FDA Regulation of Pharmaceuticals and Devices Jean TothAllen


























































- Slides: 73

FDA Regulation of Pharmaceuticals and Devices Jean Toth-Allen, Ph. D. Good Clinical Practice Program Office of Science and Health Coordination Office of the Commissioner

Overview • FDA regulations versus 45 CFR Part 46 • Pharmaceuticals and devices – What they have in common – How they differ • Bioresearch Monitoring (BIMO) • Resources • Acronyms

FDA versus 45 CFR Part 46 -1 • FDA regulations found in Title 21, Code of Federal Regulations – 21 CFR • Regulate products • Coverage includes – but not limited to: – – – – Nonclinical studies Clinical studies Human Subject Protection Institutional Review Boards (IRBs) Manufacturing Labeling Post-market adverse event reporting

FDA versus 45 CFR Part 46 -2 • FDA regulations “speak” to: – Manufacturers – Importers/exporters – Study sponsors – Nonclinical laboratory personnel – Clinical investigators – IRBs – Medical product users – hospitals, clinics, nursing homes, individual practitioners

FDA versus 45 CFR Part 46 -3 • 45 CFR Part 46 - regulates studies with human subjects that are federally funded – biomedical, sociological, behavioral, educational • Subpart A = Common Rule – general human subject protections • Remaining subparts cover research protections in studies with vulnerable populations (pregnant women, fetuses, neonates, prisoners, children) • “Speaks” to institutions and their IRBs • Crosses government agencies – enforcement led by the Office of Human Research Protections (OHRP)

FDA versus 45 CFR Part 46 -4 • Human subject protections of Common Rule covered by FDA regulations in – 21 CFR Part 50 – Protection of Human Subjects – 21 CFR Part 56 – Institutional Review Boards • 45 CFR 46, Subpart D comparable to 21 CFR 50, Subpart D – research with children • Preambles to FDA regulations identify the need for special protections for other vulnerable populations but FDA regulations do not separately address

FDA versus 45 CFR Part 46 -5 • Both may apply to a given research study • If there are regulatory differences, the more stringent requirements usually apply (e. g. , FDA regulations allow for very few exceptions from informed consent)

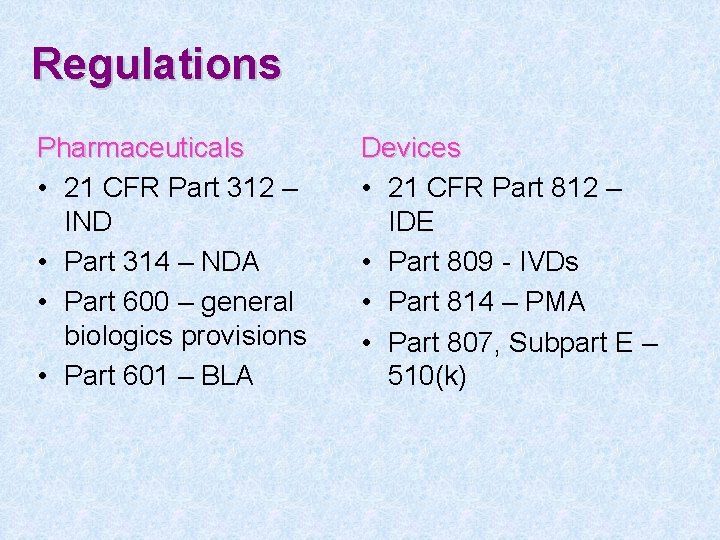
Pharmaceuticals versus devices • Pharmaceuticals (drugs and biologics) are covered by different FDA regulations from those covering devices, though some regulations are shared • Many differences result from differences among the products themselves

SHARED REGULATIONS • Part 50 – Protection of Human Subjects • Part 56 – Institutional Review Boards • Part 54 – Financial Disclosure by Clinical Investigators • Part 58 – Good Laboratory Practices for Nonclinical Laboratory Studies • Part 11 – Electronic Records; Electronic Signatures

COMPLIANCE PROGRAMS • Programs are Agency-wide (available at http: //www. fda. gov/oc/gcp/compliance. html) • Contain instructions to FDA field personnel for inspecting regulated entities • Center-specific differences are included where applicable

DIFFERENCES • Nature of product, firms, and studies • Statutory distinctions • Regulatory distinctions

