NO] [Br 2] Fast Initial Step � Because Ratef = Rater , k 1 [NO] [Br 2]](https://slidetodoc.com/presentation_image_h2/67c652c6b9a4a21662f0a7ad6b601a70/image-11.jpg)
![Fast Initial Step Substituting this expression for [NOBr 2] in the rate law for Fast Initial Step Substituting this expression for [NOBr 2] in the rate law for](https://slidetodoc.com/presentation_image_h2/67c652c6b9a4a21662f0a7ad6b601a70/image-12.jpg)
- Slides: 14


Reaction Mechanism The reaction mechanism is the series of elementary steps by which a chemical reaction occurs. ØThe sum of the elementary steps must give the overall balanced equation for the reaction Ø The mechanism must agree with the experimentally

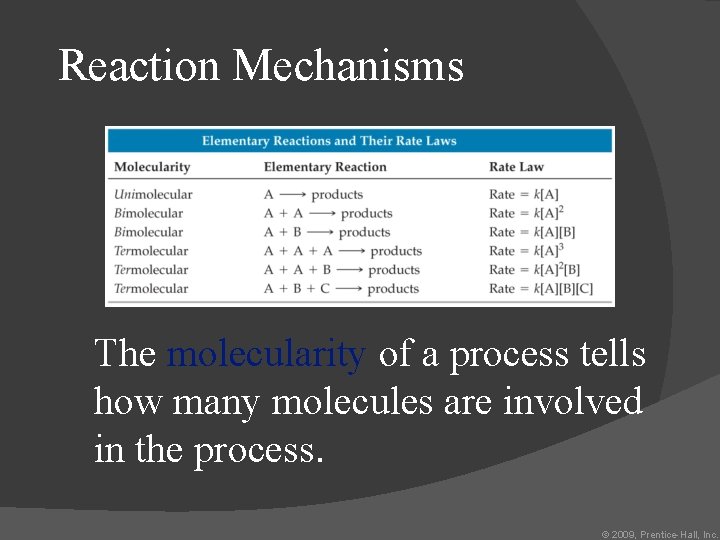
Reaction Mechanisms The molecularity of a process tells how many molecules are involved in the process. © 2009, Prentice-Hall, Inc.

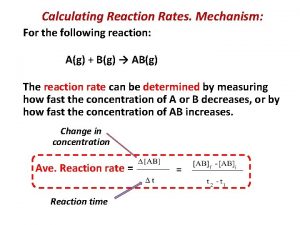
Rate-Determining Step In a multi-step reaction, the slowest step is the rate-determining step. It therefore determines the rate of the reaction. The experimental rate law must agree with the rate-determining step

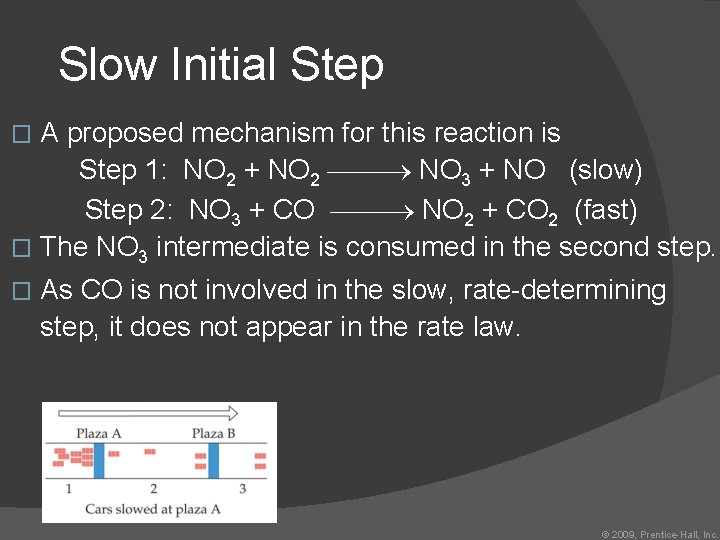
Slow Initial Step NO 2 (g) + CO (g) NO (g) + CO 2 (g) The rate law for this reaction is found experimentally to be Rate = k [NO 2]2 � CO is necessary for this reaction to occur, but the rate of the reaction does not depend on its concentration. � This suggests the reaction occurs in two steps. � © 2009, Prentice. Hall, Inc.

