Rate Law rate a Br 2 rate k
Rate Law
![rate a [Br 2] rate = k [Br 2] rate a [Br 2] rate = k [Br 2]](http://slidetodoc.com/presentation_image_h/0928efca5052dd185311280f977fd3fa/image-2.jpg)
rate a [Br 2] rate = k [Br 2]
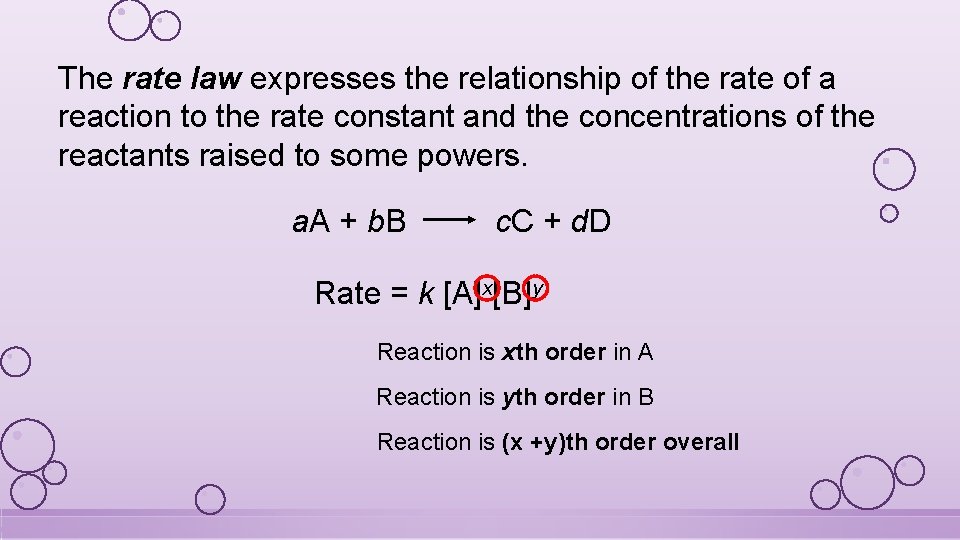
The rate law expresses the relationship of the rate of a reaction to the rate constant and the concentrations of the reactants raised to some powers. a. A + b. B c. C + d. D Rate = k [A]x[B]y Reaction is xth order in A Reaction is yth order in B Reaction is (x +y)th order overall

Examples Write the general rate law for the following chemical reactions 1. F 2(g) + 2 Cl. O 2(g) 2 FCl. O 2(g) 2. Decomposition of liquid water into hydrogen and oxygen gas
![Relationship between order and rate A B rate = k[A]x o if x=1 o Relationship between order and rate A B rate = k[A]x o if x=1 o](http://slidetodoc.com/presentation_image_h/0928efca5052dd185311280f977fd3fa/image-5.jpg)
Relationship between order and rate A B rate = k[A]x o if x=1 o Rate o If x=2 o Rate o If is directly proportional to [A] varies as the square of [A] x=0 o Rate is independent of [A]