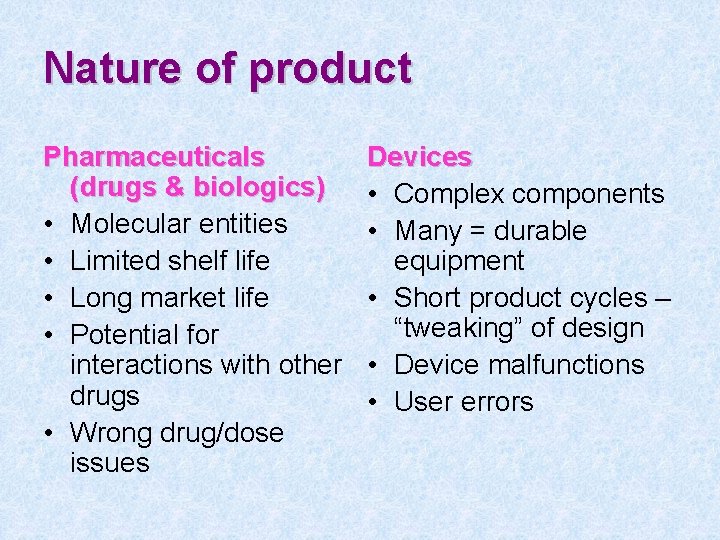
Nature of product Pharmaceuticals (drugs & biologics) • Molecular entities • Limited shelf life • Long market life • Potential for interactions with other drugs • Wrong drug/dose issues Devices • Complex components • Many = durable equipment • Short product cycles – “tweaking” of design • Device malfunctions • User errors

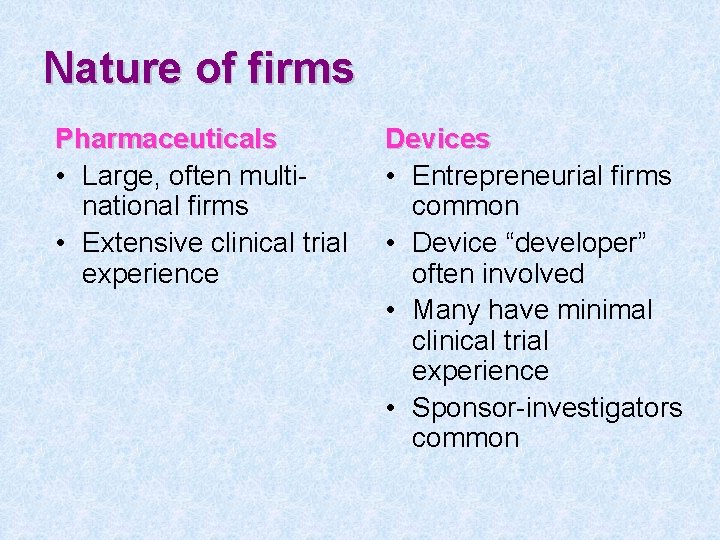
Nature of firms Pharmaceuticals • Large, often multinational firms • Extensive clinical trial experience Devices • Entrepreneurial firms common • Device “developer” often involved • Many have minimal clinical trial experience • Sponsor-investigators common

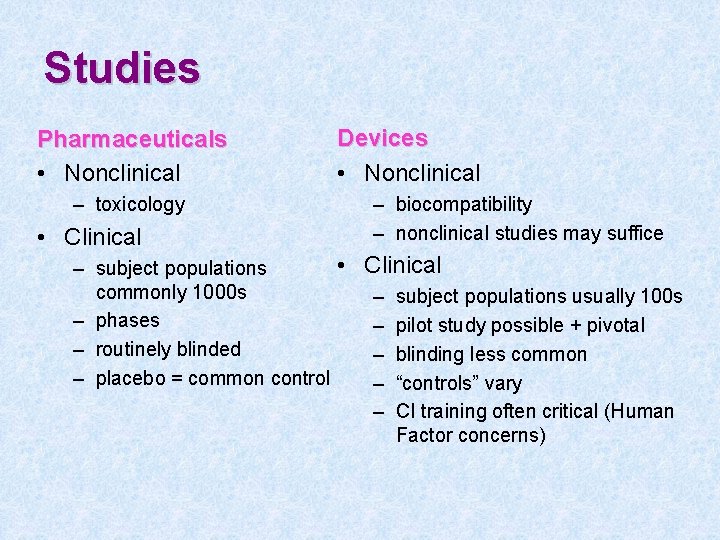
Studies Pharmaceuticals • Nonclinical – toxicology • Clinical Devices • Nonclinical – biocompatibility – nonclinical studies may suffice • Clinical – subject populations commonly 1000 s – subject populations usually 100 s – phases – pilot study possible + pivotal – routinely blinded – blinding less common – placebo = common control – “controls” vary – CI training often critical (Human Factor concerns)

Statutory Distinctions • Devices lack market exclusivity provisions – Waxman-Hatch – pediatric studies and extension of patent (drugs) – Orphan drug tax exemptions (drugs/biologics) • FDAMA (1997) – included a “least burdensome” provision for devices

Regulations Pharmaceuticals • 21 CFR Part 312 – IND • Part 314 – NDA • Part 600 – general biologics provisions • Part 601 – BLA Devices • 21 CFR Part 812 – IDE • Part 809 - IVDs • Part 814 – PMA • Part 807, Subpart E – 510(k)

Clinical Investigators -1 • Common responsibilities across products: – Personally conduct or supervise the study – Ensure site study team is properly trained – Follow FDA regulations regarding HSP, including obtaining and maintaining IRB approval and obtaining subject informed consent – Follow the approved investigational plan/protocol

Clinical Investigators -2 • CI responsibilities (cont. ): – Maintain adequate, complete, and accurate study records – Submit all required reports (e. g. , IND safety reports, study progress reports) – Maintain control of the investigational product

