Early Phase Clinical Trials Greg Pond Ph D





























































- Slides: 84

Early Phase Clinical Trials Greg Pond Ph. D. , P. Stat. Escarpment Cancer Research Institute Department of Oncology Department of Clinical Epidemiology and Biostatistics 6 November 2014

Learning Objectives 1. Understand clinical questions of interest 2. Understand statistical questions of interest 3. Understand different phases (1/2/others) 4. Able to discuss designs for early phase clinical trials

My Background (biases) • Department of Oncology (associate appointment with Clin Epi & Biostats) • 13+ years in cancer clinical trials • Minimal involvement with other diseases • Interaction (questions) are good

Your Background • Does anyone have experience designing an early phase trial? • How many have clinical trials experience? What sort? • Who is clinician / statistician / researcher / other?

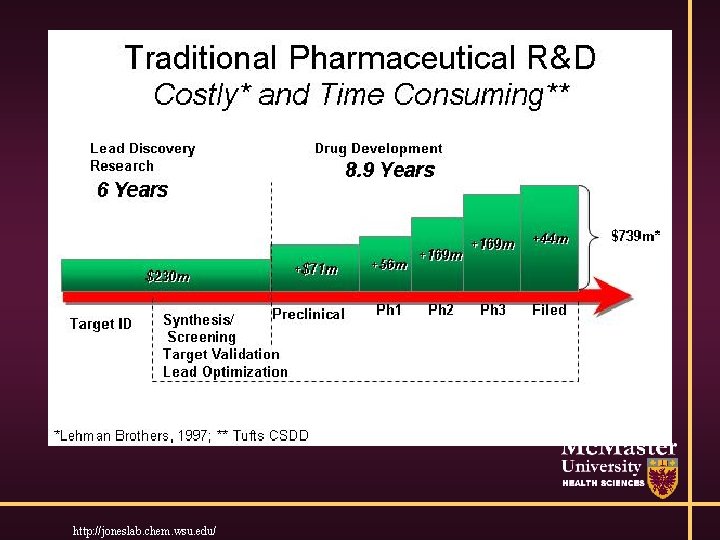
Thought Experiment • You (or collaborator) has new drug which showed astonishing activity in animals / lab experiments. Ultimately, you want to apply for regulatory approval. • You need to convince financier to fund you to get to regulatory approval. What processes will you use?

Standard Drug Development Paradigm • Phase I – safety. Find safe dose. • Phase II – preliminary efficacy. Is it active? • Phase III – efficacy. Is it superior to standard? • Phase IV – effectiveness. Is it beneficial in the real-world?

Phase I • FDA Definition: Phase 1 includes the initial introduction of an investigational new drug into humans. These studies are closely monitored and may be conducted in patients, but are usually conducted in healthy volunteer subjects. These studies are designed to determine the metabolic and pharmacologic actions of the drug in humans, the side effects associated with increasing doses, and, if possible, to gain early evidence on effectiveness. During Phase 1, sufficient information about the drug's pharmacokinetics and pharmacological effects should be obtained to permit the design of well-controlled, scientifically valid, Phase 2 studies

Phase II • FDA Definition: Phase 2 includes the early controlled clinical studies conducted to obtain some preliminary data on the effectiveness of the drug for a particular indication or indications in patients with the disease or condition. This phase of testing also helps determine the common short-term side effects and risks associated with the drug. Phase 2 studies are typically well-controlled, closely monitored, and conducted in a relatively small number of patients, usually involving several hundred people.

Clinical Trial Phases • NIH Definition: PHASE I TRIALS: Initial studies to determine the metabolism and pharmacologic actions of drugs in humans, the side effects associated with increasing doses, and to gain early evidence of effectiveness; may include healthy participants and/or patients. PHASE II TRIALS: Controlled clinical studies conducted to evaluate the effectiveness of the drug for a particular indication or indications in patients with the disease or condition under study and to determine the common short-term side effects and risks. PHASE III TRIALS: Expanded controlled and uncontrolled trials after preliminary evidence suggesting effectiveness of the drug has been obtained, and are intended to gather additional information to evaluate the overall benefit-risk relationship of the drug and provide and adequate basis for physician labeling.

Clinical Trial 1 • What question should be answered in first clinical trial? • Is it safe to administer? Is there any biological activity? How much should we give? • Give to small number of humans in a controlled environment (why? ) and check (side) effects

TGN 1412 for RA • Injected to six volunteers at sub-clinical dose on 13 March 2006, 10 min apart in London, UK • All ‘immediately’ hospitalised, six with multi-organ failure due to ‘cytokine storm’. 1 ballooned up like ‘the elephant man’. • Volunteers given ₤ 2000. All survived, but may have longterm immune system issues. • The company, Te. Genero, went bankrupt within the year.

