Vision for Clinical Trials and Clinical Trials Units























- Slides: 43

Vision for Clinical Trials and Clinical Trials Units in the Future Professor Julia Brown Leeds Institute of Clinical Trials Research, University of Leeds Institute of Clinical Trials Research

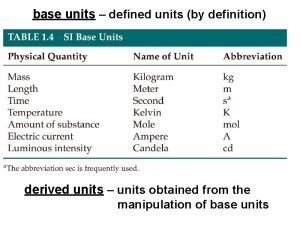
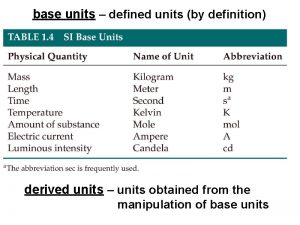
Introduction • First clinical trial in 1747, James Lind • Clinical trials are gold standard for the evaluation of new treatments/interventions in health care • Provide evidence to improve clinical service • Lead to improved patient outcomes Leeds Institute of Clinical Trials Research

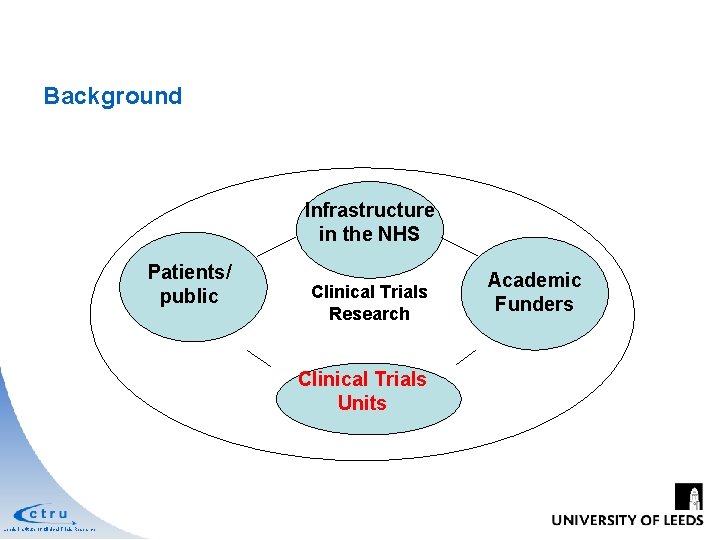
Background Significant investment in clinical trials research infrastructure and clinical trial funding over last decade Infrastructure in the NHS Patients /public INDUS TRY Leeds Institute of Clinical Trials Research Academic Funders

Background Infrastructure in the NHS Patients/ INDUSTRY public FUNDERS Clinical Trials Research Clinical Trials Units Leeds Institute of Clinical Trials Research Academic Funders

Clinical Trials Unit PLANNING • Grant prep • Study hypothesis • Study Design • Sample Size • Feasibility • Outcomes/outc ome development • Costings Leeds Institute of Clinical Trials Research SET-UP • Protocol Development • Case report Forms • Database design • Randomisation • Approvals • Contract negotiation • Sponsor liaison • Study Centres CONDUCT ANALYSIS • Trial/data management • Coordination • Adverse event management • Randomisation • Monitoring • Trial meetings • Quality assurance • Statistical analysis plan • Statistical monitoring • Programming and analysis REPORT PUBLICATION • Funder Reports • Advice and • Data consulting interpretation • Scientific • DMEC reports writing • Trial Steering Committee reports

Health Technology Assessment Programme Role of the CTU – Summary: To collaborate with clinical investigators in the successful design, development, set-up, conduct, management and analysis of clinical trials. “Clinical Trials Units are central to our vision of expanding clinical trials in this country". Professor Dame Sally C Davies, Chief Medical Officer & Chief Scientific Adviser, Department of Health Leeds Institute of Clinical Trials Research

Limitations of Clinical Trials § Clinical trials can take many years: ØLengthy process to plan, fund and set-up new trials ØIncreasing survival times mean more patients, long recruitment periods and longer follow-up times § Regulatory challenges § Rapidly changing drug development environment § Simple trials focusing on one or two treatments still dominate § Approximately 80% of registered trials 2010 -2012 simple 2 arm design § Expensive § Emerging evidence rarely incorporated into ongoing trials § Trial outcomes less relevant by time of reporting § Slow investigation of promising new therapies § Clinical trials alone rarely change practice, especially if simple experimental vs control design Leeds Institute of Clinical Trials Research

