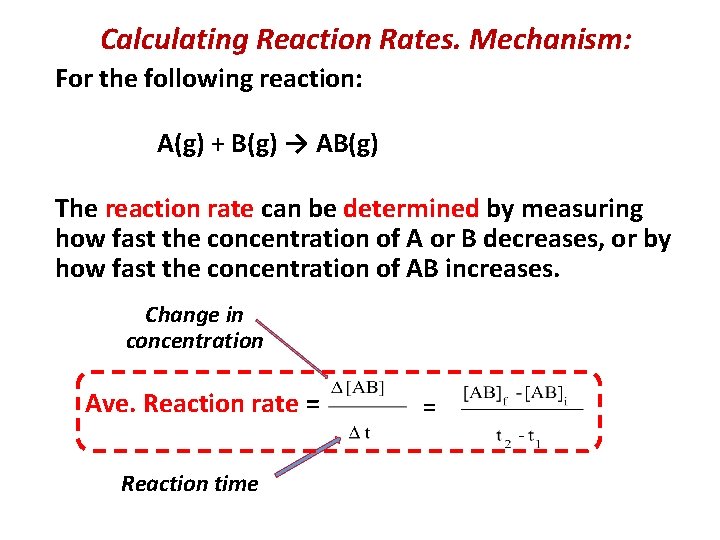
Calculating Reaction Rates Mechanism For the following reaction
![Rate = k [A] A large value of k means that A reacts rapidly Rate = k [A] A large value of k means that A reacts rapidly](https://slidetodoc.com/presentation_image_h2/d9755cd93a15183d89a74190872c1fe3/image-5.jpg)
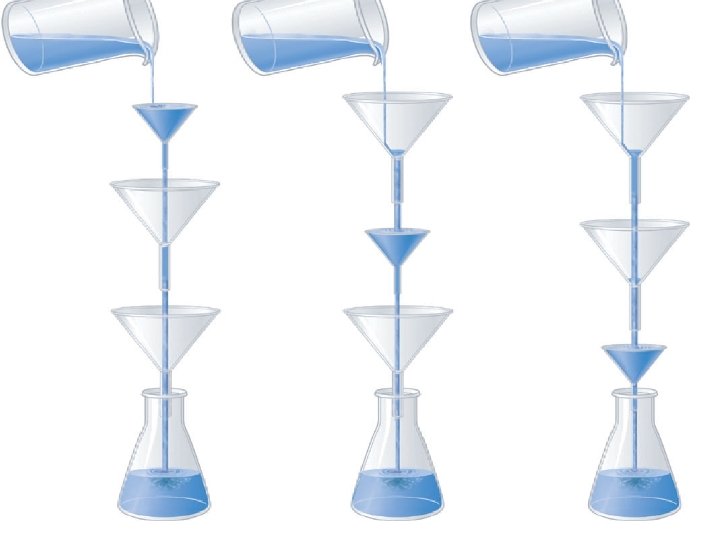
- Slides: 12

Calculating Reaction Rates. Mechanism: For the following reaction: A(g) + B(g) → AB(g) The reaction rate can be determined by measuring how fast the concentration of A or B decreases, or by how fast the concentration of AB increases. Change in concentration Ave. Reaction rate = Reaction time =