Sponsors -1 • Common responsibilities across products: – Obtain FDA approval, where necessary, before study initiation – Manufacture and label investigational products appropriately – Initiate, withhold, or discontinue clinical trials as required – Refrain from commercialization of investigational products – Maintain control of the investigational product

Sponsors -2 • Sponsor responsibilities (cont): – Select qualified investigators and disseminate appropriate information to them – Select qualified monitors and ensure the study is adequately monitored – Evaluate and report adverse experiences – Maintain adequate records – Submit progress and final reports

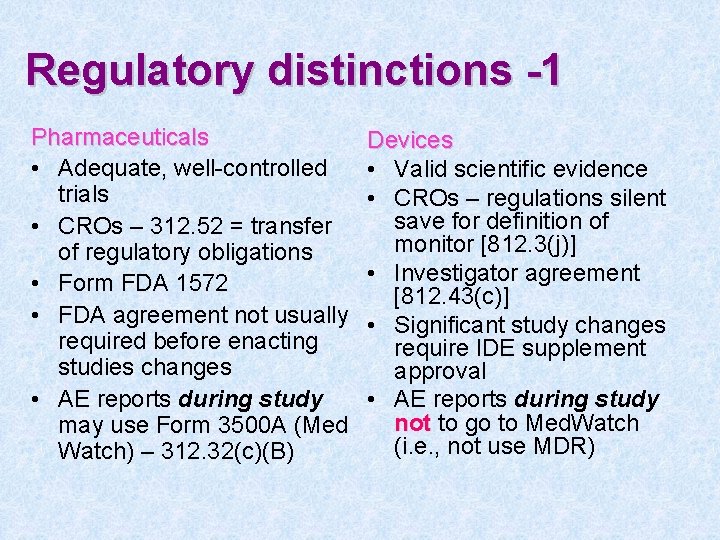
Regulatory distinctions -1 Pharmaceuticals • Adequate, well-controlled trials • CROs – 312. 52 = transfer of regulatory obligations • Form FDA 1572 • FDA agreement not usually required before enacting studies changes • AE reports during study may use Form 3500 A (Med Watch) – 312. 32(c)(B) Devices • Valid scientific evidence • CROs – regulations silent save for definition of monitor [812. 3(j)] • Investigator agreement [812. 43(c)] • Significant study changes require IDE supplement approval • AE reports during study not to go to Med. Watch (i. e. , not use MDR)

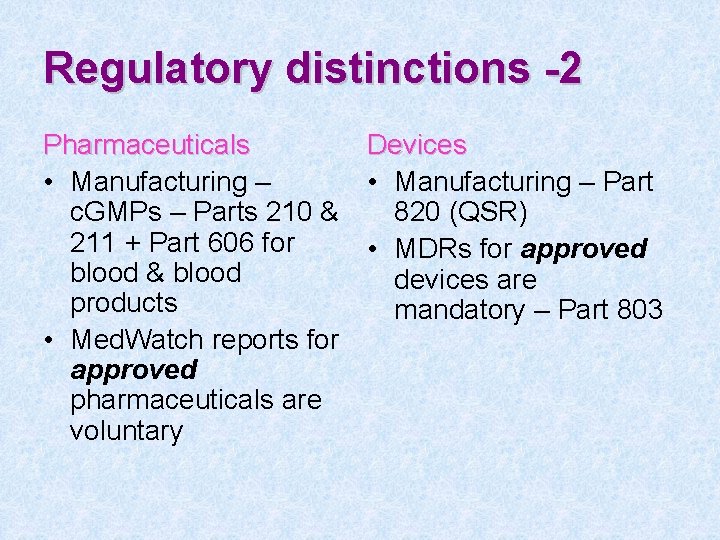
Regulatory distinctions -2 Pharmaceuticals Devices • Manufacturing – Part c. GMPs – Parts 210 & 820 (QSR) 211 + Part 606 for • MDRs for approved blood & blood devices are products mandatory – Part 803 • Med. Watch reports for approved pharmaceuticals are voluntary

Additional Device Distinctions -1 • Classes of Devices – risk-based determination – 21 CFR 860 – classification procedures – 21 CFR 862 through 892 – specific device classifications by product type

Additional Device Distinctions -2 • Cleared devices – 510(k) – 21 CFR 807, subpart E – Premarket Notification Procedures – “substantially equivalent” • Approved devices – 21 CFR Part 814 – PMA, PDP, HDE – Safety and effectiveness – PMA & PDP – Safety – HDE

Additional Device Distinctions -3 • Significant risk/non-significant risk studies • Exempt studies/in vitro diagnostics (IVDs) • Protocol changes and 5 -day notices

Significant Risk (SR) • Regulatory definition (21 CFR 812. 3(m)) – device that presents potential for serious risk to health, safety, or welfare of a subject, particularly if it • Is intended as an implant • Is purported or represented for use in supporting or sustaining life • Is for a use of substantial importance in diagnosing, curing, mitigating, or treating disease, or otherwise preventing impairment of human health

