Guidelines for Prevention and Treatment of Opportunistic Infections





























- Slides: 47

Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents Hepatitis C Virus Disease Slide Set Prepared by the AETC National Resource Center based on recommendations from the CDC, National Institutes of Health, and HIV Medicine Association/Infectious Diseases Society of America

About This Presentation These slides were developed using recommendations published in May 2013. The intended audience is clinicians involved in the care of patients with HIV. Users are cautioned that, because of the rapidly changing field of HIV care, this information could become out of date quickly. Finally, it is intended that these slides be used as prepared, without changes in either content or attribution. Users are asked to honor this intent. -AETC National Resource Center http: //www. aidsetc. org 2 May 2013 www. aidsetc. org

HCV Disease: Epidemiology § HCV disease is a leading non-AIDS cause of death in HIV-infected persons § 20 -30% of HIV-infected U. S. patients have HCV coinfection § HCV is a single-stranded RNA virus § 7 genotypes § Genotype 1: ~75% of HCV infections in United States; ~90% of HCV infections in U. S. blacks 3 May 2013 www. aidsetc. org

HCV Disease: Epidemiology (2) § Transmission: percutaneous exposure, sexual exposure, perinatal, contaminated blood products or medical equipment § Percutaneous transmission: § HCV is 10 times more infectious than HIV through percutaneous blood exposures § Injection drug use is most common risk in the U. S. (via syringes or injection paraphernalia) § HCV can survive for weeks in syringes § Other risks: intranasal cocaine use, tattoo placement 4 May 2013 www. aidsetc. org

HCV Disease: Epidemiology (3) § Sexual transmission § HIV appears to increase risk of sexual transmission of HCV § In HIV-infected MSM, multiple outbreaks of acute HCV § Risk factors: unprotected receptive anal sex, sex toys, recreational drug use, concurrent STD § In HIV-uninfected MSM, HCV transmission inefficient § Heterosexual transmission uncommon; increased risk if partner is HIV/HCV coinfected 5 May 2013 www. aidsetc. org

HCV Disease: Epidemiology (4) § Perinatal transmission § HIV appears to increase transmission risk § HCV incidence: § 1 -3% if HCV-infected mothers had detectable plasma HCV § 4 -7% if mothers had detectable plasma HCV RNA § 10 -20% if mothers had HIV/HCV coinfection 6 May 2013 www. aidsetc. org

HCV Disease: Epidemiology (5) § HIV infection speeds progression of HCV to cirrhosis, especially if CD 4 count is <200 cells/µL § HIV speeds progression from cirrhosis to endstage liver disease (ESLD) and hepatocellular carcinoma (HCC) 7 May 2013 www. aidsetc. org

HCV Disease: Clinical Manifestations Acute hepatitis C: § Usually asymptomatic or mildly symptomatic; usually not recognized § <20% have symptoms of acute hepatitis (eg, fever, right upper quadrant pain, nausea, vomiting, anorexia, jaundice) § Liver transaminases may be elevated § Recognizing possible acute HCV is important, given greater efficacy of treatment in early HCV 8 May 2013 www. aidsetc. org

HCV Disease: Clinical Manifestations (2) Chronic hepatitis C: § Often asymptomatic § Fatigue is common § With progression, stigmata of portal hypertension (eg, spider angiomata, temporal wasting, splenomegaly, caput medusa, ascites, jaundice, pruritus, encephalopathy) § May see skin abnormalities (leukocytoclastic vasculitis, porphyria cutanea tarda), renal disease 9 May 2013 www. aidsetc. org

HCV Disease: Diagnosis § Screen all HIV-infected patients for HCV at entry into care: sensitive immunoassay § For at-risk HCV uninfected, retest annually or as indicated by risk exposure § To confirm infection: HCV RNA by sensitive quantitative assay § HCV RNA does not correlate with HCV disease; should not be monitored serially unless on HCV treatment § HCV RNA correlated with likelihood of response to HCV treatment 10 May 2013 www. aidsetc. org

HCV Disease: Diagnosis (2) § False-negative HCV antibody results are possible in HIV- infected persons with advanced immunosuppression (<1%) § Negative HCV antibody result can occur during acute infection § Window period before seroconversion is 2 -12 weeks § Test for HCV RNA if risk of HCV, high ALT, but negative or indeterminate serologic test 11 May 2013 www. aidsetc. org