Dose Levels • DL 1 usually based on animal data (e. g. 1/10 th LD 50 in mice) • Escalation of doses uses Fibonacci sequence (x 2, x 1. 67, x 1. 5, x 1. 33, …) • Oral medications: based on availability of doses (i. e. if only come in 50 mg pills) Penel N and Kramar A. BMC Med Res Meth 12: 103, 2012

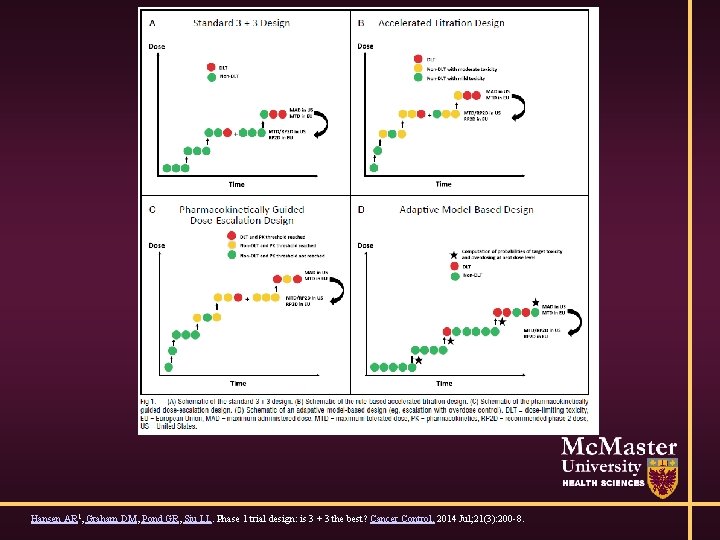
Phase I: Cancer (3+3) • Give to 3 patients • If 0/3 have dose limiting toxicity - escalate • If 1/3 has DLT, expand to 3 more patients • If 1/6 has DLT – escalate • If ≥ 2/3 or ≥ 2/6 – reached maximum tolerated dose (MTD) • DLT: serious or life-threatening AE occurring in first cycle – not standard Hansen, Graham, Pond, Siu, Phase I Trial Design: Is 3+3 the Best? Cancer Control. 21(3): 2014

DLTs • Serious or life-threatening AE occurring in first cycle – not standard • Grade of AE (1=mild, 2, 3, 4, 5=death). Grade 3+ • Leukemia patients almost all have grade 4 hematological AE • Attributable to drug? • Chronic grade 2 AE (fatigue, nausea, etc)

Phase I: Cancer (3+3) • Recommended phase II dose is the dose below MTD • Europe: MTD=RP 2 D • DEFINE!!!!!!

Phase I: Non-cancer • Give drug to paid volunteers • How much would you need to be paid to take a drug with a 33% chance of giving you a serious/life-threatening AE? • What is compensation if 33% of volunteers get SAE? • Luckily, most treatments expected to have few/none AE

Phase I: Non-cancer • How do you measure safety/outcomes? • Measure the effects on body (pharmacokinetics, pharmacodynamics) • Ensure it is within acceptable limits

Phase I: Non-cancer • Statistical tests on differences in variability • Differences in mean less important (why? ) • How do you define sample size? • Often want to ensure biodistribution

Cancer Phase I Ethics • Often start ‘too low’ for safety reasons • Suboptimal dose reduces chance of response • Phase I response rates quoted as ~5% • Patients do not enter these trials altruistically

Ethics • Should you allow intrapatient dose escalation? • Chance of response for patient • How to evaluate late AE? • How likely is it for a patient with worsening disease to stay on treatment for >2 cycles? • Generally No.

Ethics • Average patient survival may be a few months • What if drug requires weekly IV injections? • How to assess long-term toxicities?

Correct RP 2 D • Too low reduced chance of efficacy in later trials • Too high excess toxicity / dose reductions • Based on 3+3=6 patients