Review of Concepts A reaction is second order in A and first order in B. If the concentration of A doubles and the concentration of B is halved, overall what has happened to the rate?
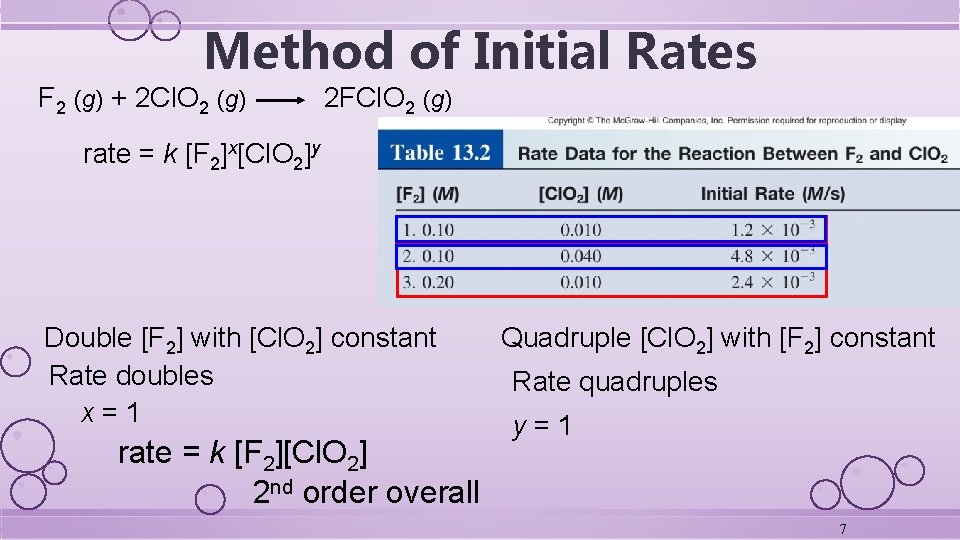
Method of Initial Rates F 2 (g) + 2 Cl. O 2 (g) 2 FCl. O 2 (g) rate = k [F 2]x[Cl. O 2]y Double [F 2] with [Cl. O 2] constant Rate doubles x=1 rate = k [F 2][Cl. O 2] 2 nd order overall Quadruple [Cl. O 2] with [F 2] constant Rate quadruples y=1 7
![Example 1 3 A B Trial Initial [A] (M) Initial Rate (M/s) 1 0. Example 1 3 A B Trial Initial [A] (M) Initial Rate (M/s) 1 0.](http://slidetodoc.com/presentation_image_h/0928efca5052dd185311280f977fd3fa/image-8.jpg)
Example 1 3 A B Trial Initial [A] (M) Initial Rate (M/s) 1 0. 050 3. 0 x 10 -4 2 0. 10 12 x 10 -4 3 0. 20 48 x 10 -4 Use the data provided to identify the order with respect to A, overall order, and rate law.
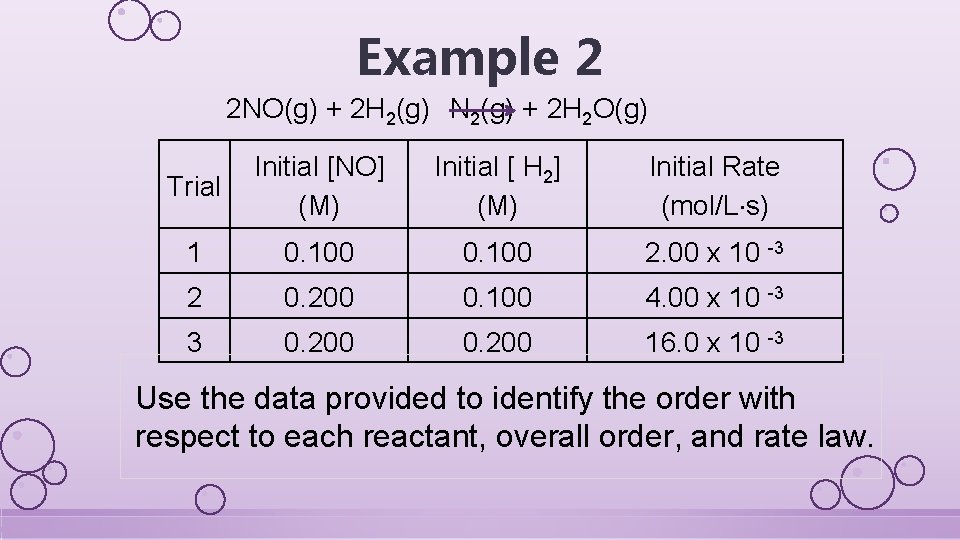
Example 2 2 NO(g) + 2 H 2(g) N 2(g) + 2 H 2 O(g) Trial Initial [NO] (M) Initial [ H 2] (M) Initial Rate (mol/L s) 1 0. 100 2. 00 x 10 -3 2 0. 200 0. 100 4. 00 x 10 -3 3 0. 200 16. 0 x 10 -3 Use the data provided to identify the order with respect to each reactant, overall order, and rate law.
![General rate law: rate = k [NO]x[H 2]y Pick appropriate experiments to compare *For General rate law: rate = k [NO]x[H 2]y Pick appropriate experiments to compare *For](http://slidetodoc.com/presentation_image_h/0928efca5052dd185311280f977fd3fa/image-10.jpg)
General rate law: rate = k [NO]x[H 2]y Pick appropriate experiments to compare *For NO, pick trials 1&2 because the H 2 concentration doesn’t change Exp 2: 4. 00× 10− 3 = k [0. 200]X [0. 100]Y *Same terms on the top and bottom Exp 1: 2. 00× 10− 3 = k [0. 100]X [0. 100]Y cancel out 2 = 2 x X=1; 1 st order in NO

For H 2, pick trials 2&3 because the concentration of NO doesn’t change Exp 3: 16. 0× 10− 3 = k [0. 200]X [0. 200]Y Exp 2: 4. 0× 10− 3 = k [0. 200]X [0. 100]Y 4 = 2 y y=2; 2 nd order in H 2 (better) rate=k[NO][H 2]2 *Same terms on the top and bottom cancel out 3 rd order overall Pick any trial, plug in all the numbers, solve for k k=2. 00 1/M 2 s *Calculating the units FINIAL ANSWER for k is a bonus rate=2. 00[NO][H 2]2
- Slides: 11