Slow Initial Step A proposed mechanism for this reaction is Step 1: NO 2 + NO 2 NO 3 + NO (slow) Step 2: NO 3 + CO NO 2 + CO 2 (fast) � The NO 3 intermediate is consumed in the second step. � � As CO is not involved in the slow, rate-determining step, it does not appear in the rate law. © 2009, Prentice-Hall, Inc.

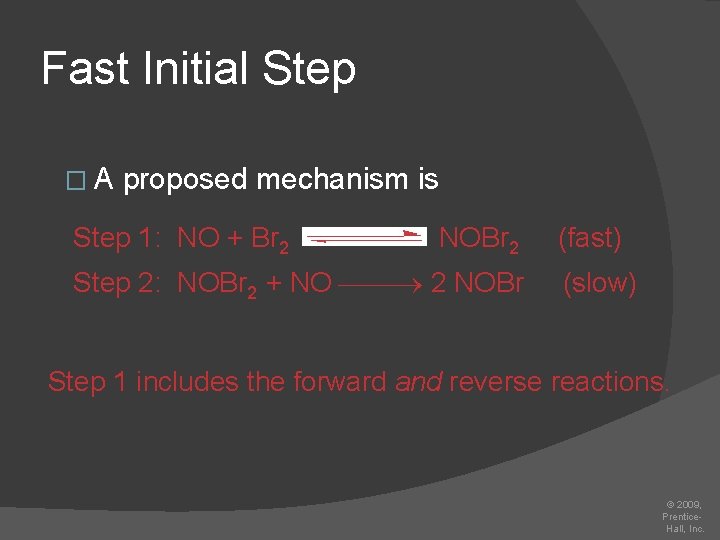
Fast Initial Step 2 NO (g) + Br 2 (g) 2 NOBr (g) � The rate law for this reaction is found to be Rate = k [NO]2 [Br 2] � Because termolecular processes are rare, this rate law suggests a two-step mechanism. © 2009, Prentice. Hall, Inc.

Fast Initial Step �A proposed mechanism is Step 1: NO + Br 2 NOBr 2 Step 2: NOBr 2 + NO 2 NOBr (fast) (slow) Step 1 includes the forward and reverse reactions. © 2009, Prentice. Hall, Inc.

Fast Initial Step � The rate of the overall reaction depends upon the rate of the slow step. � The rate law for that step would be Rate = k 2 [NOBr 2] [NO] � But how can we find [NOBr 2]? © 2009, Prentice. Hall, Inc.

Fast Initial Step � NOBr 2 can react two ways: �With NO to form NOBr �By decomposition to reform NO and Br 2 � The reactants and products of the first step are in equilibrium with each other. � Therefore, Ratef = Rater © 2009, Prentice. Hall, Inc.
![Fast Initial Step Because Ratef Rater k 1 NO Br 2 Fast Initial Step � Because Ratef = Rater , k 1 [NO] [Br 2]](https://slidetodoc.com/presentation_image_h2/67c652c6b9a4a21662f0a7ad6b601a70/image-11.jpg)
Fast Initial Step � Because Ratef = Rater , k 1 [NO] [Br 2] = k− 1 [NOBr 2] � Solving for [NOBr 2] gives us k 1 [NO] [Br ] = [NOBr ] 2 2 k− 1 © 2009, Prentice. Hall, Inc.
![Fast Initial Step Substituting this expression for NOBr 2 in the rate law for Fast Initial Step Substituting this expression for [NOBr 2] in the rate law for](https://slidetodoc.com/presentation_image_h2/67c652c6b9a4a21662f0a7ad6b601a70/image-12.jpg)
Fast Initial Step Substituting this expression for [NOBr 2] in the rate law for the rate-determining step gives Rate = k 2 k 1 k− 1 [NO] [Br 2] [NO] = k [NO]2 [Br 2] © 2009, Prentice. Hall, Inc.