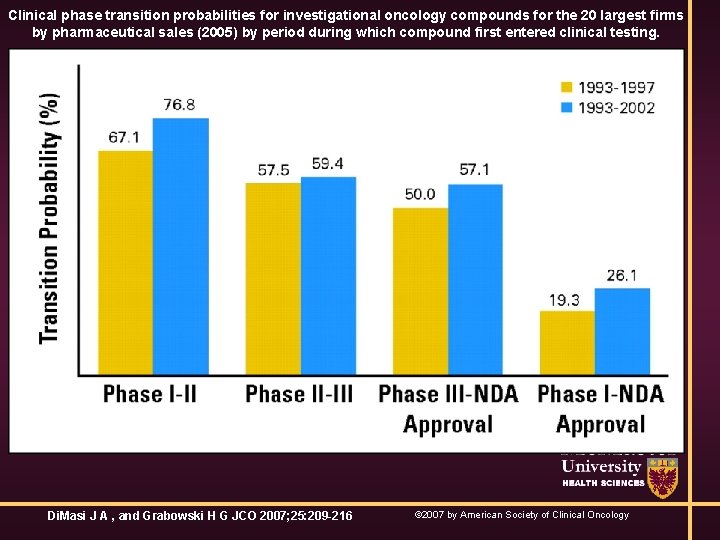
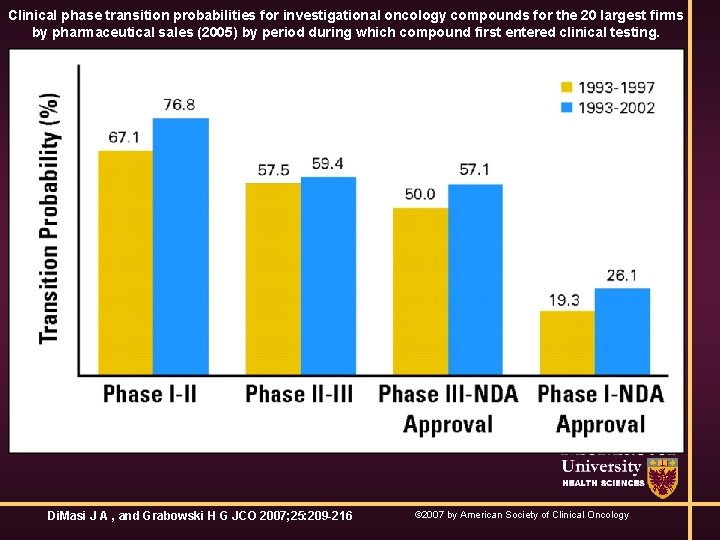
Clinical phase transition probabilities for investigational oncology compounds for the 20 largest firms by pharmaceutical sales (2005) by period during which compound first entered clinical testing. Di. Masi J A , and Grabowski H G JCO 2007; 25: 209 -216 © 2007 by American Society of Clinical Oncology

http: //joneslab. chem. wsu. edu/

Modified Designs • Rapid early escalation and intra-patient escalation permitted • 1 pt / dose level until grade 2 AE, then 3+3 • Reduce # of patients in trial (slightly), # at suboptimal dose. More likely to give patients too toxic dose

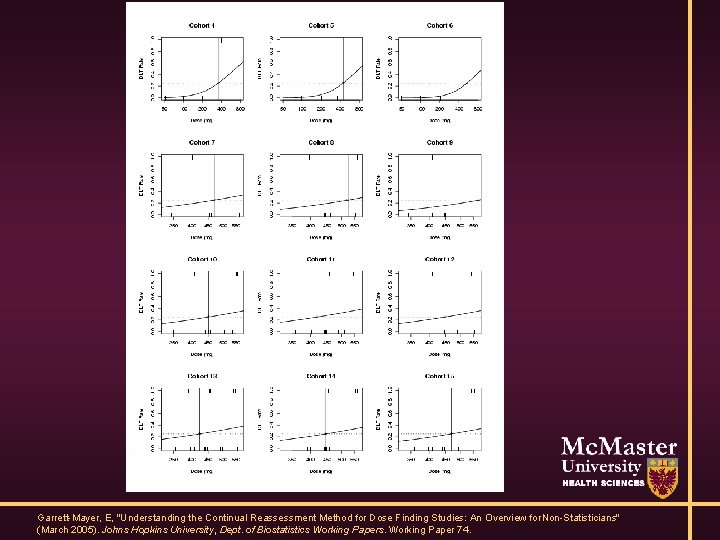
Model-Based Designs • ‘Recent’ innovation • Specify dose-toxicity relationship • Update based on data

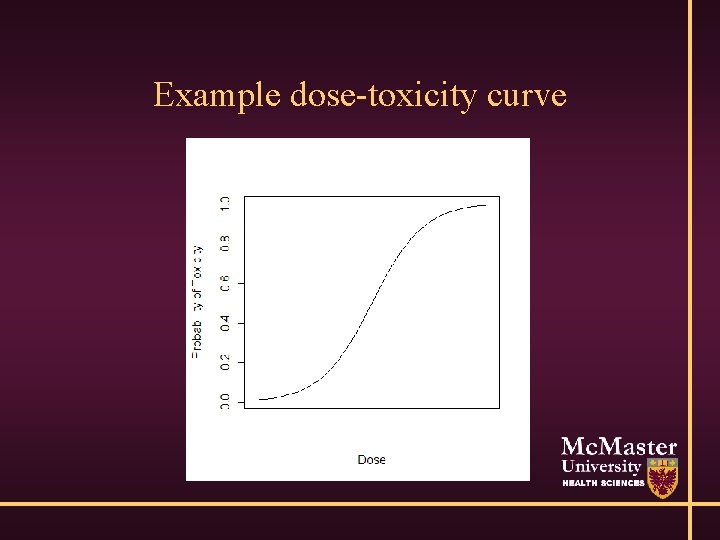
Example dose-toxicity curve

Garrett-Mayer, E, "Understanding the Continual Reassessment Method for Dose Finding Studies: An Overview for Non-Statisticians" (March 2005). Johns Hopkins University, Dept. of Biostatistics Working Papers. Working Paper 74.