HCV Disease: Preventing Exposure § Encourage injection drug users to enter substance abuse treatment program § Advise IDUs not to share needles or drug preparation equipment if unable to stop using § Needle exchange may facilitate access to sterile equipment § Inform patients of risks associated with nonsterile body piercing, tattooing § Encourage safer sex, especially condom use, to reduce sexual transmission of HCV 12 May 2013 www. aidsetc. org

HCV Disease: Preventing Disease § No vaccine or recommended postexposure prophylaxis § After acute HCV, treatment within 6 -12 months may prevent chronic infection; high rates of HCV clearance § Acutely infected patients should be offered treatment, unless contraindications § Peginterferon (Peg. IFN) +/– ribavirin (RBV) § Some experts recommend observation for ~3 -6 months to see if HCV will clear spontaneously 13 May 2013 www. aidsetc. org

HCV Disease: Preventing Disease Prevent liver damage: § Avoid alcohol consumption § Avoid hepatotoxins; limit acetaminophen intake (<2 g/day) § Avoid iron supplementation unless iron deficiency § Vaccinate against HAV, HBV if nonimmune § If cirrhosis, consult with specialist § Serial screening for HCC: § Optimal strategy unknown; some recommend ultrasound every 6 -12 months § AFP has poor specificity and sensitivity; should not be used as the only screening method 14 May 2013 www. aidsetc. org

HCV Disease: Preventing Disease (2) § Liver transplant is not absolutely contraindicated in HIV/HCV coinfection § May refer coinfected patients with well-controlled HIV and liver decompensation or early HCC § ART associated with reduced risk of liver disease progression § Treat with ART in accordance with usual ART guidelines § Dosage adjustment of some ARVs may be needed for patients with decompensated cirrhosis 15 May 2013 www. aidsetc. org

HCV Disease: Treatment § Goal of HCV therapy: sustained virologic response (SVR), absence of detectable viremia ≥ 6 months after discontinuation of HCV treatment § SVR associated with decreased risk of endstage liver disease, HCC, death; may decrease risk of ARV-related liver injury 16 May 2013 www. aidsetc. org

HCV Disease: Treatment (2) § HCV treatment should be considered for all HIV/HCV patients § Assessments before HCV treatment: § Genotype (GT): should be done before treatment § Important determinant of likelihood of response to IFN-based treatment § GT 2 or 3 disease has higher rate of response to treatment than do GT 1 or GT 4 § For GT 1, subtype (A or B) also may influence treatment response § HCV NS 3/4 A protease inhibitors (PIs) approved for GT 1 treatment only 17 May 2013 www. aidsetc. org

HCV Disease: Treatment (3) § Assessments before HCV treatment (cont’d): § IL 28 B genotype § C/C genotype associated with better treatment outcomes (vs C/T or T/T) § Utility and cost effectiveness uncertain § Liver disease stage § Baseline and serial ALT and AST: higher levels predictive of faster liver disease progression § Cirrhosis can be present in persons with normal ALT § Liver biopsy: preferred test to evaluate disease stage, exclude other causes, and guide treatment decisions § Not required before treatment 18 May 2013 www. aidsetc. org

HCV Disease: Treatment (4) Treatment Regimens § Peg. IFN + ribavirin recommended backbone of treatment for HIV/HCV-coinfected patients, regardless of HCV genotype § For GT 1, limited clinical trial data in HIV/HCV coinfection show HCV NS 3/4 A PIs (boceprevir, telaprevir) + Peg. IFN/ribavirin have greater efficacy than Peg. IFN/ribavirin alone § Recommended if not on ARVs with significant drug interactions with HCV PIs Note: Many new agents for treatment of HCV are in development; some are expected to be available in the near future. 19 May 2013 www. aidsetc. org

HCV Disease: Treatment (5) § For chronic HCV, decision to treat should involve assessment of potential risks and benefits of current therapy § Medical urgency for treatment based largely on degree of fibrosis; if minimal liver disease (no or mild portal fibrosis), may consider deferring therapy in anticipation of availability of new HCV drugs 20 May 2013 www. aidsetc. org