Leeds Institute of Clinical Trials Research

Leeds Institute of Clinical Trials Research

What’s next? • Streamline processes • Coordination of trials • Collaboration • Not reinventing wheel • Bust bureaucracy • Risk adapted approaches • Better data capture • Maximise information gained from trials • Efficient design • Better prediction of successful treatments • Precision medicine • Multiple treatments • Rolling designs • Data sharing Leeds Institute of Clinical Trials Research

Coordination of Clinical Trials Leeds Institute of Clinical Trials Research

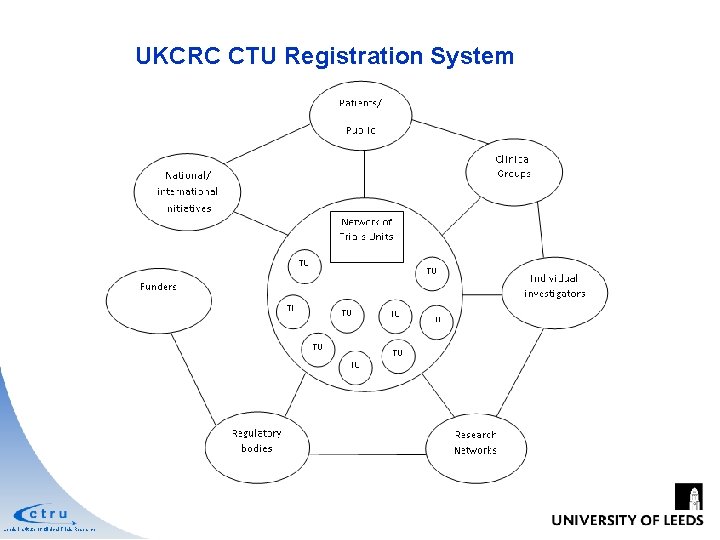
UKCRC CTU Registration System • CTU Core competencies • Track record of coordinating multicentre clinical trials from design through to publication, as evidenced by publication in peer reviewed journal • Established multidisciplinary team • Quality assurance systems • Robust and secure IT • Robust statistical input • Regular review (3 -5 years) by International Peer Review Panel Leeds Institute of Clinical Trials Research

UKCRC CTU Registration System Leeds Institute of Clinical Trials Research

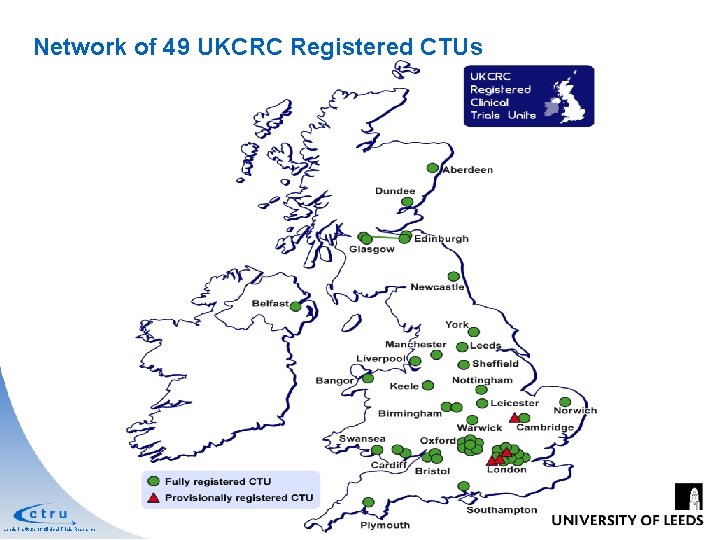
Network of 49 UKCRC Registered CTUs Leeds Institute of Clinical Trials Research

UKCRC Registered CTU Network Aims • Develop and embed the CTU Registration Process as a recognised standard for high quality clinical trials research • Maintain a cohesive network of high quality CTUs • Maintain a visible profile to facilitate access to and integration of UKCRC Registered CTUs with key stakeholders, in particular research funders (including industry), research networks, regulators, and investigators • Influence 2018 EU Directive Update BREXIT! Leeds Institute of Clinical Trials Research