Non-Significant Risk (NSR) • • Decision based on use of device in study Sponsor makes initial assessment IRB makes determination FDA can disagree If NSR study, no IDE application to FDA Informed consent required Abbreviated requirements apply (21 CFR 812. 2(b)) • Considered to have an IDE

Exempt device studies • 21 CFR 812. 2 (c) • Studies with cleared devices, used as specified in clearance • By policy, extended to approved devices, with same conditions • Diagnostic devices that meet requirements specified – basically IVDs, as references labeling conditions of 809. 10

In Vitro Diagnostics (IVDs) • SR/NSR/exempt studies • Exempt if: • labeled according to 21 CFR 809. 10 • noninvasive sampling or no significant risk • does not introduce energy into a subject • not used as the diagnostic for determination of treatment

Significant Risk IVD Studies • If study involves invasive sampling that presents a significant risk • If results from use of an investigational IVD will determine treatment, could inaccurate results: - be life-threatening - result in permanent functional impairment - result in permanent structural damage - necessitate medical or surgical intervention to prevent impairment or damage

IVD Studies & HSP Issues • Studies on specimens – included in device definition of a subject (812. 3(p)) • Expedited review by IRB possible • Confusion with 45 CFR Part 46 • Privacy & confidentiality • FDA data audits

Additional IVD issues -1 • Drug-diagnostic co-development concept paper – April 2005 – http: //www. fda. gov/cder/genomics/ pharmacoconceptfn. pdf • Guidance on use of left-over specimens that are not individually identifiable – April 2006 – http: //www. fda. gov/cdrh/oivd/guidance/ 1588. html

Additional IVD issues -2 • Interim final rule regarding exception from informed consent (bioterrorism, emerging diseases) – September 2006 http: //www. fda. gov/OHRMS/DOCKETS /98 fr/E 6 -8790. pdf • Draft guidance on Analyte Specific Reagents (ASRs) – September 2006 http: //www. fda. gov/cdrh/oivd/guidance/ 1590. pdf

Additional IVD issues -3 • Draft guidance on Multivariate Index Assays (MIA) – September 2006 http: //www. fda. gov/cdrh/oivd/guidance/1610. html – Public meeting held February 8, 2007

WEB PAGE Office of In Vitro Diagnostic Device Evaluation and Safety (OIVD) www. fda. gov/cdrh/oivd/

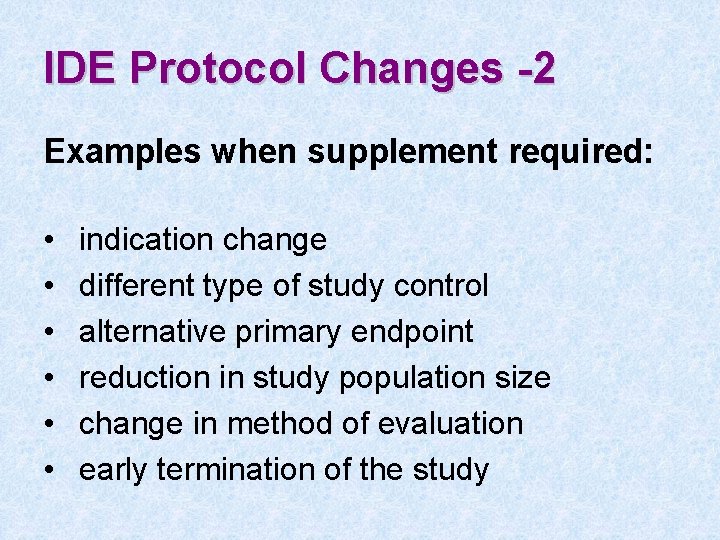
IDE Protocol Changes -1 • IDE Supplement required if changes significantly affect: • validity of data • scientific soundness of study • rights, safety, or welfare of subjects

IDE Protocol Changes -2 Examples when supplement required: • • • indication change different type of study control alternative primary endpoint reduction in study population size change in method of evaluation early termination of the study

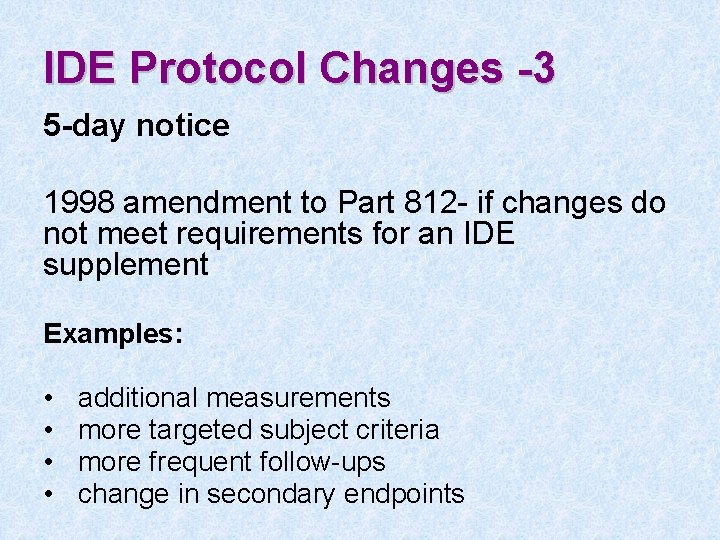
IDE Protocol Changes -3 5 -day notice 1998 amendment to Part 812 - if changes do not meet requirements for an IDE supplement Examples: • • additional measurements more targeted subject criteria more frequent follow-ups change in secondary endpoints