Others • EWOC – curve based on probability of observing a SAE • TITE-CRM – time to event CRM • Mixed effects proportional odds model – odds of severe toxicity per cycle • Some gain in assessing accurate dose. May use more patients (pre-define limit on sample size)

Hansen AR 1, Graham DM, Pond GR, Siu LL. Phase 1 trial design: is 3 + 3 the best? Cancer Control. 2014 Jul; 21(3): 200 -8.

Secondary Outcomes • Small numbers of patients • Want correlative (p. K/p. D/biological effects) • Patients receive different doses • Many correlative outcomes are binary/ordinal • Consider cost/benefit

Secondary Outcomes • Does biology change pre- to post-treatment? • Is patient healthy enough post-treatment? • Ethics of paired biopsies • What is accuracy of assay?

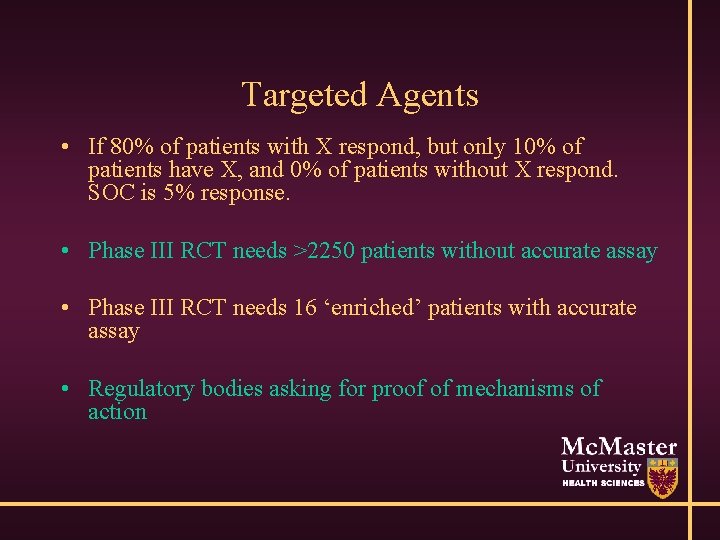
However… • Some argue no reason to pursue development of new drug if no marker of target • Molecularly targeted agents • Drug A targets marker X, highly prevalent in cancer • If patient does not express X, then drug useless

Targeted Agents • If 80% of patients with X respond, but only 10% of patients have X, and 0% of patients without X respond. SOC is 5% response. • Phase III RCT needs >2250 patients without accurate assay • Phase III RCT needs 16 ‘enriched’ patients with accurate assay • Regulatory bodies asking for proof of mechanisms of action

Phase II • Is there any evidence of activity? • Might this agent be potentially useful? • Do not want to test if drug is ‘better’ than standard treatment (why not? ) • Proof of principle

Clinical phase transition probabilities for investigational oncology compounds for the 20 largest firms by pharmaceutical sales (2005) by period during which compound first entered clinical testing. Di. Masi J A , and Grabowski H G JCO 2007; 25: 209 -216 © 2007 by American Society of Clinical Oncology

Phase II: non-cancer • Compare efficacy – often of a surrogate outcome • Small, randomised trial • Does blood pressure go down – surrogate for MI • Do MS patients improve activity levels, walk? – long-term abilities

Placebo 5 mg R. 10 mg 20 mg 50 mg

Phase II: non-cancer • Size – few hundred • Multiple comparison adjustments • α=0. 05, β=0. 80

Phase II: non-cancer • Select optimal dose / regimen • Highest dose may be unsafe / not tolerable • Ideally want dose-response relationship

Phase II: non-cancer • Trial design: standard statistical analyses • T-tests, χ2 tests, Wilcoxon rank-sum, Fisher’s exact tests • Estimation of differences also important • Some subgroup analyses performed, though power is small – pre-define

Phase II: non-cancer • Secondary outcomes: safety, tolerability, treatment completion rate • Preliminary evidence of OS (or phase III outcome) • Use for designing phase III trial

Phase II: Cancer • Placebo generally considered unethical • Any disease which is terminal, or requires intervention of some sort (schizophrenia, HIV) • Best supportive care may be given for palliation

Phase II: Cancer • How would you measure if there is ‘any evidence of activity’? • Most cancer drugs historically fail and have substantial side effects • Remember: 33% have serious/life-threatening AE in 1 st 28 days alone and ~5% respond at all • Thoughts?

Phase II: Cancer • Give to small numbers of patients (single-arm) • If anyone responds => evidence of activity • Add a few more patients and estimate RR • Gehan - 1960

Phase II: Cancer • Two stages, formalize based on α and β (Fleming, 1982) • Optimal designs (Simon, 1989) • Assuming drug is ineffective, what is minimum number of patients needed • Reduces total sample size across all trials

Design Issues • Some advances – some patients respond to std • Ethically can not give novel therapy with less chance of response • Novel therapies designed not to shrink tumour, but only prevent it from growing • How to proceed?