UKCRC Registered CTU Network Aims • Enhance capacity and capability by continuing to support and promote the development of collaborative solutions to CTU issues • Task and finish groups • Industry collaboration • Costings and infrastructure funding • Efficient trial conduct • Patient public involvement • Insurance • Data sharing • Operational Groups • Statistics • Information Systems • Quality Assurance • Roll out risk adapted procedures Leeds Institute of Clinical Trials Research

Better data capture • Remote data capture/mobile technology • Patient reported outcomes • Report UK • Real-time Electronic Patient Outcome Repor. Ting of adverse events in UK cancer trials (REPORT-UK) • Eprime • Real time Electronic Patient Outcome Reporting of adverse events in early phase trials • Real time monitoring (apps, wearable devices) • Sensium patch - Vital signs monitoring postoperation Leeds Institute of Clinical Trials Research

Better Data Capture • Use of Routine data • Benefits • Identification of patients • Assess feasibility • Adapt conduct • Enable follow-up • Aid interpretation • Cost reduction • Challenges/concerns • Difficulty accessing and extracting data • Lack of uniformity • Poor data quality, completeness and accuracy • Complicated models required for evidence synthesis with many (sometimes untestable) assumptions Cook J, Collins G. The rise of big clinical databases BJS 2015 102(2) 93 -101 Leeds Institute of Clinical Trials Research

The SHIFT trial (Self Harm Intervention: Family Therapy) ‘A pragmatic, randomised, controlled trial, comparing family therapy with treatment as usual for young people seen after second or subsequent episodes of self-harm’ • Individually randomised 1: 1 between Family Therapy and Treatment As Usual • 832 young people from 30+ centres (CAMHS) across England (Yorkshire, Greater Manchester, London). • Young people and their primary care-giver are followed up by trial Researchers via: • face-to-face meetings • collection of clinical outcome data Leeds Institute of Clinical Trials Research

Primary outcome • Primary outcome: Repetition of self-harm leading to hospital attendance within 18 months of randomisation • Objective rather than subjective • Can be obtained from hospital records even if contact has been lost with participants • SHIFT researchers visit hospitals in ‘SHIFT areas’ to manually interrogate local medical records Leeds Institute of Clinical Trials Research

Challenges to the collection of primary outcome data • Resource intensive • Differential frequency of access between researchers • Hospital attendance episodes may be missed • Different search processes for different hospitals (paper vs electronic) • Lack of data linkage within Trusts – between A&E and Admissions • Various obstacles and levels of access to different hospital trusts • • Leeds Institute of Clinical Trials Research Approvals obtained from 25/33 Trusts identified for SHIFT Data accessed from hospitals within ~ 22 Trusts

Benefits of data collection utilising Hospital Episode Statistics If reliable data can be obtained, benefits to the SHIFT trial include: • Regular, fast, England-wide data retrieval from a central source • Avoidance of potentially biased data collection • Save on researcher resources Leeds Institute of Clinical Trials Research

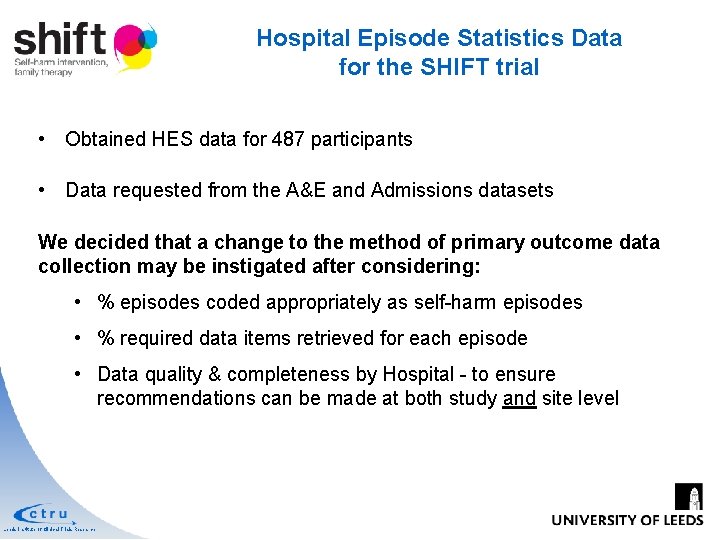
Hospital Episode Statistics Data for the SHIFT trial • Obtained HES data for 487 participants • Data requested from the A&E and Admissions datasets We decided that a change to the method of primary outcome data collection may be instigated after considering: • % episodes coded appropriately as self-harm episodes • % required data items retrieved for each episode • Data quality & completeness by Hospital - to ensure recommendations can be made at both study and site level Leeds Institute of Clinical Trials Research