IDE Protocol Changes -4 GUIDANCE DOCUMENT – issued by Office of Device Evaluation (ODE) Changes or Modifications During the Conduct of a Clinical Investigation issued May 29, 2001 www. fda. gov/cdrh/ode/guidance/1337. html www. fda. gov/cdrh/ode/guidance/1337. pdf

BIMO Program Comprehensive program of on-site inspections and data audits designed to monitor all aspects of the conduct and reporting of FDA-regulated research

BIMO Program Objectives • To ensure – the integrity of data supporting submissions to the Agency – the rights, safety, and welfare of study subjects

Bioresearch Monitoring (BIMO) -1 • Specific group in each Center to oversee BIMO program – Bioresearch Monitoring Branch/Division of Inspections and Surveillance/Office of Compliance – CBER – Division of Scientific Investigations (DSI)/Office of Compliance - CDER – Division of Bioresearch Monitoring (DBM)/Office of Compliance – CDRH

Bioresearch Monitoring (BIMO) -2 Present BIMO contacts • CBER – Pat Holobaugh – Branch Phone # - (301) 827 -6220 • CDER – Gary Della’Zanna – Division Phone # - (240) 276 -8817 • CDRH – Michael Marcarelli – Division Phone # - (240) 276 -0125

Bioresearch Monitoring (BIMO) -3 Headquarters BIMO staff: • Interact with Center reviewers • Issue inspection assignments • Interact with ORA BIMO investigators • Review and classify EIRs • Issue post-inspectional correspondence • Take part in regulatory actions (AIP, DQ) • Provide staff for ORA BIMO investigator training • Provide speakers for outreach activities

Inspection assignments • Assigned by HQ BIMO staff • Majority issued on receipt of an application or submission – When for marketing, supporting study usually completed • “for cause” – usually when suspicion of integrity or human subject protection (HSP) issue – often for on-going studies

BIMO Compliance Programs • Good Laboratory Practice – CP 7348. 808 • Institutional Review Board – CP 7348. 809 • Sponsor, Contract Research Organizations (CROs), Monitors – CP 7348. 810 • Clinical Investigator – CP 7348. 811 • In Vivo Bioequivalence – CP 7348. 001 http: //www. fda. gov/oc/gcp/compliance. html

Inspectional follow-up • Final inspection classification made by HQ • Post-inspectional correspondence issued to inspected party • Administration/regulatory options vary by party inspected • Recommendations may also be sent to those reviewing a research or marketing application/submission

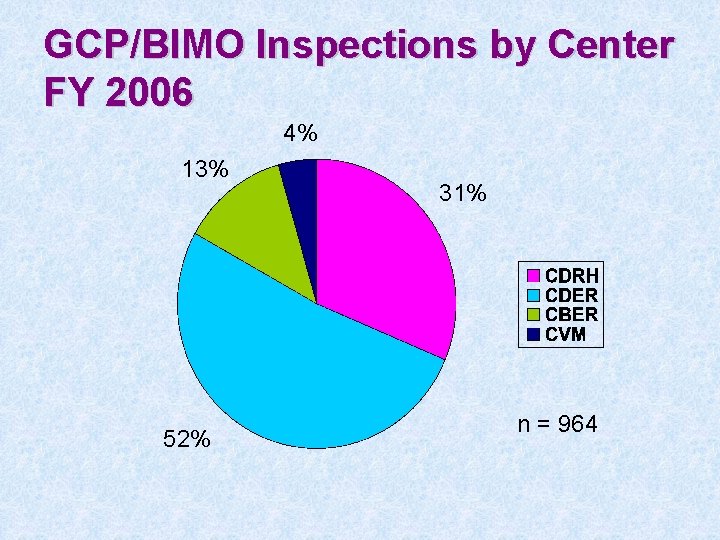
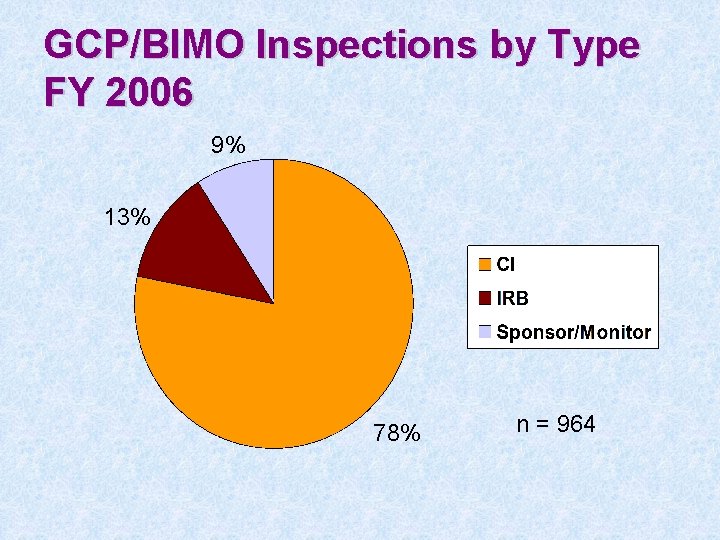
GCP/BIMO Inspections Completed FY 2006 Center CI CBER 108 8 5 121 CDER 401 66 32 499 CDRH 203 48 51 302 0 0 CVM 41 n/a 1 42 All Centers 753 122 89 964 CFSAN IRB Spon/Mon Total