HCV Disease: Treatment (6) § Factors that favor treatment initiation: § High likelihood of SVR § HCV GT 2 or 3 § HCV GT 1 and low-level HCV RNA (<400, 000 IU/m. L) § HCV GT 1 and favorable IL-28 B (C/C) § Medical necessity § Advanced fibrosis (bridging fibrosis or cirrhosis) § Vasculitis (owing to HCV) § Membranoproliferative glomerulonephritis (owing to HCV) § Patient with high motivation for treatment Note: Many new agents for treatment of HCV are in development; future HCV regimens may include options with greater efficacy and less toxicity. 21 May 2013 www. aidsetc. org

HCV Disease: Treatment (7) § Factors that favor treatment deferral: § Untreated HIV with advanced immunosuppression (CD 4 <200 cells/µL) § Hepatic decompensation § Severe concurrent medical conditions § Severe uncontrolled psychiatric illness § Current alcohol or substance abuse § Hgb <10 g/d. L, ANC <1000/µL, platelets <50, 000/µL § Pregnancy or breast-feeding § Women who may become pregnant, and their male partners: unwillingness to practice contraception during treatment and for 6 months afterward § Sarcoidosis § Uncontrolled autoimmune disease § Hemoglobinopathies 22 May 2013 www. aidsetc. org

HCV Disease: Treatment (8) § Acute HCV: § Peg. IFN-2 a 180 mg SQ weekly, or Peg. IFN-2 b 1. 5 mcg/kg SQ weekly Plus § Ribavirin: § GT 1: 600 mg PO BID if weight ≥ 75 kg (600 mg QAM, 400 mg QPM if weight <75 kg) § GT 2, 3, 4, 5, 6: 400 mg BID § Duration: 24 -48 weeks § HCV PIs should not be routinely used: high SVR rates with standard therapy; no data for HCV PIs in acute HCV 23 May 2013 www. aidsetc. org

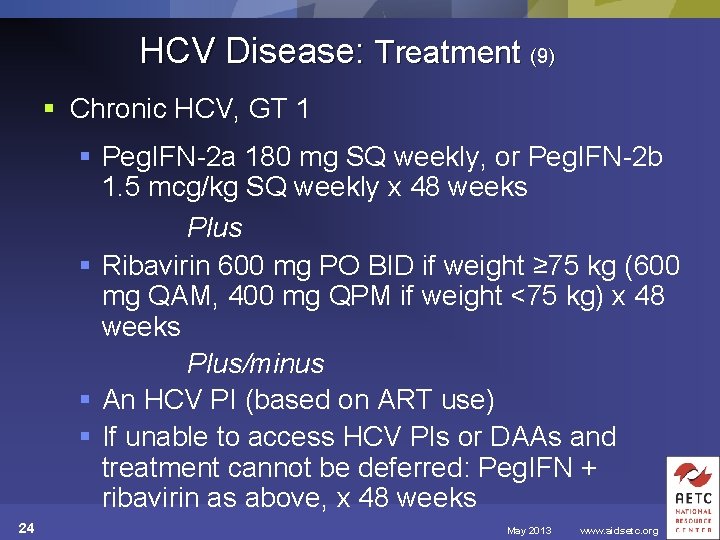
HCV Disease: Treatment (9) § Chronic HCV, GT 1 § Peg. IFN-2 a 180 mg SQ weekly, or Peg. IFN-2 b 1. 5 mcg/kg SQ weekly x 48 weeks Plus § Ribavirin 600 mg PO BID if weight ≥ 75 kg (600 mg QAM, 400 mg QPM if weight <75 kg) x 48 weeks Plus/minus § An HCV PI (based on ART use) § If unable to access HCV PIs or DAAs and treatment cannot be deferred: Peg. IFN + ribavirin as above, x 48 weeks 24 May 2013 www. aidsetc. org

HCV Disease: Treatment (10) § Chronic HCV; GT 2, 3, 4, 5, or 6 § Peg. IFN-2 a 180 mg SQ weekly, or Peg. IFN-2 b 1. 5 mcg/kg SQ weekly Plus § Ribavirin: 400 mg BID § Duration: 48 weeks (some recommend response-guided therapy) § HCV PIs should not be used 25 May 2013 www. aidsetc. org