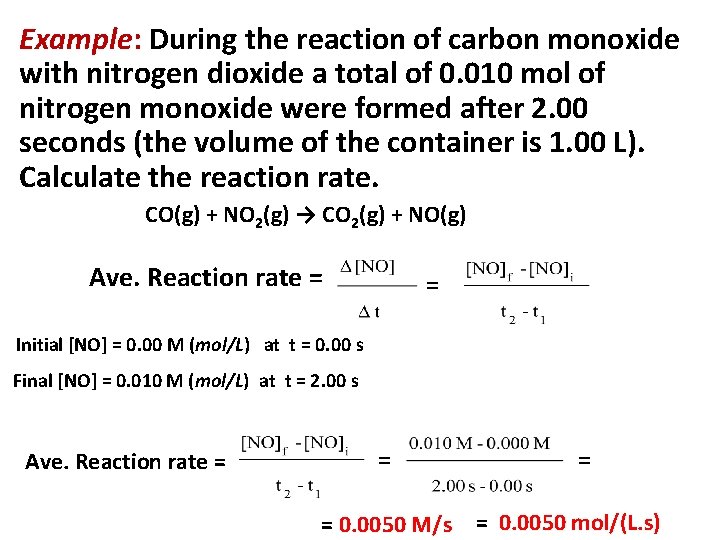
Example: During the reaction of carbon monoxide with nitrogen dioxide a total of 0. 010 mol of nitrogen monoxide were formed after 2. 00 seconds (the volume of the container is 1. 00 L). Calculate the reaction rate. CO(g) + NO 2(g) → CO 2(g) + NO(g) Ave. Reaction rate = = Initial [NO] = 0. 00 M (mol/L) at t = 0. 00 s Final [NO] = 0. 010 M (mol/L) at t = 2. 00 s Ave. Reaction rate = = 0. 0050 M/s = 0. 0050 mol/(L. s)

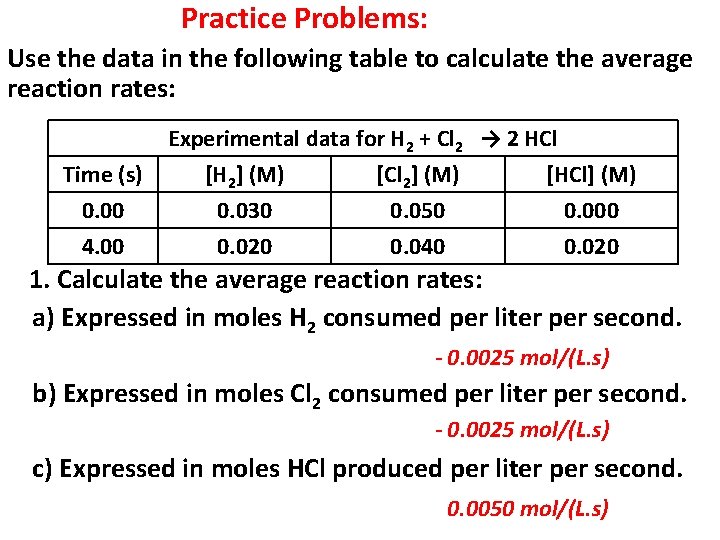
Practice Problems: Use the data in the following table to calculate the average reaction rates: Time (s) 0. 00 4. 00 Experimental data for H 2 + Cl 2 → 2 HCl [H 2] (M) [Cl 2] (M) [HCl] (M) 0. 030 0. 050 0. 000 0. 020 0. 040 0. 020 1. Calculate the average reaction rates: a) Expressed in moles H 2 consumed per liter per second. - 0. 0025 mol/(L. s) b) Expressed in moles Cl 2 consumed per liter per second. - 0. 0025 mol/(L. s) c) Expressed in moles HCl produced per liter per second. 0. 0050 mol/(L. s)

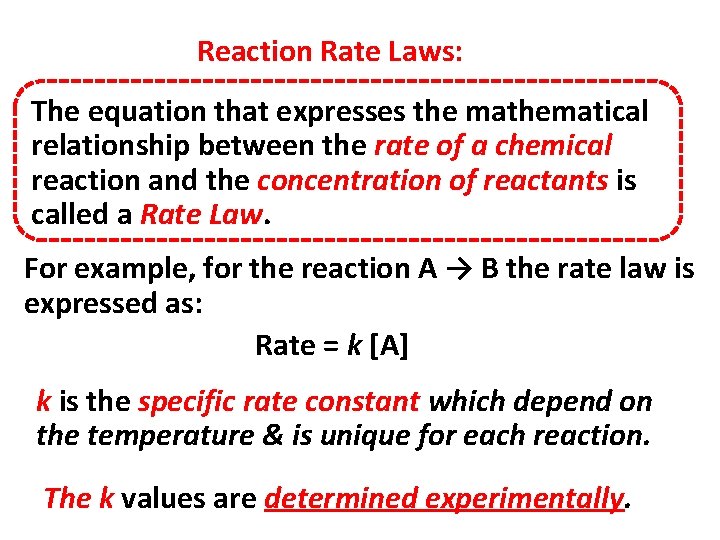
Reaction Rate Laws: The equation that expresses the mathematical relationship between the rate of a chemical reaction and the concentration of reactants is called a Rate Law. For example, for the reaction A → B the rate law is expressed as: Rate = k [A] k is the specific rate constant which depend on the temperature & is unique for each reaction. The k values are determined experimentally.
![Rate k A A large value of k means that A reacts rapidly Rate = k [A] A large value of k means that A reacts rapidly](https://slidetodoc.com/presentation_image_h2/d9755cd93a15183d89a74190872c1fe3/image-5.jpg)
Rate = k [A] A large value of k means that A reacts rapidly to form B. Reaction Order: For a reactant defines how the rate is affected by the concentration of that reactant. For example in the equation above [A] means the same as [A]1. For reactant A the exponent 1 is called the reaction order. In other words, the reaction is the first order in A.