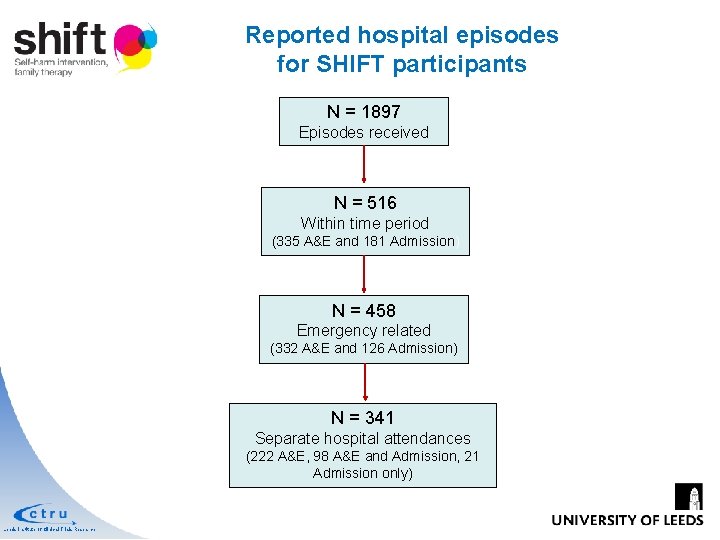
Reported hospital episodes for SHIFT participants N = 1897 Episodes received N = 516 Within time period (335 A&E and 181 Admission) N = 458 Emergency related (332 A&E and 126 Admission) N = 341 Separate hospital attendances (222 A&E, 98 A&E and Admission, 21 Admission only) Leeds Institute of Clinical Trials Research

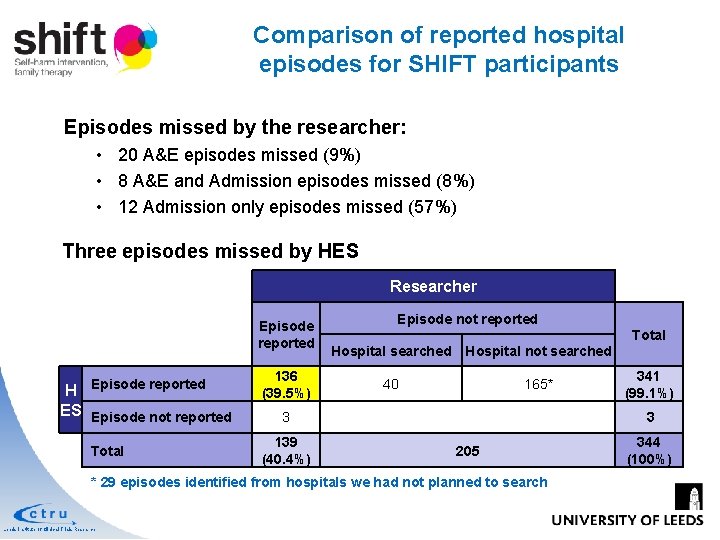
Comparison of reported hospital episodes for SHIFT participants Episodes missed by the researcher: • 20 A&E episodes missed (9%) • 8 A&E and Admission episodes missed (8%) • 12 Admission only episodes missed (57%) Three episodes missed by HES Researcher Episode reported H ES Episode not reported Total 136 (39. 5%) Episode not reported Total Hospital searched Hospital not searched 40 165* 3 139 (40. 4%) 3 205 * 29 episodes identified from hospitals we had not planned to search Leeds Institute of Clinical Trials Research 341 (99. 1%) 344 (100%)