GCP/BIMO Inspections by Center FY 2006 4% 13% 52% 31% n = 964

GCP/BIMO Inspections by Type FY 2006 9% 13% 78% n = 964

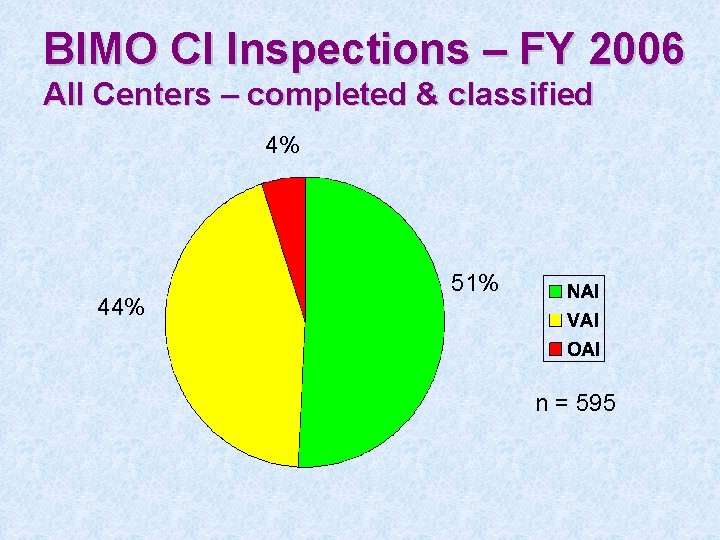
Clinical Investigators • Compliance inspection program covers study specific inspections and audits of CIs (physicians, veterinarians, others) conducting clinical trials on human and veterinary products • Usually preannounced • Inspection includes an interview with the clinical investigator and pertinent study staff + an in-depth study/data audit – to validate study findings and verify compliance with regulations

BIMO CI Inspections – FY 2006 All Centers – completed & classified 4% 44% 51% n = 595

Most Common CI Deficiencies • • Failure to follow the investigational plan Protocol deviations Inadequate recordkeeping Inadequate accountability for the investigational product • Inadequate subject protection – including informed consent issues

Administrative/regulatory options • Untitled or Warning letter • Initiation of disqualification procedures • Sharing information with Office of Criminal Investigations (OCI) for pursuit of prosecution • Recommendation for rejection of site/study data

Institutional Review Boards (IRBs) • Board, committee, or other group formally designated by an institution to – review – approve the initiation of – conduct periodic review of research involving human subjects • Primary purpose of review = ensure protection of rights, safety, and welfare of the human subjects

Applicable regulations • 21 CFR Part 50 – Protection of Human Subjects – contains informed consent requirements • 21 CFR Part 56 – Institutional Review Boards – includes specifics of IRB’s makeup and duties

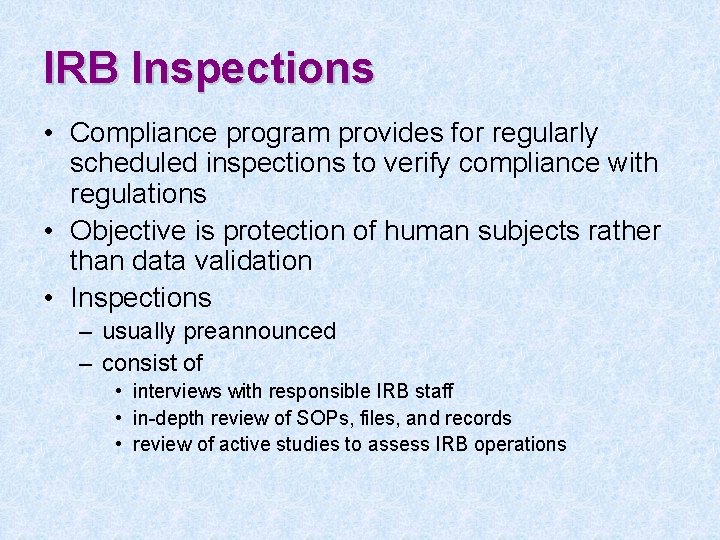
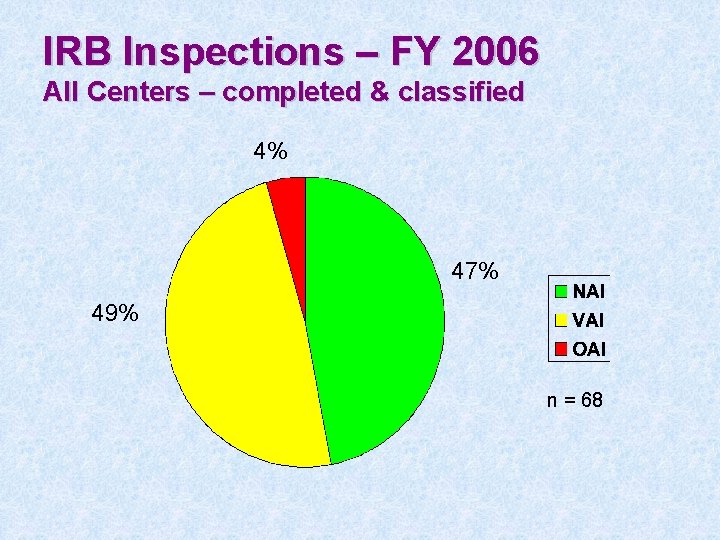
IRB Inspections • Compliance program provides for regularly scheduled inspections to verify compliance with regulations • Objective is protection of human subjects rather than data validation • Inspections – usually preannounced – consist of • interviews with responsible IRB staff • in-depth review of SOPs, files, and records • review of active studies to assess IRB operations