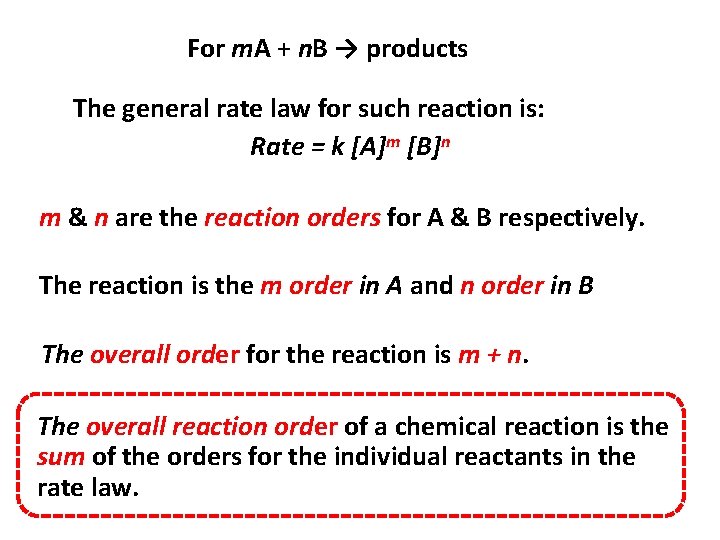
For m. A + n. B → products The general rate law for such reaction is: Rate = k [A]m [B]n m & n are the reaction orders for A & B respectively. The reaction is the m order in A and n order in B The overall order for the reaction is m + n. The overall reaction order of a chemical reaction is the sum of the orders for the individual reactants in the rate law.

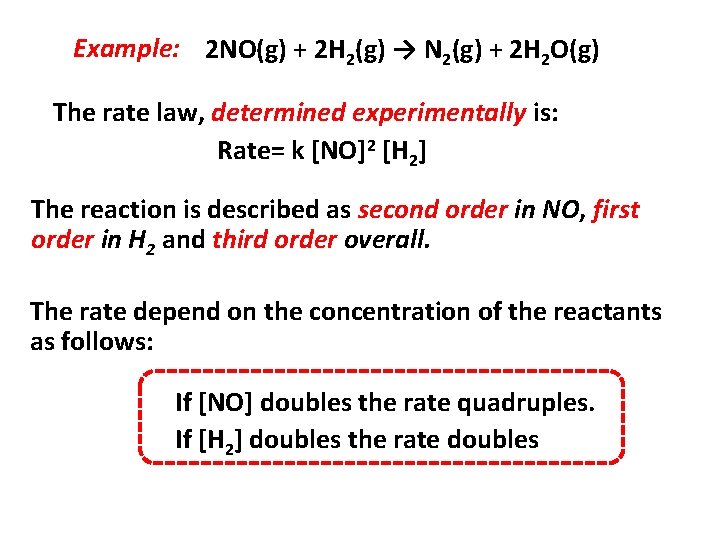
Example: 2 NO(g) + 2 H 2(g) → N 2(g) + 2 H 2 O(g) The rate law, determined experimentally is: Rate= k [NO]2 [H 2] The reaction is described as second order in NO, first order in H 2 and third order overall. The rate depend on the concentration of the reactants as follows: If [NO] doubles the rate quadruples. If [H 2] doubles the rate doubles

