Excitedstate dynamics in the S 1 state of


![Higher bands of S 1←S 0 transition [P lines] Higher bands of S 1←S 0 transition [P lines]](https://slidetodoc.com/presentation_image_h2/b8aa64ce99e2974248bbe1704ed75883/image-26.jpg)
- Slides: 27

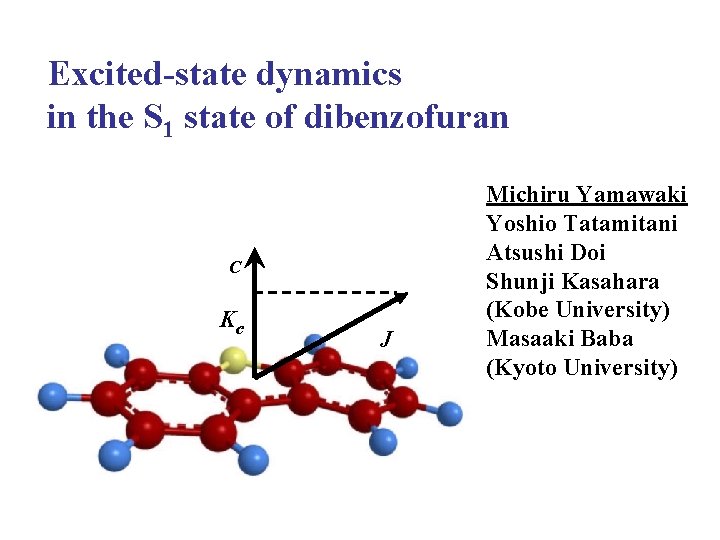
Excited-state dynamics in the S 1 state of dibenzofuran C Kc J Michiru Yamawaki Yoshio Tatamitani Atsushi Doi Shunji Kasahara (Kobe University) Masaaki Baba (Kyoto University)

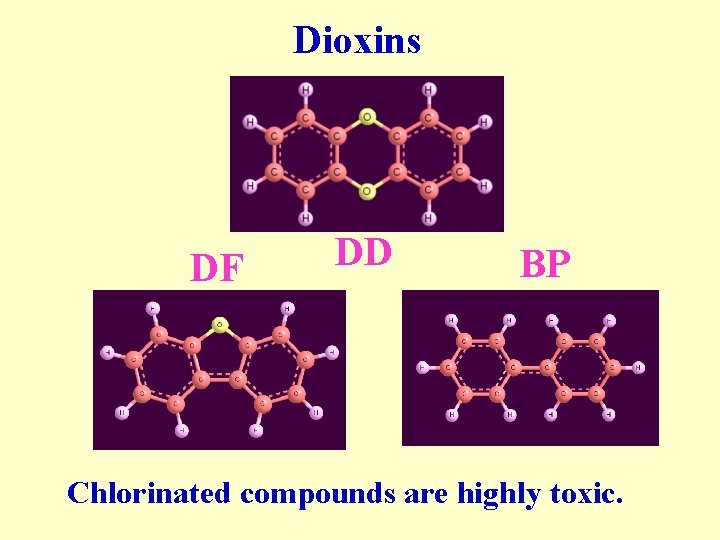
Dioxins DF DD BP Chlorinated compounds are highly toxic.

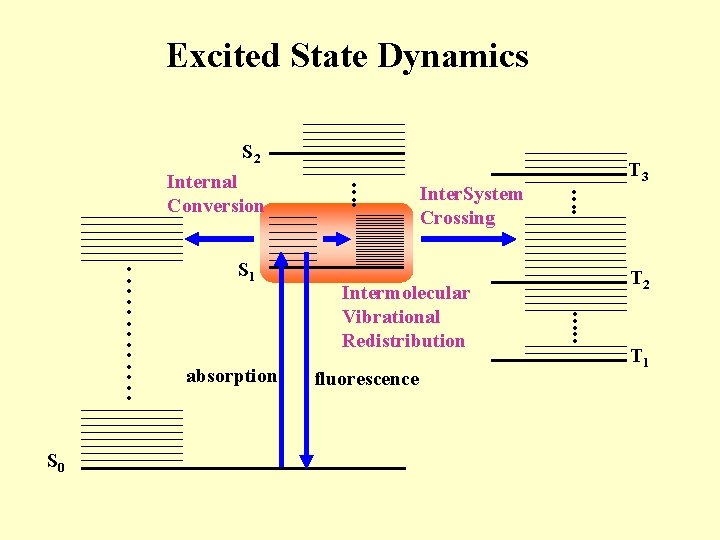
Excited State Dynamics S 2 Internal Conversion S 0 S 1 absorption Inter. System Crossing Intermolecular Vibrational Redistribution fluorescence T 3 T 2 T 1