IRB Inspections – FY 2006 All Centers – completed & classified 4% 47% 49% n = 68

Most common IRB deficiencies • • Inadequate initial and/or continuing review Inadequate SOPs Inadequate membership rosters Inadequate meeting minutes Specific to devices – lack of or incorrect SR/NSR determination

Administrative/regulatory options • Untitled or Warning letter • Restriction of functions – prohibiting increase of subject population in on -going FDA-regulated studies – prohibiting review of new FDA-regulated studies • Initiation of disqualification procedures

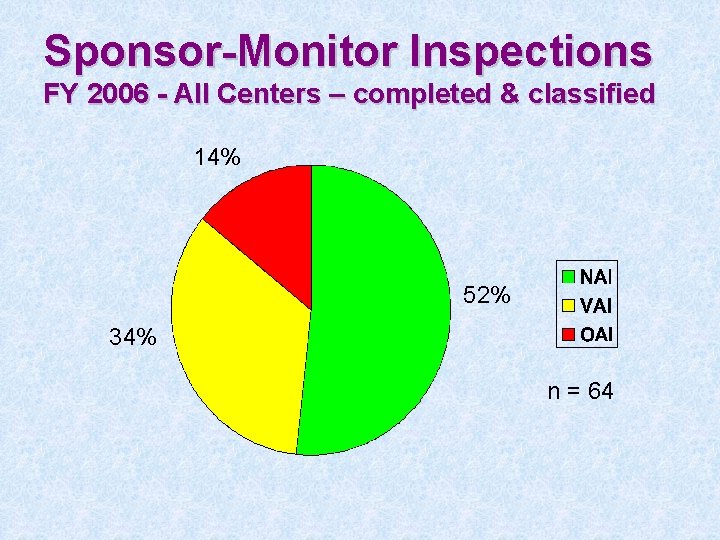
Sponsors/CROs/Monitors • Compliance program – covers parties responsible for initiating and overseeing research and for submitting research results to FDA – lists sponsor responsibilities • Inspections – usually preannounced – consist of interviews and audits of study records – objective is to both evaluate compliance with regulations and validate data – commonly assigned for NDAs for new molecular entities (NMEs) and for PMAs

Sponsor-Monitor Inspections FY 2006 - All Centers – completed & classified 14% 52% 34% n = 64

Most common S/M deficiencies • Inadequate monitoring • Failure to bring investigators into compliance • Inadequate accountability for the investigational product

Administrative/regulatory options • Untitled or Warning letter • Invocation of the Application Integrity Policy (AIP) • Refusal to accept site or study data • Denial of NDA/BLA/PMA • Sharing information with Office of Criminal Investigations (OCI) for pursuit of prosecution

Bioequivalence (BEQ) studies • Primarily support – Abbreviated drug applications (ANDA) for generic drugs – Applications for new form or formulation of marketed drugs • Compliance program – Provides for inspection of both clinical facilities and analytical laboratories involved with BEQ studies – Focuses on inspecting • • • New facilities Previously violative sites Suspicious data Non-conventional studies Studies pivotal to NDA decision-making

BEQ inspections • Conducted by an inspection team, including a laboratory chemist and an ORA field investigator • May involve multiple facilities • Include – physical inspection and technical evaluation of laboratory facilities and methods – audits of analytical and clinical data

Resources - 1 • GCP website – http: //www. fda. gov/oc/gcp/ – Links include • pertinent regulations and guidance • FDA contacts • related sites with HSP/GCP information • Recent documents of interest relate to – – – Data monitoring committees Use of a centralized IRB AE reporting CI supervisory responsibilities Computerized systems in clinical trials

Resources - 2 • GCP queries e-mail account (about 1, 200 queries answered per year) – gcp. questions@fda. hhs. gov • Previous answers captured – http: //www. fda. gov/oc/gcp/redacted. Emails/default. htm • Listserve – via GCP website – notice of updates on FDA’s GCP/HSP activities • Site maintained by Good Clinical Practice Program (GCPP)