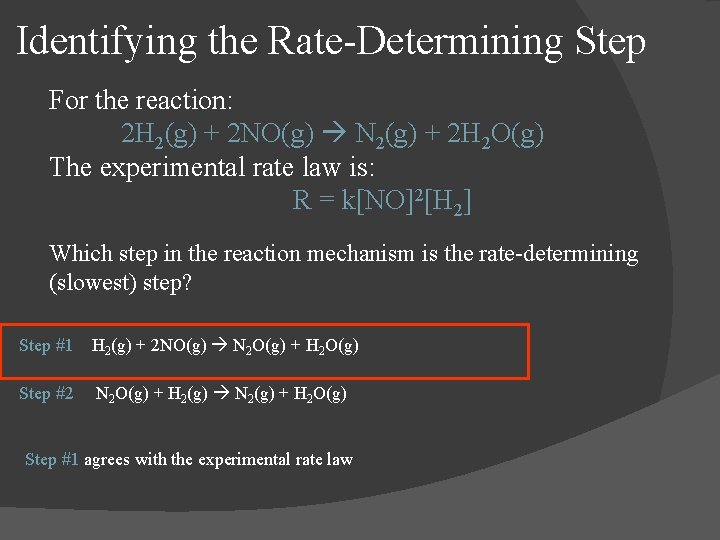
Identifying the Rate-Determining Step For the reaction: 2 H 2(g) + 2 NO(g) N 2(g) + 2 H 2 O(g) The experimental rate law is: R = k[NO]2[H 2] Which step in the reaction mechanism is the rate-determining (slowest) step? Step #1 H 2(g) + 2 NO(g) N 2 O(g) + H 2 O(g) Step #2 N 2 O(g) + H 2(g) N 2(g) + H 2 O(g) Step #1 agrees with the experimental rate law

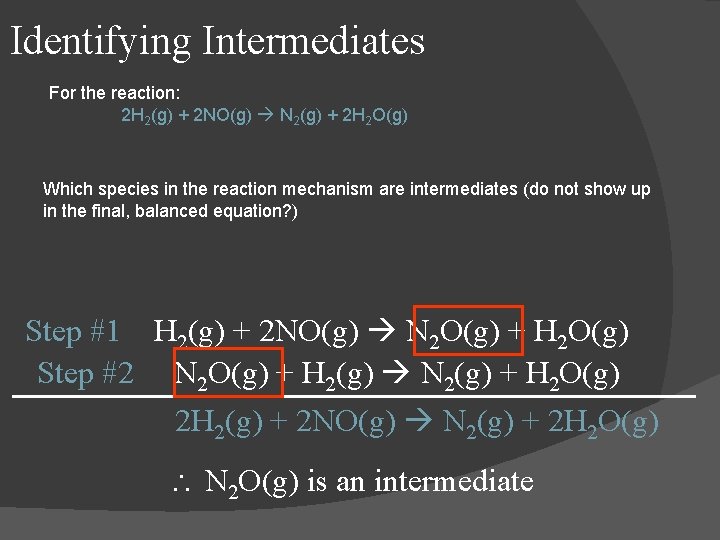
Identifying Intermediates For the reaction: 2 H 2(g) + 2 NO(g) N 2(g) + 2 H 2 O(g) Which species in the reaction mechanism are intermediates (do not show up in the final, balanced equation? ) Step #1 H 2(g) + 2 NO(g) N 2 O(g) + H 2 O(g) Step #2 N 2 O(g) + H 2(g) N 2(g) + H 2 O(g) 2 H 2(g) + 2 NO(g) N 2(g) + 2 H 2 O(g) N 2 O(g) is an intermediate
 Taylor vs maclaurin
Taylor vs maclaurin Arithmetic sum formula
Arithmetic sum formula Maclaurin series vs taylor series
Maclaurin series vs taylor series Ibm p series
Ibm p series Balmer series lyman series
Balmer series lyman series Series shunt feedback amplifier
Series shunt feedback amplifier Taylor series of composite functions
Taylor series of composite functions Series aiding and series opposing
Series aiding and series opposing Solid rock
Solid rock Metamorphic rocks
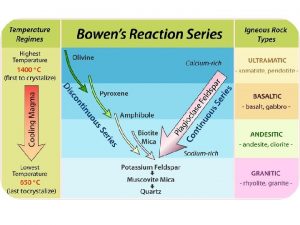
Metamorphic rocks Bowen's reaction series chart
Bowen's reaction series chart Chemical reaction engineering
Chemical reaction engineering Bowen reaction series
Bowen reaction series Bowen's reaction series describes...
Bowen's reaction series describes... Series reaction example
Series reaction example