Questions • Often novel therapies are given in addition to standard of care • Outcome of response changed to time to progression • Randomization

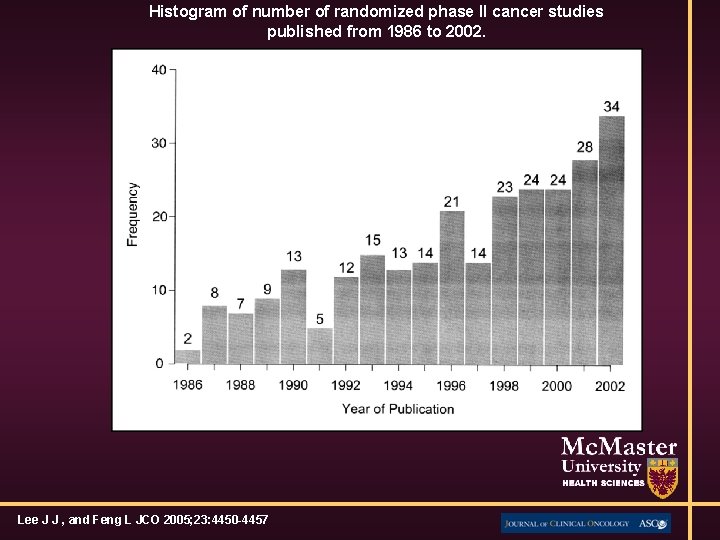
Histogram of number of randomized phase II cancer studies published from 1986 to 2002. Lee J J , and Feng L JCO 2005; 23: 4450 -4457

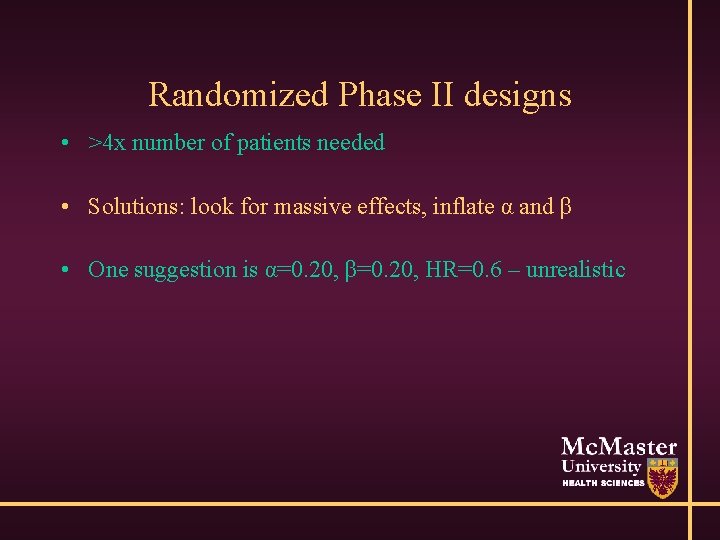
Randomized Phase II designs • >4 x number of patients needed • Solutions: look for massive effects, inflate α and β • One suggestion is α=0. 20, β=0. 20, HR=0. 6 – unrealistic

Randomized Phase II Outcomes • PFS instead of OS • PFS often a poor surrogate • May be difficult to run phase III if large differences observed

Other designs • ‘Pick-the-winner’ • 2: 1 allocation, with control only to ‘ensure H 0 is accurate’ • No formal comparison, different regimens • None widely accepted

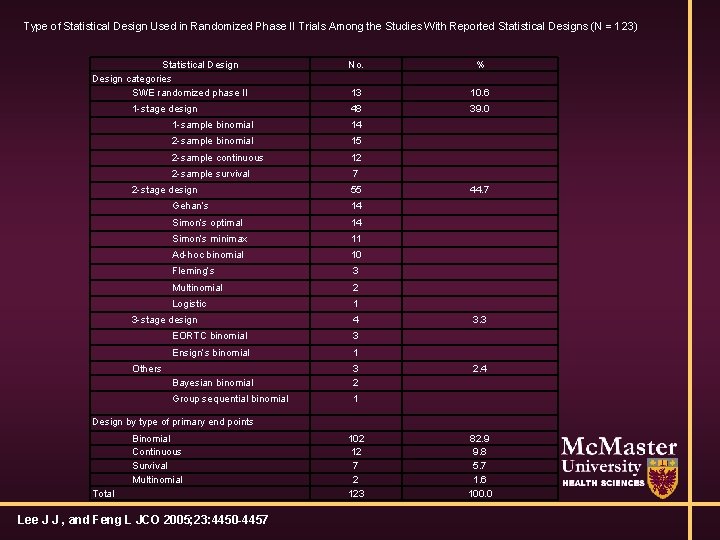
Type of Statistical Design Used in Randomized Phase II Trials Among the Studies With Reported Statistical Designs (N = 123) Statistical Design categories SWE randomized phase II 1 -stage design No. % 13 10. 6 48 39. 0 1 -sample binomial 14 2 -sample binomial 15 2 -sample continuous 12 2 -sample survival 7 2 -stage design 55 Gehan’s 14 Simon’s optimal 14 Simon’s minimax 11 Ad-hoc binomial 10 Fleming’s 3 Multinomial 2 Logistic 1 3 -stage design 4 EORTC binomial 3 Ensign’s binomial 1 Bayesian binomial 3 2 Group sequential binomial 1 Others 44. 7 3. 3 2. 4 Design by type of primary end points Binomial Continuous Survival Multinomial Total Lee J J , and Feng L JCO 2005; 23: 4450 -4457 102 12 7 2 123 82. 9 9. 8 5. 7 1. 6 100. 0