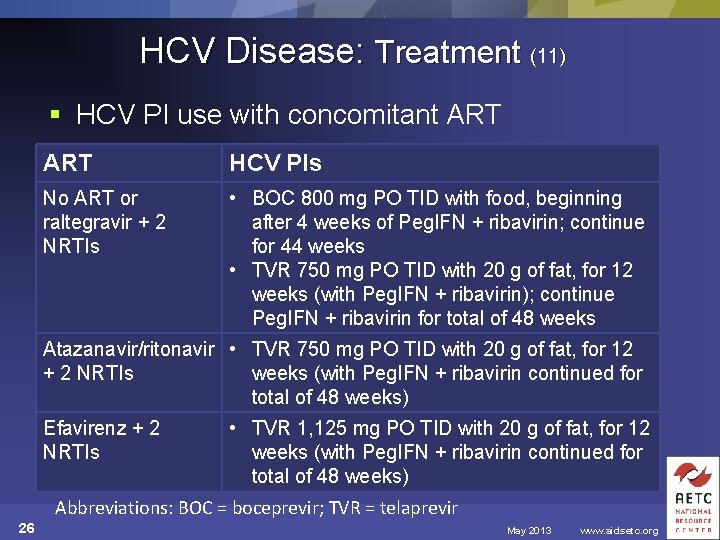
HCV Disease: Treatment (11) § HCV PI use with concomitant ART HCV PIs No ART or raltegravir + 2 NRTIs • BOC 800 mg PO TID with food, beginning after 4 weeks of Peg. IFN + ribavirin; continue for 44 weeks • TVR 750 mg PO TID with 20 g of fat, for 12 weeks (with Peg. IFN + ribavirin); continue Peg. IFN + ribavirin for total of 48 weeks Atazanavir/ritonavir • TVR 750 mg PO TID with 20 g of fat, for 12 + 2 NRTIs weeks (with Peg. IFN + ribavirin continued for total of 48 weeks) Efavirenz + 2 NRTIs • TVR 1, 125 mg PO TID with 20 g of fat, for 12 weeks (with Peg. IFN + ribavirin continued for total of 48 weeks) Abbreviations: BOC = boceprevir; TVR = telaprevir 26 May 2013 www. aidsetc. org

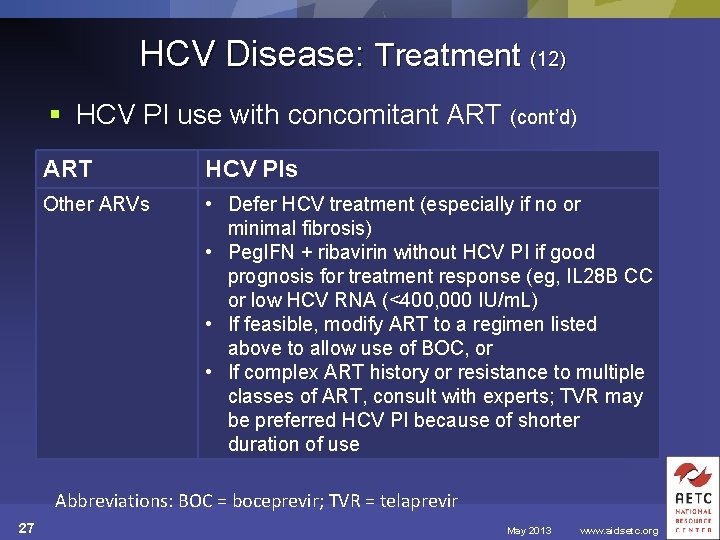
HCV Disease: Treatment (12) § HCV PI use with concomitant ART (cont’d) ART HCV PIs Other ARVs • Defer HCV treatment (especially if no or minimal fibrosis) • Peg. IFN + ribavirin without HCV PI if good prognosis for treatment response (eg, IL 28 B CC or low HCV RNA (<400, 000 IU/m. L) • If feasible, modify ART to a regimen listed above to allow use of BOC, or • If complex ART history or resistance to multiple classes of ART, consult with experts; TVR may be preferred HCV PI because of shorter duration of use Abbreviations: BOC = boceprevir; TVR = telaprevir 27 May 2013 www. aidsetc. org