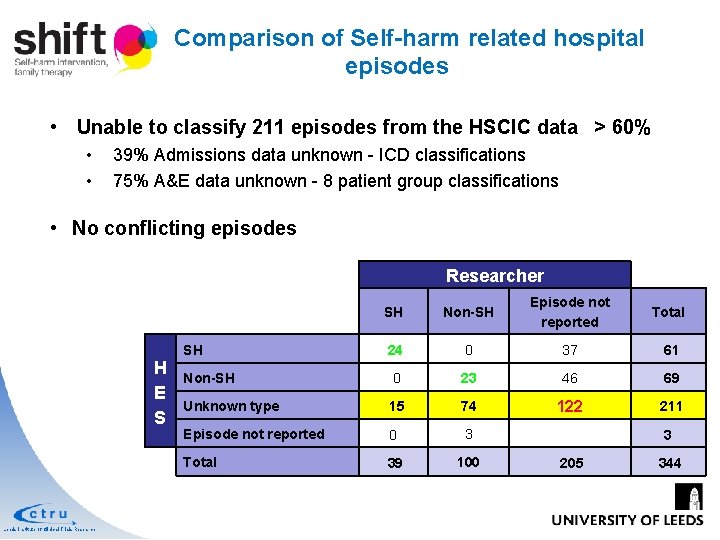
Comparison of Self-harm related hospital episodes • Unable to classify 211 episodes from the HSCIC data > 60% • • 39% Admissions data unknown - ICD classifications 75% A&E data unknown - 8 patient group classifications • No conflicting episodes Researcher SH Non-SH Episode not reported Total 24 0 37 61 Non-SH 0 23 46 69 Unknown type 15 74 122 211 Episode not reported 0 3 Total 39 100 SH H E S Leeds Institute of Clinical Trials Research 3 205 344

Conclusion • Outcome: continued collection of primary outcome data via this method every 6 months plus targeted researcher searching • Development of data search algorithm and data manipulation intensive task • Incorporate a pilot download to test searches and understand data • Requires a multidisciplinary team to develop and interpret • Advantages for the SHIFT trial outweigh disadvantages Ø Statistics time increased by 3 -4 weeks per download Ø More comprehensive and accurate trial outcome data Ø Reduced cost of data collection Ø Research time substantially reduced Ø Data management time reduced Leeds Institute of Clinical Trials Research

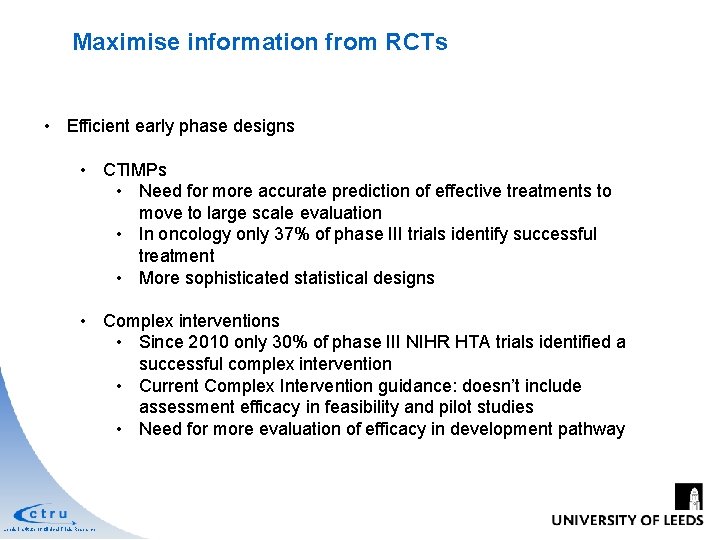
Maximise information from RCTs • Efficient early phase designs • CTIMPs • Need for more accurate prediction of effective treatments to move to large scale evaluation • In oncology only 37% of phase III trials identify successful treatment • More sophisticated statistical designs • Complex interventions • Since 2010 only 30% of phase III NIHR HTA trials identified a successful complex intervention • Current Complex Intervention guidance: doesn’t include assessment efficacy in feasibility and pilot studies • Need for more evaluation of efficacy in development pathway Leeds Institute of Clinical Trials Research

Maximise information from RCTs • Adapting phase II CTIMP trial ideas to the feasibility/pilot setting could help in assessing efficacy, thereby increasing the success rate of phase III trials of complex interventions. • Major challenges to such adaptation include allowing for multi component interventions, multiple endpoints, multilevel data. • Bayesian approaches being investigated Stat Methods Med Res. 2016 Jun; 25(3): 997 -1009. Statistical challenges in assessing potential efficacy of complex interventions in pilot or feasibility studies. Wilson DT 1, Walwyn RE 2, Brown J 2, Farrin AJ 2, Brown SR 2 Leeds Institute of Clinical Trials Research