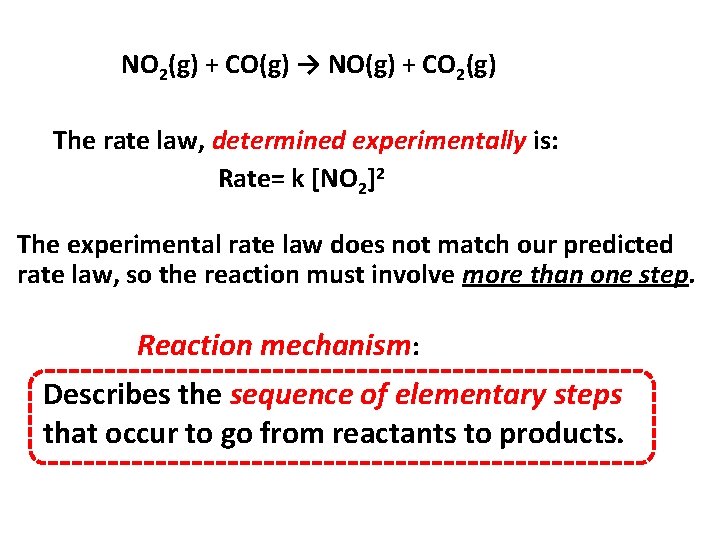
NO 2(g) + CO(g) → NO(g) + CO 2(g) The rate law, determined experimentally is: Rate= k [NO 2]2 The experimental rate law does not match our predicted rate law, so the reaction must involve more than one step. Reaction mechanism: Describes the sequence of elementary steps that occur to go from reactants to products.

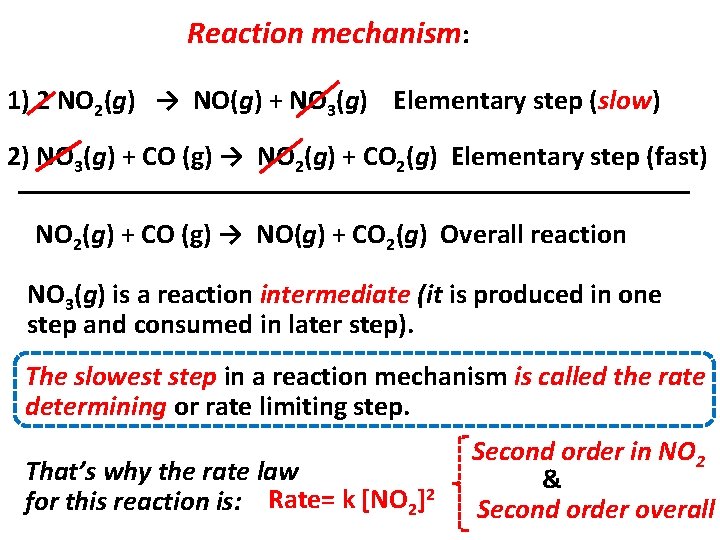
Reaction mechanism: 1) 2 NO 2(g) → NO(g) + NO 3(g) Elementary step (slow) 2) NO 3(g) + CO (g) → NO 2(g) + CO 2(g) Elementary step (fast) NO 2(g) + CO (g) → NO(g) + CO 2(g) Overall reaction NO 3(g) is a reaction intermediate (it is produced in one step and consumed in later step). The slowest step in a reaction mechanism is called the rate determining or rate limiting step. That’s why the rate law for this reaction is: Rate= k [NO 2]2 Second order in NO 2 & Second order overall
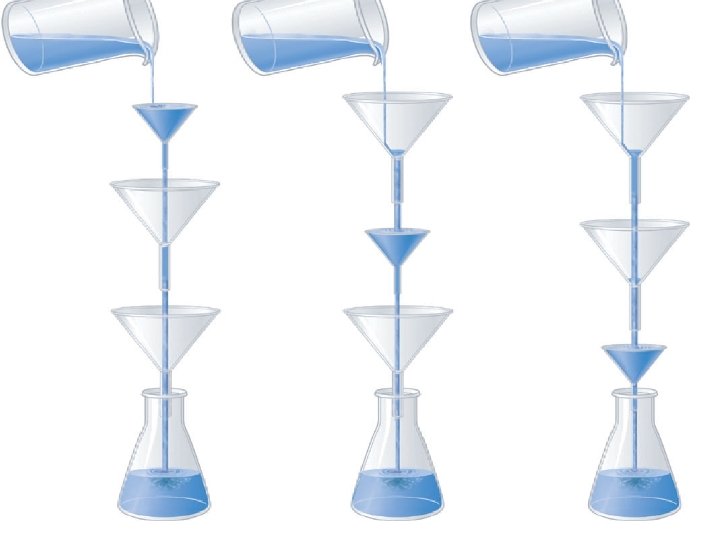