HCV Disease: Treatment (13) § If ribavirin is contraindicated: § Peg. IFN-2 a 180 mg SQ weekly, or Peg. IFN-2 b 1. 5 mcg/kg SQ weekly § Likelihood of SVR without ribavirin is markedly lower § HCV PI should not be given without ribavirin (high risk of virologic failure) § If decompensated liver disease § Peg. IFN is contraindicated § Liver transplantation if feasible 28 May 2013 www. aidsetc. org

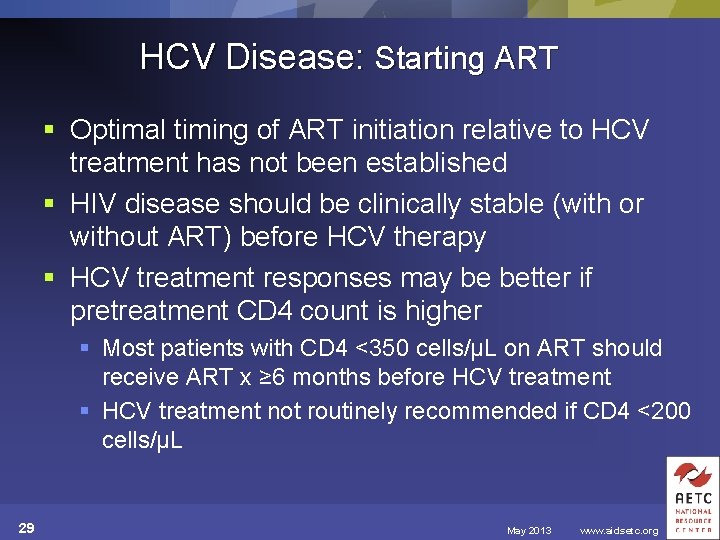
HCV Disease: Starting ART § Optimal timing of ART initiation relative to HCV treatment has not been established § HIV disease should be clinically stable (with or without ART) before HCV therapy § HCV treatment responses may be better if pretreatment CD 4 count is higher § Most patients with CD 4 <350 cells/µL on ART should receive ART x ≥ 6 months before HCV treatment § HCV treatment not routinely recommended if CD 4 <200 cells/µL 29 May 2013 www. aidsetc. org

HCV Disease: Starting ART (2) § Concurrent treatment of HIV and HCV may involve complex medical regimens, high pill burdens, potential drug-drug interactions, and increased toxicities § AZT: should be avoided with Peg. IFN/ribavirin because of higher rate of severe anemia § Didanosine: strictly contraindicated for use with ribavirin-increased didanosine toxicity including lactic acidosis § Abacavir: conflicting data re association with lower likelihood of SVR; routine discontinuation is not recommended 30 May 2013 www. aidsetc. org

HCV Disease: Starting ART (3) § Substantial risk of drug-drug interactions between ARVs and HCV PIs (BOC and TVR) § PK and/or clinical data available for some but not all ARVs § BOC can be given with raltegravir (PK data show significant bidirectional interactions with PIs) § TVR can be given with efavirenz (with dose increase of efavirenz), ritonavir-boosted atazanavir, and raltegravir § If feasible, change to acceptable ART regimen before treatment with BOC or TVR § For patients with CD 4 >500 cells/µL, some experts recommend completion of HCV PI phase of HCV treatment before ART initiation 31 May 2013 www. aidsetc. org

HCV Disease: Monitoring of Response to Therapy § Treatment response is determined by HCV RNA response; monitoring required before, during, and after treatment § Treatment week 4: change in HCV RNA gives early assessment of likelihood of SVR § With Peg. IFN/ribavirin: § If <1 log 10 decrease, <5% probability of SVR § If undetectable HCV RNA (rapid virologic response, RVR), 80% probability of SVR § With Peg. IFN/ribavirin + TVR, stop treatment if HCV RNA >1, 000 IU/m. L § Treatment week 8 § With Peg. IFN/ribavirin + BOC: § If undetectable HCV RNA (RVR), greater likelihood of SVR 32 May 2013 www. aidsetc. org

HCV Disease: Monitoring of Response to Therapy (2) § Treatment week 12: § With Peg. IFN/ribavirin: § Null virologic response: If <2 log 10 reduction from baseline: discontinue Peg. IFN/ribavirin as sole treatment § Partial early virologic response (EVR): ≥ 2 log 10 decrease but detectable § Complete EVR: undetectable HCV RNA: 60 -65% probability of SVR § With Peg. IFN/ribavirin + TVR, stop treatment if HCV RNA >1, 000 IU/m. L § With Peg. IFN/ribavirin + BOC, stop treatment if HCV RNA >100 IU/m. L 33 May 2013 www. aidsetc. org