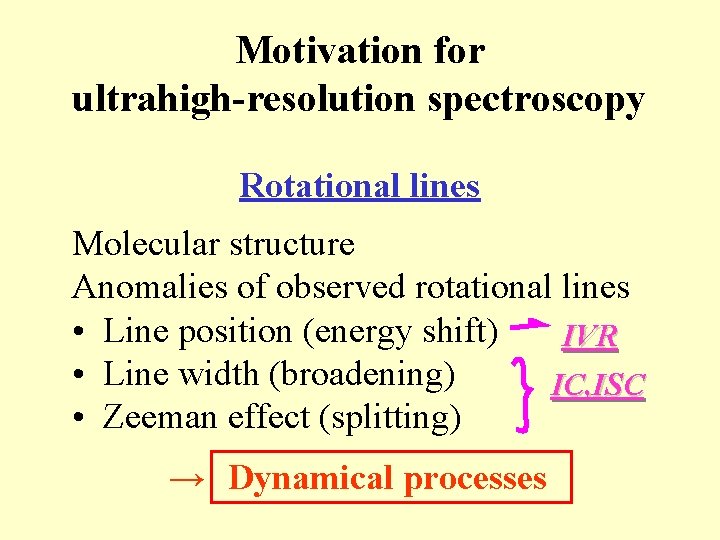
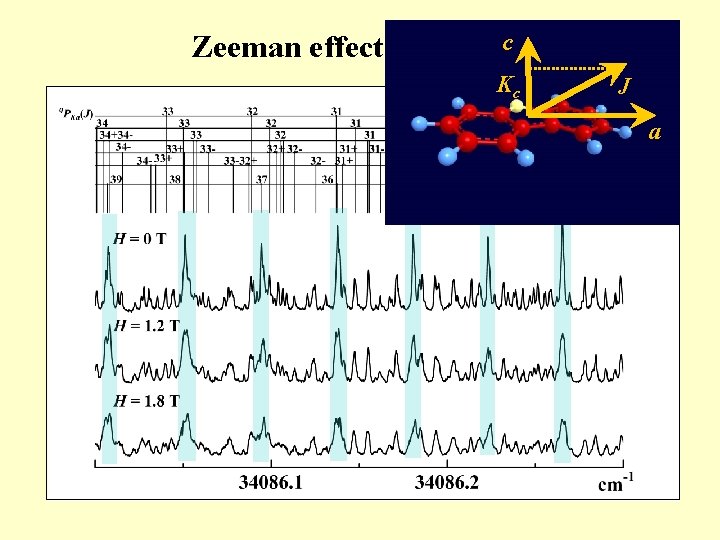
Motivation for ultrahigh-resolution spectroscopy Rotational lines Molecular structure Anomalies of observed rotational lines • Line position (energy shift) IVR • Line width (broadening) IC, ISC • Zeeman effect (splitting) → Dynamical processes