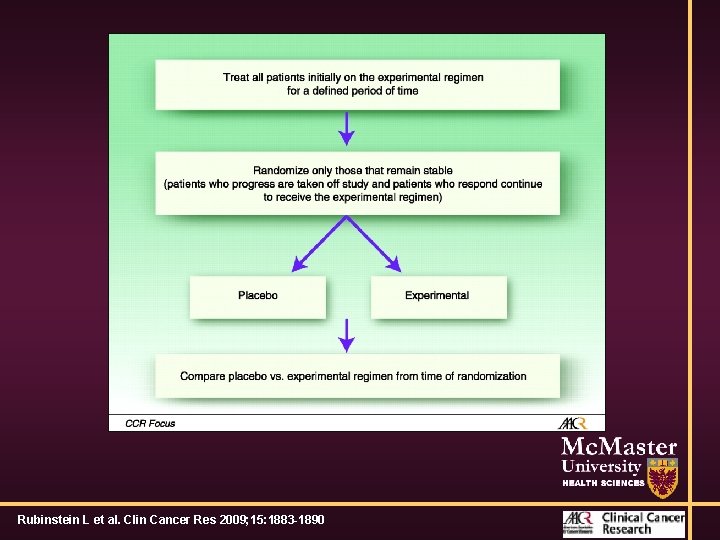
‘Novel’ designs • Randomised discontinuation trials • Treat all patients with agent for 1 cycle • Keep treating patients with a response • Stop treating patients who are progressing • Randomize patients with stable disease to treatment versus placebo

Summary of phase II trial designs. Rubinstein L et al. Clin Cancer Res 2009; 15: 1883 -1890

RDT Trials • Primary analysis is comparison of randomized patients • Re-challenge placebo patients who progress • Ethics? • Can fail miserably if # of patients with SD is small

Crossover • Trial is novel therapy versus BSC • Patients who fail BSC ‘crossover’ to receive novel therapy • Ethically, all pts receive trt

Crossover • Problem is study is really treat now, or treat later • All patients are progressing at start of study • Tumour grows and becomes more resistant • Cannot determine long-term OS advantage. If trial is + and regulatory approval, difficult for funders/insurance (OHIP)