Acronyms -1 • • 510(k) – premarket notification AE – adverse event (or effect) AIP – Application Integrity Policy BEQ – bioequivalence BIMO – Bioresearch Monitoring BLA – biologics license application CBER – Center for Biologics Evaluation and Research • CDER – Center for Drug Evaluation and Research

Acronyms -2 • CDRH – Center for Devices and Radiological Health • CFR – Code of Federal Regulations • CI – clinical investigator • c. GMPs – current good manufacturing practices • CRO – contract research organization • DBM – Division of Bioresearch Monitoring • DSI – Division of Scientific Investigations • DQ – disqualification

Acronyms -3 • EIR – establishment inspection report • FDAMA – Food and Drug Administration Modernization Act (1997) • GCP – Good Clinical Practice • GCPP – Good Clinical Practice Program • HDE – humanitarian device exemption • HSP – human subject protection • HQ – headquarters • IDE – investigational device exemption • IND – investigational new drug

Acronyms -4 • • • IRB – institutional review board IVD – in vitro diagnostic MDR – medical device report NAI – no action indicated NDA – new drug application NME – new molecular entity NSR – non-significant risk OAI – official action indicated OHRP – Office of Human Research Protections

Acronyms -5 • OIVD – Office of In Vitro Diagnostic Device Evaluation and Safety • ORA – Office of Regulatory Affairs • PDP – product development protocol • PMA – premarket approval • QSR – quality system regulation • SOPs – standard operating procedures • SR – significant risk • VAI – voluntary action indicated
 Jean omer marie gabriel monnet
Jean omer marie gabriel monnet Jean monnet jean-gabriel monnet
Jean monnet jean-gabriel monnet Triumph pharmaceuticals
Triumph pharmaceuticals What is contoso
What is contoso Contoso pharmaceuticals
Contoso pharmaceuticals Contoso pharmaceuticals
Contoso pharmaceuticals Contoso pharmaceuticals
Contoso pharmaceuticals Contoso pharmaceuticals powerpoint
Contoso pharmaceuticals powerpoint Mirjam nilsson contoso
Mirjam nilsson contoso Ambalal sarabhai
Ambalal sarabhai Vddi cardio
Vddi cardio Samy shanmugam
Samy shanmugam Newbury pharmaceuticals keskustelu
Newbury pharmaceuticals keskustelu Contoso pharmaceuticals
Contoso pharmaceuticals Isis
Isis Lavet pharmaceuticals
Lavet pharmaceuticals Vertex pharmaceuticals (canada) incorporated
Vertex pharmaceuticals (canada) incorporated Phony pharmaceuticals speech
Phony pharmaceuticals speech Levolta pharmaceuticals
Levolta pharmaceuticals Tamper resistant packaging examples
Tamper resistant packaging examples Report pharma fraud
Report pharma fraud Contoso pharmaceuticals
Contoso pharmaceuticals Vddi pharmaceuticals
Vddi pharmaceuticals Devices in literature
Devices in literature Computer system input
Computer system input Fda v brown and williamson
Fda v brown and williamson Changfu wu fda
Changfu wu fda Nicole gillette fda
Nicole gillette fda Leonard sacks fda
Leonard sacks fda Real world evidence
Real world evidence Dq design qualification
Dq design qualification Eclampismo
Eclampismo Klasyfikacja fda
Klasyfikacja fda Institut za kardiovaskularne bolesti sremska kamenica
Institut za kardiovaskularne bolesti sremska kamenica John marler fda
John marler fda Clasificacion fda teratogenia
Clasificacion fda teratogenia Fda cgmp training
Fda cgmp training Leonard sacks fda
Leonard sacks fda Nicole ibrahim fda
Nicole ibrahim fda Fda
Fda Fda chemist
Fda chemist Viveca livezey md
Viveca livezey md Dr todd simpson
Dr todd simpson Fda ora org chart
Fda ora org chart Fda cfsan organizational chart
Fda cfsan organizational chart Fda reviewer jobs
Fda reviewer jobs Fda registration number example
Fda registration number example Fda qsit
Fda qsit Good documentation practices error codes
Good documentation practices error codes Nsde file
Nsde file Iso tr 24971
Iso tr 24971 Estimand fda
Estimand fda Fda authorizes first to
Fda authorizes first to 513g fda
513g fda E copy example
E copy example Clinical investigator training program
Clinical investigator training program Garnett wood fda
Garnett wood fda Fda open data
Fda open data Fda dmc guidance
Fda dmc guidance Fda ssed database
Fda ssed database Ashley boam fda
Ashley boam fda Oopd database
Oopd database Blincyto fda
Blincyto fda Melissa torres fda
Melissa torres fda Neumedix
Neumedix Thalia mills fda
Thalia mills fda Fda caers
Fda caers Fda approval means nothing
Fda approval means nothing Fda approval means nothing
Fda approval means nothing Hec-fda
Hec-fda Bimo audit checklist
Bimo audit checklist Irbmakeup
Irbmakeup Fda disqualification list
Fda disqualification list Fda bimo inspection checklist
Fda bimo inspection checklist