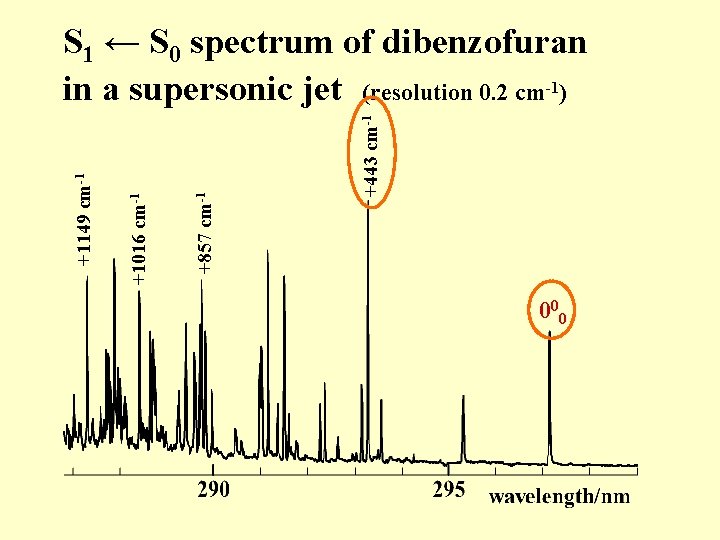
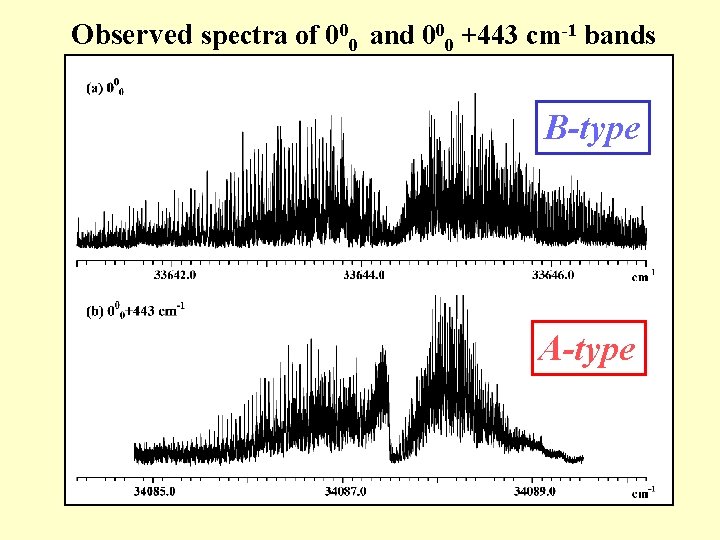
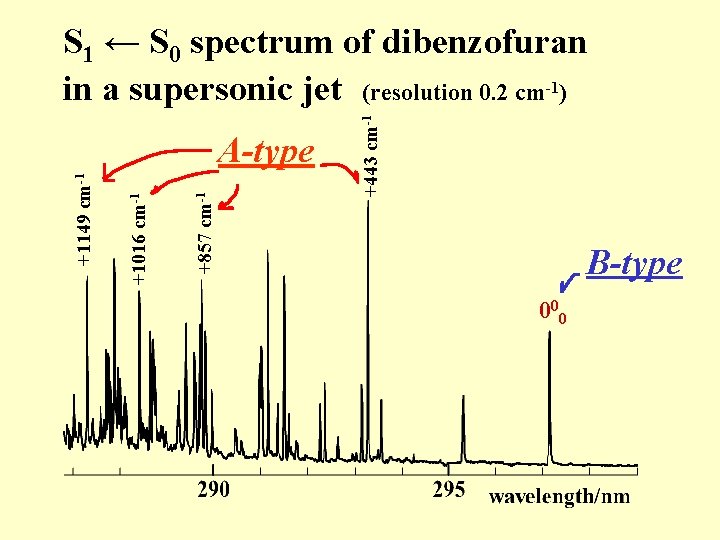
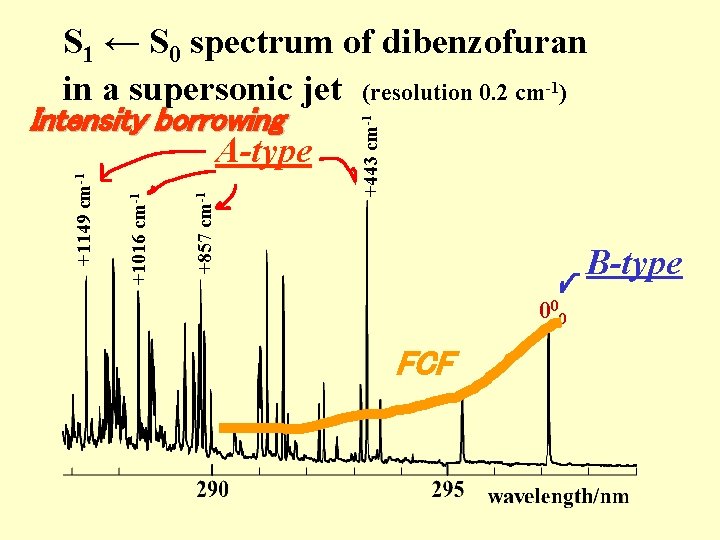
+443 cm-1 +857 cm-1 +1016 cm-1 +1149 cm-1 S 1 ← S 0 spectrum of dibenzofuran in a supersonic jet (resolution 0. 2 cm-1) 000