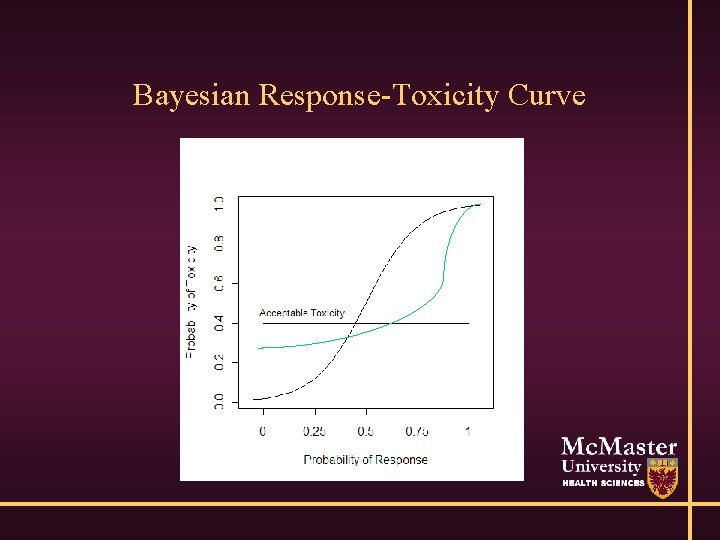
Bayesian Response-Toxicity Curve

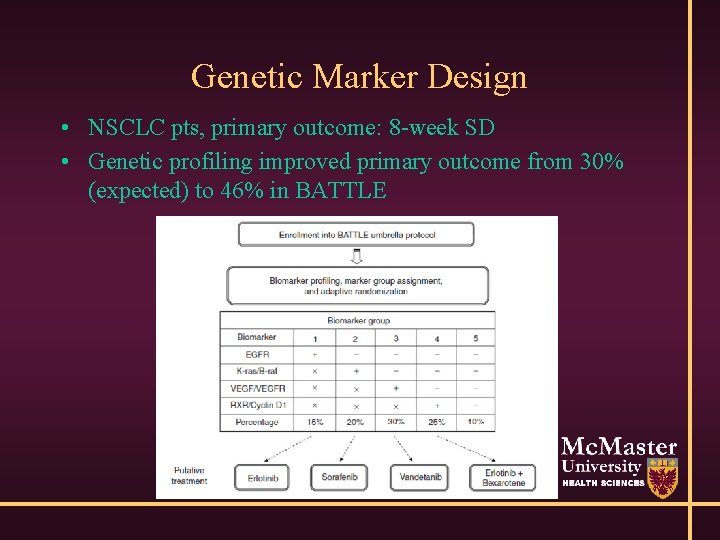
Genetic Marker Design • NSCLC pts, primary outcome: 8 -week SD • Genetic profiling improved primary outcome from 30% (expected) to 46% in BATTLE

Bayesian Adaptive Design • BATTLE: randomize pts 1: 1: 1: 1 initially • As info increases, allocate patients preferentially to treatment/marker combination with best performance • Outcome is Prob(8 week SD|marker & treatment) • Large, complex, need real-time data/histology

Marker Designs • I-SPY 2 underway in breast cancer • Lots of information; essentially many single-arm trials • May reject treatment/marker combo with small numbers • Costs ~$27 million over 5 years for 800 patients

Combined Phase Trials • Phase I/II trials • Include phase I patients in phase II analysis • Only pts treated at RP 2 D • Population must be the same, thus, may reduce #s evaluable for phase I

Combined Phase Trials • Phase II/III • Do randomised phase II study using a surrogate endpoint • Stop at analysis 1 if lack of efficacy • Continue to phase III if some evidence

Combined Phase Trials • Does not affect the α since interim analysis based on different endpoint • However, must be willing to commit resources for phase III study • Phase III tends to have large # of institutions; if stop at IA, much work for little gain • 3 -6 months to get study started

Secondary Outcomes • Safety / toxicity / cost / QOL / ease of treatment • Many not known after phase I • May inform phase III design, but may be added costs with no benefit if phase II fails • Can add support for regulatory approval of positive trials

Registration Information for US Food and Drug Administration (FDA) Oncology Drug Approvals for Solid Tumors 1998 Through 2008 Inclusive Basis of All Initial Approvals Cytotoxic Drugs (n = 13 drugs in 34 indications) Targeted Drugs (n = 11 drugs in 26 indications) No. % Phase III data* 30 88 20 77 Single-arm phase II data only 4† 12 4‡ 15 Randomized phase II data only 0 0 2§ 8 Hui K. Gan, Axel Grothey, Gregory R. Pond, Malcolm J. Moore, Lillian L. Siu⇓ and Daniel Sargent Randomized Phase II Trials: Inevitable or Inadvisable? JCO 28(15): 2641 -2647; 2010

Phase 0 • Looking for biological effects only • Give agent as a single-agent for 1 week/day • Evaluate biological markers only • Some then combine agent with standard in full trial • No clinical benefit

Phase 0 • Preferentially given as part of phase I study (can identify dose effect) • Not necessarily given to all patients in study • Occasionally given in isolation (give 1 month of treatment to 5 -10 patients) • Recruitment is a major issue

Additional Considerations • Benefit to patient is of concern • Considerable risk of harm • Small numbers of patients • Benefit is that may identify assay / target

Additional Considerations • Phase I and II are supposed to be ‘quick’ • Can take 2 -3 years (or more) each • What will landscape look like in 10 years (when applying for regulatory approval)?

Additional Considerations • Up to 1 year to get study activated after protocol finalized • Research Ethics Boards • Legal contracts with all sites • Database / data collection • Site scientific and finance reviews

Data Collection • Up to 1000 data items collected / trial • Average of <20% used (<100 / trial) • Massive wastage O’Leary, Seow, Julian, Levine, Pond. Data collection in cancer clinical trials: Too much of a good thing? Clin Trials 10(4): 624 -632; Aug 2013

Data Collection • • • Clinical trials nurse collects data Data manager enters data Trial coordinator reviews data / finds issue Coordinator sends DCF to data manager Data manager reviews with nurse Data manager sends revised data Trial coordinator re-reviews data Study investigator reviews and signs off Database is locked • All at >$50 / hour

Data Collection • Is it critical to the study? • How likely will it be for planning future study? • Data clarification is important Weight=110 (kg/lbs? ) What date is 11/6/14 ? How important is family history if half patients answer=UNK?

Privacy • Large restrictions on data captured due to privacy • Date of birth / diagnosis – restricted to year? • Genetic markers • Want tissue sample sent to third party for QA review – did you specify in the consent?