HCV Disease: Monitoring of Response to Therapy (3) § Treatment week 24: § With Peg. IFN/ribavirin: § For patients with partial EVR, retest at week 24; if undetectable HCV RNA, 20% probability of SVR § For all treatment regimens (including those with BOC or TPV), stop treatment if detectable HCV § End of treatment (week 24 or 48): § End of treatment response: undetectable HCV RNA § 24 weeks posttreatment § SVR: undetectable HCV RNA 34 May 2013 www. aidsetc. org

HCV Disease: Adverse Events Peg. IFN: § Side effects common; severity variable § “Flulike” symptoms (fever, myalgia, headache), fatigue, nausea, alopecia, depression (may be severe), insomnia, cognitive dysfunction, cytopenias including reversible CD 4 decrease, retinopathy, neuropathy, autoimmune disorders, hypo- or hyperthyroidism (monitor TSH), ophthalmic complications § May limit dosage or limit ability to complete therapy 35 May 2013 www. aidsetc. org

HCV Disease: Adverse Events (2) Ribavirin: § Hemolytic anemia, cough, dyspepsia § Anemia may limit dosage § Teratogenic; women with potential for pregnancy and men receiving ribavirin must use contraception consistently during ribavirin therapy and for 6 months after completion of therapy 36 May 2013 www. aidsetc. org

HCV Disease: Adverse Events (3) Boceprevir: § Anemia, neutropenia, dysgeusia 37 May 2013 www. aidsetc. org

HCV Disease: Adverse Events (4) § Telaprevir: § Rash (up to 56% of patients, may be severe), pruritus, anemia, nausea, vomiting, diarrhea, anal discomfort or burning, hyperbilirubinemia (with atazanavir-ritonavir coadministration) § Patients with rash should be followed for progression, or development of systemic symptoms (SJS and DRESS have been reported) § If severe rash or systemic symptoms, discontinue TPV; if no improvement in 7 days, discontinue Peg. IFN and ribavirin § If SJS or DRESS, stop all medications and refer for urgent care 38 May 2013 www. aidsetc. org

HCV Disease: Adverse Events (5) § Monitor with clinical and laboratory exams before treatment and at least monthly (more frequently during the initial 12 weeks of HCV PI therapy) § Pretreatment: § CBC, electrolytes, creatinine, ALT, AST, albumin, total bilirubin, TSH, HIV RNA, CD 4, pregnancy test for women of child-bearing potential § Depression screen § During treatment: § Monthly: clinical visits, CBC, electrolytes, creatinine, ALT, AST, albumin, total bilirubin, pregnancy test, depression screen § Every 3 months: HIV RNA, CD 4, TSH 39 May 2013 www. aidsetc. org

HCV Disease: Adverse Events (6) § If cytopenias: manage with dosage reduction of IFN (neutropenia, thrombocytopenia) and/or ribavirin (anemia) § Growth factors if dosage reduction is not sufficient § If adverse neuropsychiatric effects: antidepressants, other mood stabilizers 40 May 2013 www. aidsetc. org

HCV Disease: Treatment Failure § Limited data in HIV/HCV coinfected patients with treatment failure; with Peg. IFN/ribavirin, SVR rates generally markedly lower than for treatment-naive patients § Retreatment of HCV GT 1 monoinfected patients with HCV PIs + Peg. IFN/ribavirin: SVR depends on previous virologic response § SVR rates higher if previous virologic relapse or partial virologic response, lowest if null response § Retreatment of coinfected patients with HCV PIs: few data § Evaluate carefully for need for retreatment; refer for studies of investigational DAAs when possible 41 May 2013 www. aidsetc. org

HCV Disease: Preventing Recurrence § No protective immunity after infection; reinfection possible if new exposure to HCV (eg, via injection drug use or unprotected sex) § Patients who achieve SVR should be counseled to avoid reinfection § Methods that prevent sexual transmission of HIV should prevent sexual transmission of HCV 42 May 2013 www. aidsetc. org