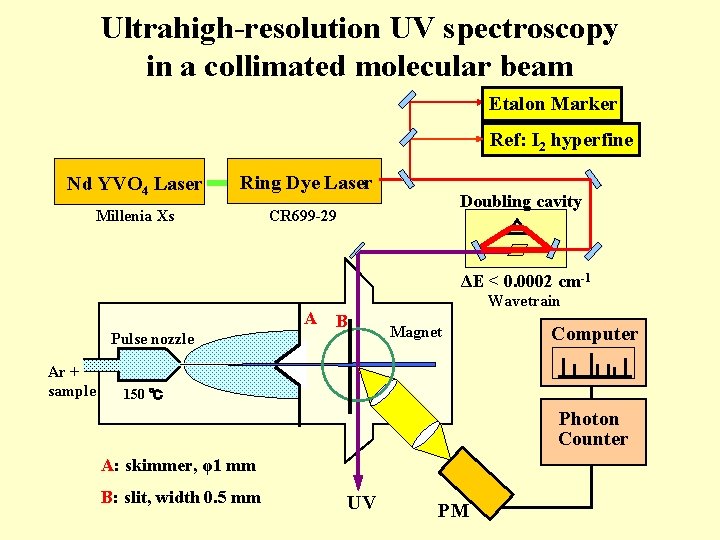
Ultrahigh-resolution UV spectroscopy in a collimated molecular beam Etalon Marker Ref: I 2 hyperfine Nd YVO 4 Laser Ring Dye Laser Millenia Xs CR 699 -29 Doubling cavity ΔE < 0. 0002 cm-1 A Pulse nozzle Ar + sample Wavetrain B Magnet Computer 150 ℃ Photon Counter A: skimmer, φ1 mm B: slit, width 0. 5 mm UV PM

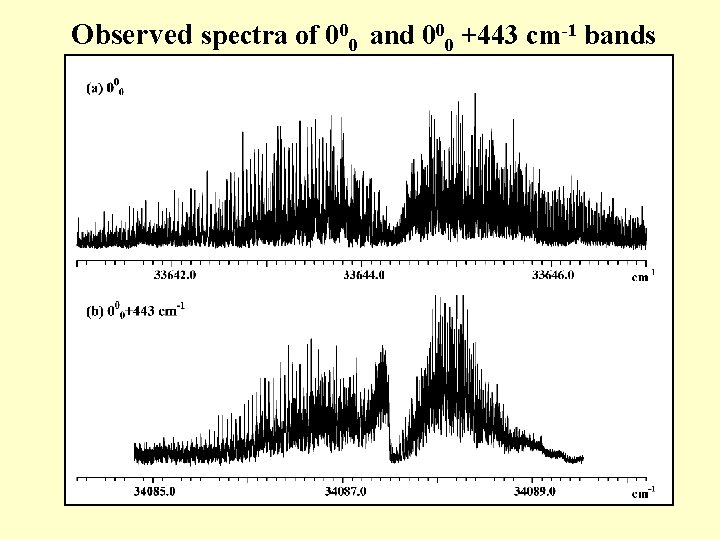
Observed spectra of 000 and 000 +443 cm-1 bands

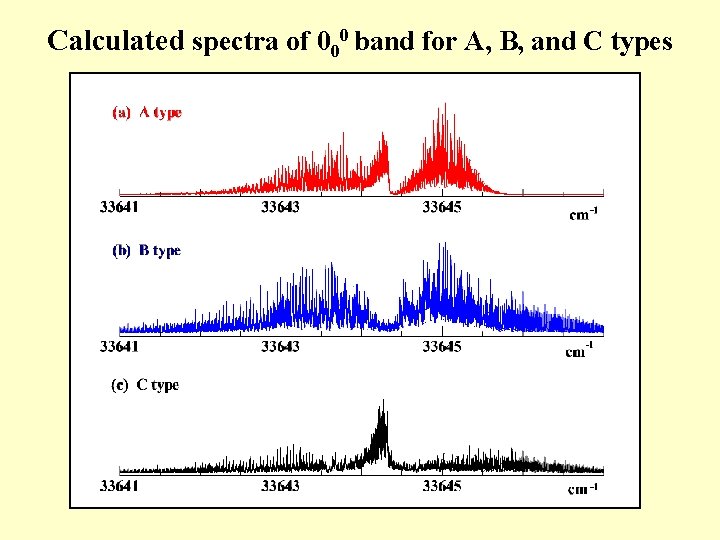
Calculated spectra of 000 band for A, B, and C types