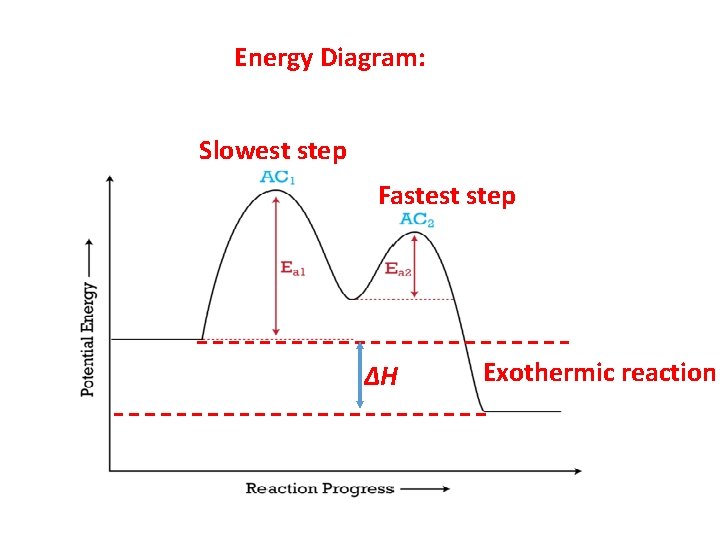
Energy Diagram: Slowest step Fastest step ∆H Exothermic reaction

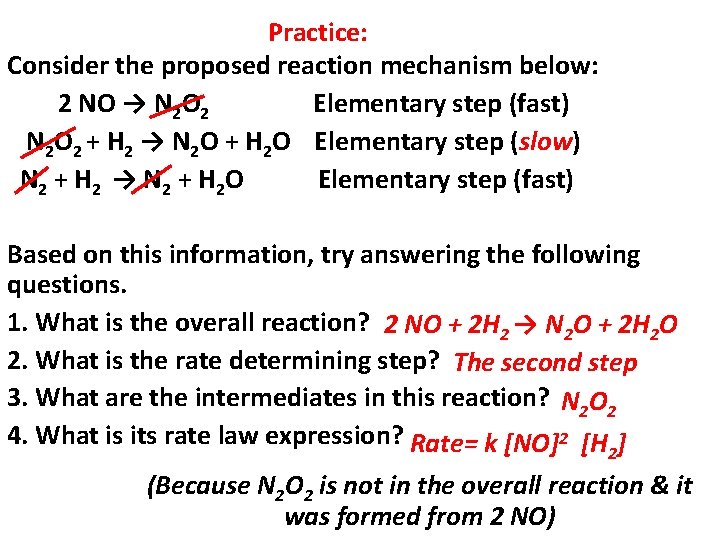
Practice: Consider the proposed reaction mechanism below: 2 NO → N 2 O 2 Elementary step (fast) N 2 O 2 + H 2 → N 2 O + H 2 O Elementary step (slow) N 2 + H 2 → N 2 + H 2 O Elementary step (fast) Based on this information, try answering the following questions. 1. What is the overall reaction? 2 NO + 2 H 2 → N 2 O + 2 H 2 O 2. What is the rate determining step? The second step 3. What are the intermediates in this reaction? N 2 O 2 4. What is its rate law expression? Rate= k [NO]2 [H ] 2 (Because N 2 O 2 is not in the overall reaction & it was formed from 2 NO)