Consent Form • Legal document describing procedures / goals / information needed / options for patient • Very detailed (I have seen >30 page consents) • Sub-consents for biomarker research • Signed by patient / personnel (not in position of authority)

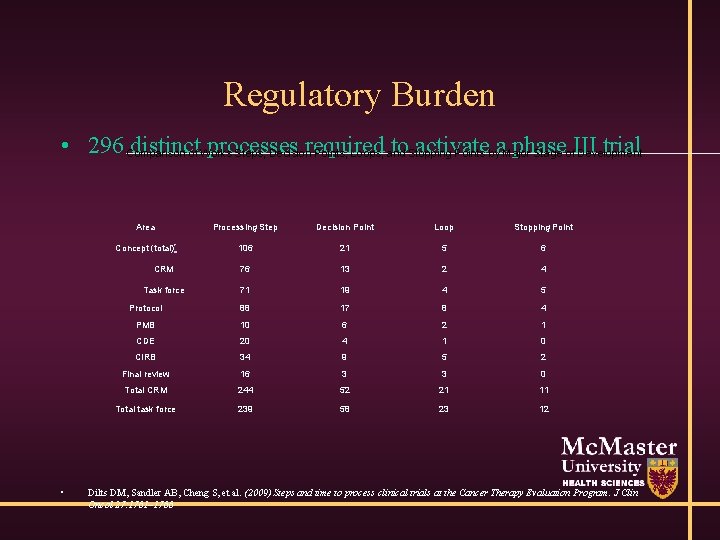
Regulatory Burden • 296 Comparison distinctof Works processes required to. Stopping activate phase trial Steps, Decision Points, Loops, and Points bya. Major Stage of. III Development • Area Processing Step Decision Point Loop Stopping Point Concept (total)* 106 21 5 6 CRM 76 13 2 4 Task force 71 19 4 5 Protocol 88 17 8 4 PMB 10 6 2 1 CDE 20 4 1 0 CIRB 34 9 5 2 Final review 16 3 3 0 Total CRM 244 52 21 11 Total task force 239 58 23 12 Dilts DM, Sandler AB, Cheng S, et al. (2009) Steps and time to process clinical trials at the Cancer Therapy Evaluation Program. J Clin Oncol 27: 1761– 1766

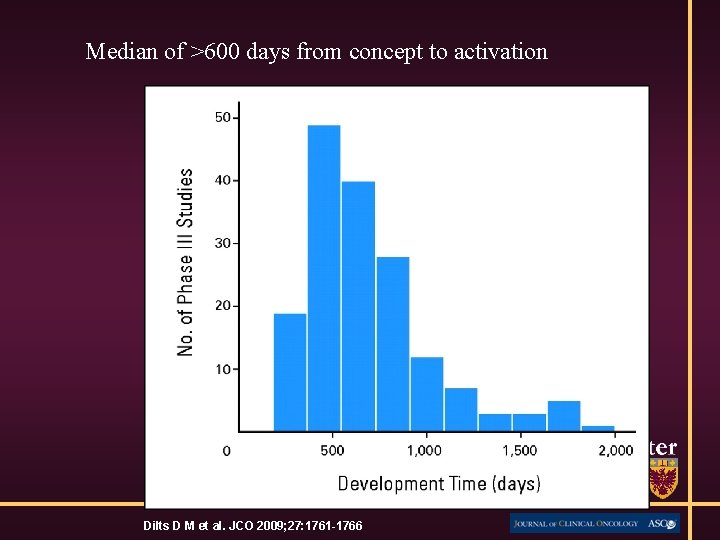
Median of >600 days from concept to activation Dilts D M et al. JCO 2009; 27: 1761 -1766

Costs per Year of Life Gained by Selected Interventions Procedure Clinical trials regulations Cost/Life-Year Saved* $2, 700, 000 Hemodialysis 29 $43, 000 -$104, 000 Statins for heart disease (moderate- to high-risk patients)30 $19, 000 -$25, 000 Colorectal cancer screening by colonoscopy 32 $14, 000 Adjuvant trastuzumab breast cancer 31 $20, 000 Bevacizumab advanced non–small -cell lung cancer 33 $380, 000 Paclitaxel/cisplatin for advanced ovarian cancer 34 $26, 000 David J. Stewart, Simon N. Whitney, Razelle Kurzrock Equipoise Lost: Ethics, Costs, and the Regulation of Cancer Clinical Research. JCO, 28(17): 2925 -2935; 2010

DSMB • Debate about if they are needed • Phase I probably not • Cancer phase II – depends on design (randomized or single -arm, # of patients) • Non-cancer phase II - probably

DSMB • 1 -2 sites: investigators know each patient • DSMB knows just numbers • Steering committee should meet before every stage/dose escalation decision, etc • Larger studies/more sites may need independent review

Final thoughts • Early phase studies often have ‘simple’ designs • More complex logistical / practical issues than late stage designs • These must be incorporated in analysis, design, interpretation, inference

Final thoughts • Must, as a biostatistician, have understanding of nonstatistical aspects, and • Must be able to communicate effectively with nonstatisticians (clinicians, nurses, CRA, ethics, regulatory, pathologists, radiologists, lab technicians, scientists, IT, geneticists, …)