Observed spectra of 000 and 000 +443 cm-1 bands B-type A-type

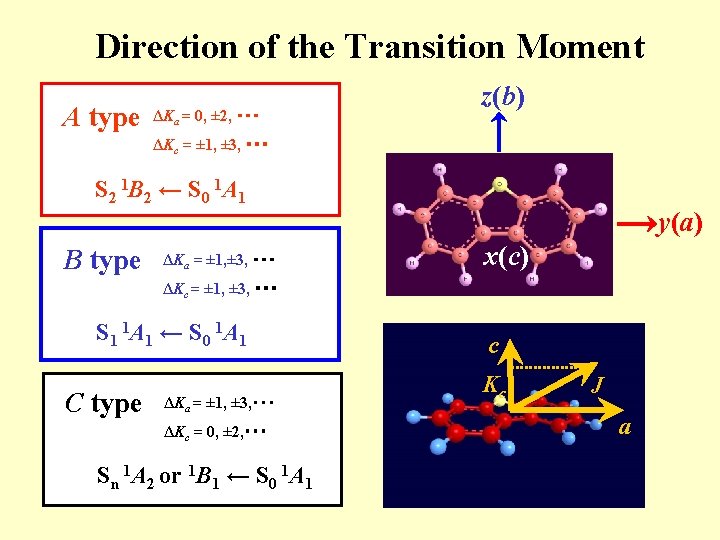
Direction of the Transition Moment A type ΔKa = 0, ± 2, ・・・ z(b) ΔKc = ± 1, ± 3, ・・・ S 2 1 B 2 ← S 0 1 A 1 B type ΔKa = ± 1, ± 3, ・・・ y(a) x(c) ΔKc = ± 1, ± 3, ・・・ S 1 1 A 1 ← S 0 1 A 1 C type ΔKa = ± 1, ± 3, ・・・ ΔKc = 0, ± 2, ・・・ Sn 1 A 2 or 1 B 1 ← S 0 1 A 1 c Kc J a

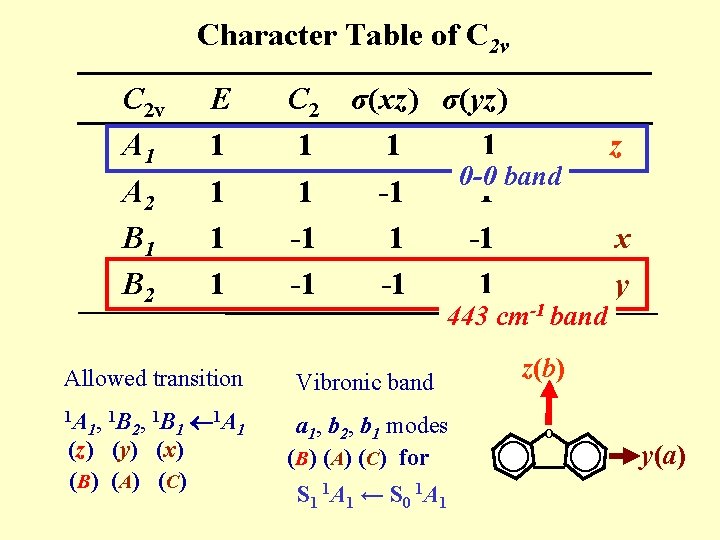
Character Table of C 2 v A 1 A 2 B 1 B 2 E 1 1 Allowed transition 1 A 1, 1 B 2, 1 B 1 (z) (y) (x) (B ) ( A ) ( C ) 1 A 1 C 2 σ(xz) σ(yz) 1 1 1 0 -0 band 1 -1 -1 -1 1 443 cm-1 band Vibronic band a 1, b 2, b 1 modes (B) (A) (C) for S 1 1 A 1 ← S 0 1 A 1 z x y z(b) O y(a)

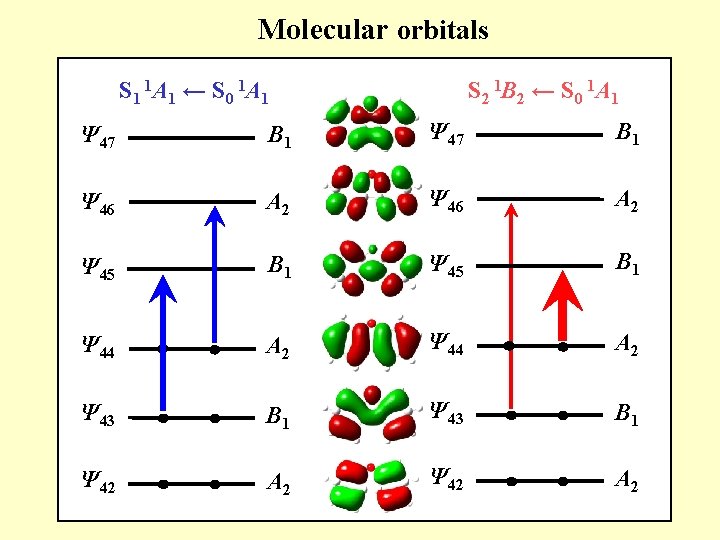
Molecular orbitals S 1 1 A 1 ← S 0 1 A 1 S 2 1 B 2 ← S 0 1 A 1 Ψ 47 B 1 Ψ 46 A 2 Ψ 45 B 1 Ψ 44 A 2 Ψ 43 B 1 Ψ 42 A 2