HCV Disease: Considerations in Pregnancy § All HIV-infected pregnant women should be tested for HCV § Evaluation, including liver biopsy, can be delayed ≥ 3 months after delivery (pregnancy-related changes in HCV activity should resolve) § Hepatitis A and hepatitis B vaccination can be given; should be given if not immune 43 May 2013 www. aidsetc. org

HCV Disease: Considerations in Pregnancy (2) § HCV treatment with Peg. IFN and ribavirin is contraindicated during pregnancy § IFN: has antigrowth and antiproliferative effects; is abortifacient in monkeys § Ribavirin: FDA category X; teratogenic at low dosages in many animal species § Both women and men must be counseled about risks and need for consistent and effective contraception during ribavirin therapy and for 6 months after completion of therapy § BOC, TPV: pregnancy category B, but must be used with IFN and ribavirin, which are contraindicated 44 May 2013 www. aidsetc. org

HCV Disease: Considerations in Pregnancy (3) § Perinatal HCV transmission: higher risk for HIVcoinfected women § Limited data on efficacy of medical or surgical preventive measures § Cesarean delivery does not decrease risk of perinatal HCV transmission, and may increase risk of maternal morbidity in HIV-infected women § Cesarean delivery in HIV/HCV-coinfected women can be considered based on HIV-related indications; data insufficient to support routine use for prevention of HCV transmission 45 May 2013 www. aidsetc. org

Websites to Access the Guidelines § http: //www. aidsetc. org § http: //aidsinfo. nih. gov 46 May 2013 www. aidsetc. org

About This Slide Set § This presentation was prepared by Susa Coffey, MD for the AETC National Resource Center in May 2013 § See the AETC NRC website for the most current version of this presentation: http: //www. aidsetc. org 47 May 2013 www. aidsetc. org
 Retroviruses and opportunistic infections
Retroviruses and opportunistic infections Opportunistic infections
Opportunistic infections Opportunistic infections
Opportunistic infections Primary prevention secondary prevention tertiary prevention
Primary prevention secondary prevention tertiary prevention Opportunistic approach adalah model proses
Opportunistic approach adalah model proses Nation and macalister 2010
Nation and macalister 2010 A bacterial std that usually affects mucous membranes
A bacterial std that usually affects mucous membranes Johnson and johnson botnet infections
Johnson and johnson botnet infections Bone and joint infections
Bone and joint infections Methotrexate and yeast infections
Methotrexate and yeast infections Transtubular potassium gradient
Transtubular potassium gradient Odg guidelines texas
Odg guidelines texas Sbp paracentesis
Sbp paracentesis Management of allergic rhinitis
Management of allergic rhinitis Allergic rhinitis treatment guidelines
Allergic rhinitis treatment guidelines Ich treatment guidelines
Ich treatment guidelines Hepatic encephalopathy staging
Hepatic encephalopathy staging Storch infections
Storch infections Storch infections
Storch infections Infections opportunistes digestives
Infections opportunistes digestives Eye infections
Eye infections Postpartum infections
Postpartum infections Genital infections
Genital infections Amber blumling
Amber blumling Nosocomial infections
Nosocomial infections Classification of acute gingival infections
Classification of acute gingival infections Fspos vägledning för kontinuitetshantering
Fspos vägledning för kontinuitetshantering Typiska drag för en novell
Typiska drag för en novell Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Vad står k.r.å.k.a.n för
Vad står k.r.å.k.a.n för Varför kallas perioden 1918-1939 för mellankrigstiden
Varför kallas perioden 1918-1939 för mellankrigstiden En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Personalliggare bygg undantag
Personalliggare bygg undantag Personlig tidbok
Personlig tidbok Sura för anatom
Sura för anatom Vad är densitet
Vad är densitet Datorkunskap för nybörjare
Datorkunskap för nybörjare Boverket ka
Boverket ka Att skriva en debattartikel
Att skriva en debattartikel Delegerande ledarstil
Delegerande ledarstil Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Formel för lufttryck
Formel för lufttryck Offentlig förvaltning
Offentlig förvaltning Jag har nigit för nymånens skära text
Jag har nigit för nymånens skära text Presentera för publik crossboss
Presentera för publik crossboss Argument för teckenspråk som minoritetsspråk
Argument för teckenspråk som minoritetsspråk Kanaans land
Kanaans land