+857 cm-1 +1016 cm-1 +1149 cm-1 A-type +443 cm-1 S 1 ← S 0 spectrum of dibenzofuran in a supersonic jet (resolution 0. 2 cm-1) B-type 000

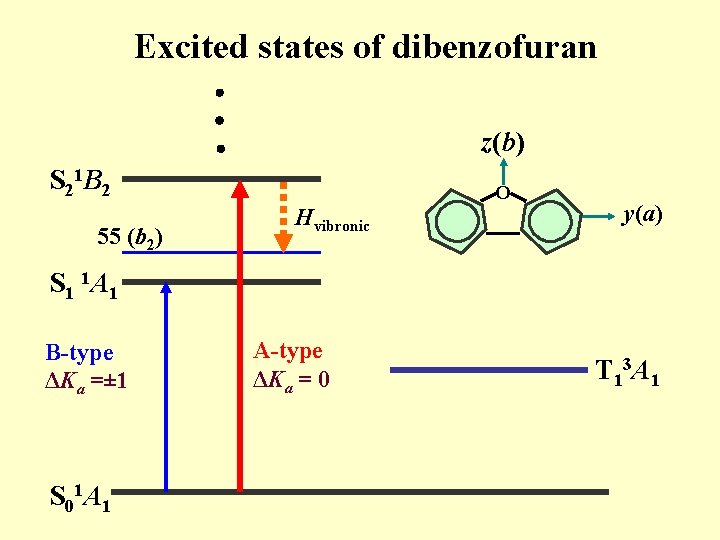
Excited states of dibenzofuran z(b) S 21 B 2 55 (b 2) Hvibronic O y(a) S 1 1 A 1 B-type ΔKa =± 1 S 01 A 1 A-type ΔKa = 0 T 13 A 1

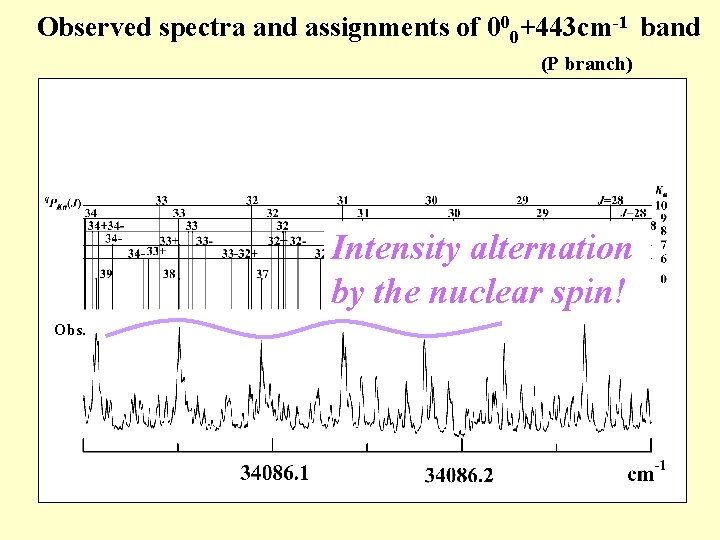
Observed spectra and assignments of 000+443 cm-1 band (P branch) Calc. Intensity alternation by the nuclear spin! Obs.

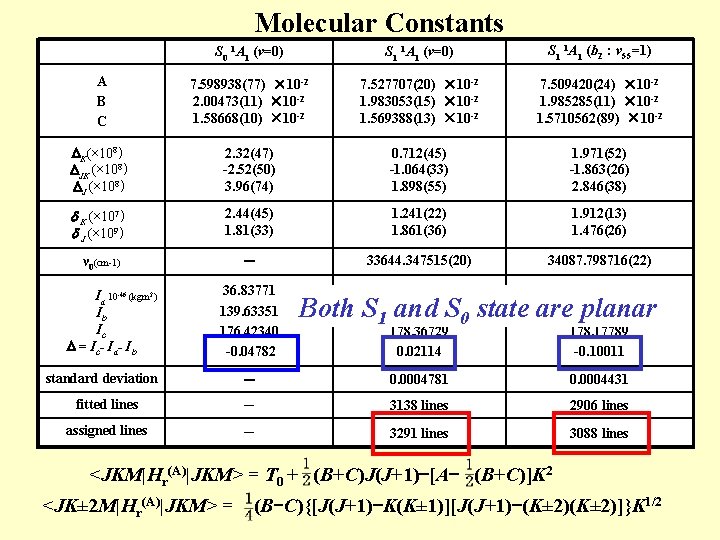
Molecular Constants S 0 1 A 1 (v=0) S 1 1 A 1 (b 2:v 55=1) A B C 7. 598938(77) × 10 -2 2. 00473(11) × 10 -2 1. 58668(10) × 10 -2 7. 527707(20) × 10 -2 1. 983053(15) × 10 -2 1. 569388(13) × 10 -2 7. 509420(24) × 10 -2 1. 985285(11) × 10 -2 1. 5710562(89) × 10 -2 K(× 108) JK (× 108) J (× 108) 2. 32(47) -2. 52(50) 3. 96(74) 0. 712(45) -1. 064(33) 1. 898(55) 1. 971(52) -1. 863(26) 2. 846(38) K (× 107) J (× 109) 2. 44(45) 1. 81(33) 1. 241(22) 1. 861(36) 1. 912(13) 1. 476(26) ν 0(cm-1) - 33644. 347515(20) 34087. 798716(22) 37. 18629 1 141. 15986 178. 36729 0. 02114 37. 27684 141. 00116 178. 17789 -0. 10011 - 0. 0004781 0. 0004431 fitted lines - 3138 lines 2906 lines assigned lines - 3291 lines 3088 lines Ia 10 -46 (kgm 2) Ib Ic = Ic - Ia - Ib 36. 83771 139. 63351 176. 42340 -0. 04782 standard deviation Both S and S 0 state are planar <JKM|Hr(A)|JKM> = T 0 + (B+C)J(J+1)-[A- (B+C)]K 2 <JK± 2 M|Hr(A)|JKM> = (B-C){[J(J+1)-K(K± 1)][J(J+1)-(K± 2)]}K 1/2

Intensity borrowing +857 cm-1 +1016 cm-1 +1149 cm-1 A-type +443 cm-1 S 1 ← S 0 spectrum of dibenzofuran in a supersonic jet (resolution 0. 2 cm-1) B-type 000 FCF

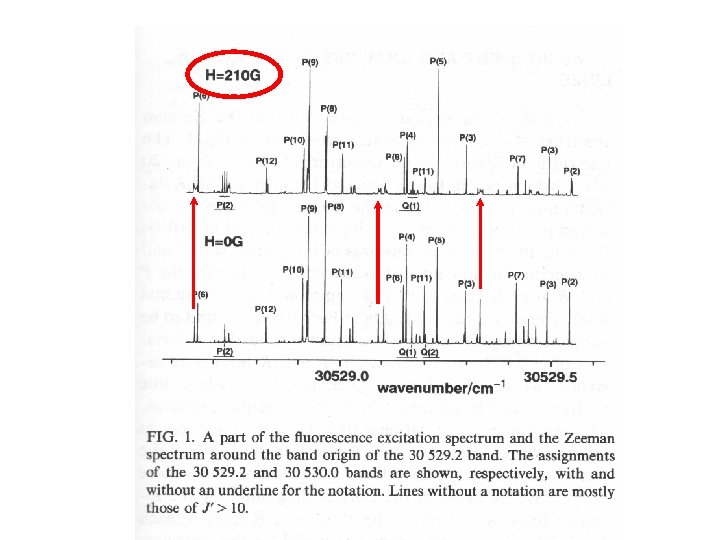
c Zeeman effect (443 cm-1 band) Kc J a

J- and K- dependences of the observed Zeeman splitting (443 cm-1 band) ZS∝Kc 2 ZS∝J

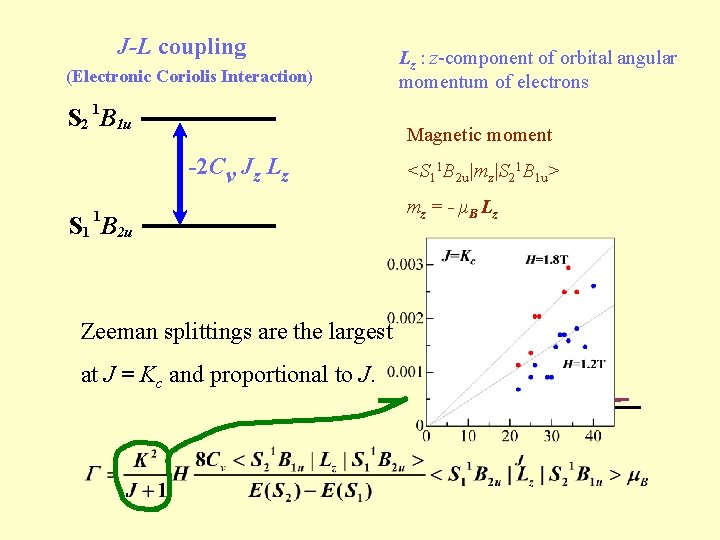
J-L coupling (Electronic Coriolis Interaction) S 2 1 B 1 u Lz : z-component of orbital angular momentum of electrons Magnetic moment -2 Cv Jz Lz 1 S 1 B 2 u <S 11 B 2 u|mz|S 21 B 1 u> mz = - μ B L z M= Zeeman splittings are the largest at J = Kc and proportional to J. -J Γ M=+J

Conclusions The S 1 S 0 000 spectrum of Dibenzofuran (DF) is B type and 000+443 cm-1 is A type. The S 1 state is assigned to be the 1 A 1 (ππ*) state. The DF molecule is planar both in the ground state and in the S 1 1 A 1 state. The intensity arises from the vibronic coupling with the S 2 1 B 2 state. The Zeeman effect depends on J, K and Zeeman splitting is small, which suggests that rotationally resolved levels are not mixed with a triplet state largely.

Zeeman effect (0 -0 band)

J- and K- dependence of the observed Zeeman Splitting 443 cm-1 band 0 -0 band 443 cm-1 band の異なる J においても、 0 -0 band においても、 同じ傾向が現れた


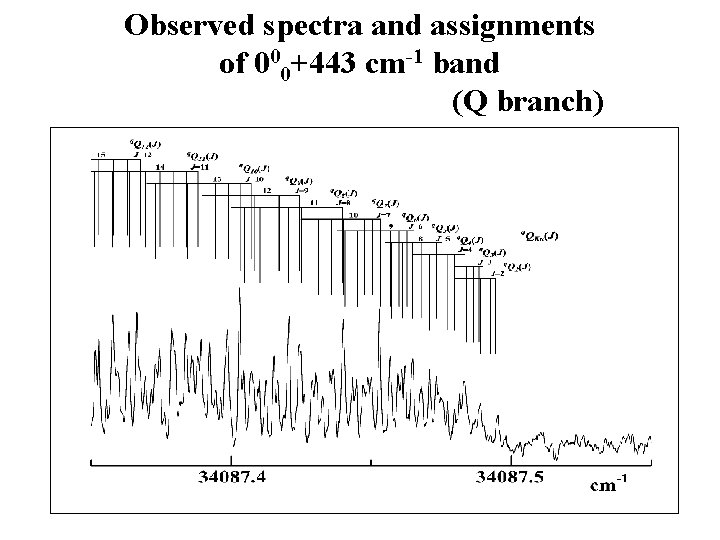
Observed spectra and assignments of 000+443 cm-1 band (Q branch)
![Higher bands of S 1S 0 transition P lines Higher bands of S 1←S 0 transition [P lines]](https://slidetodoc.com/presentation_image_h2/b8aa64ce99e2974248bbe1704ed75883/image-26.jpg)
Higher bands of S 1←S 0 transition [P lines]

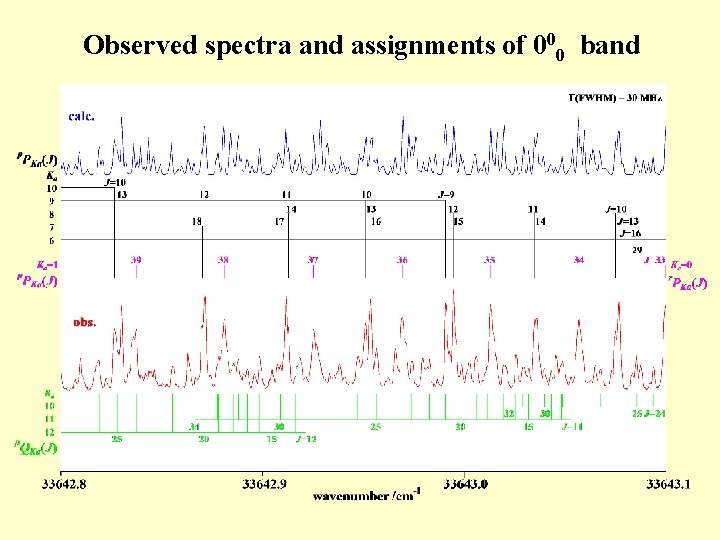
Observed spectra and assignments of 000 band