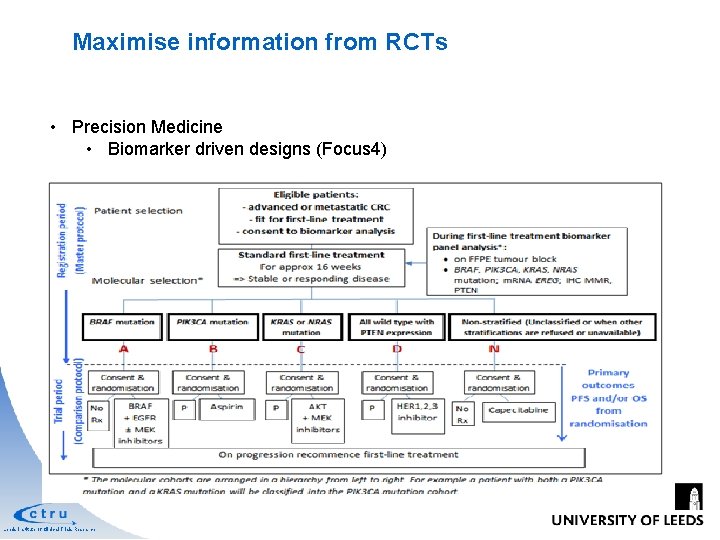
Maximise information from RCTs • Precision Medicine • Biomarker driven designs (Focus 4) Leeds Institute of Clinical Trials Research

Maximise information from RCTs • Multiple questions within a trial Ø Access for patients to more promising therapies within trials Ø Trials outcomes remain relevant Ø Greater chance of a successful outcome from the trial Ø Faster adoption of successful therapies into practice Ø Reduced overall patient numbers, trial costs and resources Leeds Institute of Clinical Trials Research

Maximise information from RCTs: MIDFUT Multi-arm Multi stage trial design Phase III N=108 N=54 IC N=54 N=166 (+112) ID Numbers in () indicate number of additional patients recruited in Phase III Leeds Institute of Clinical Trials Research Analysis (time to complete healing (by 52 weeks)) IB N=54 Randomisation (1: 1: 1) IA N=54 N=220 (+112) Analysis (≥ 50% reduction in area at 4 weeks) Randomisation (1: 1: 2) C

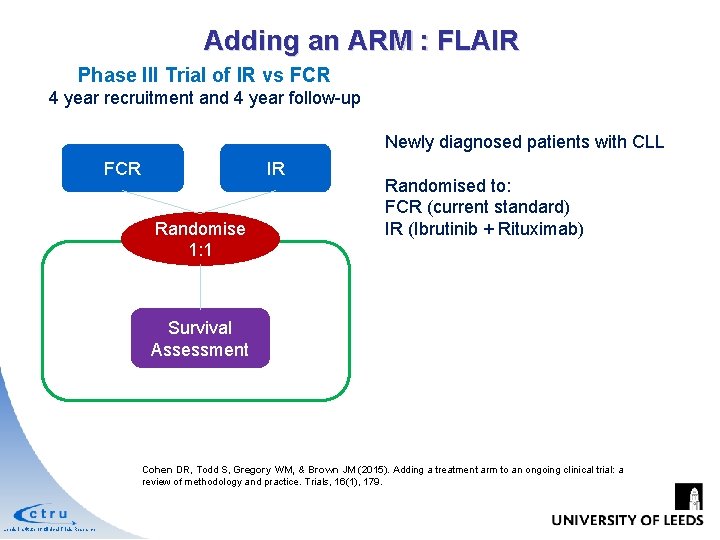
Adding an ARM : FLAIR Phase III Trial of IR vs FCR 4 year recruitment and 4 year follow-up Newly diagnosed patients with CLL FCR IR Randomise 1: 1 Randomised to: FCR (current standard) IR (Ibrutinib + Rituximab) Survival Assessment Cohen DR, Todd S, Gregory WM, & Brown JM (2015). Adding a treatment arm to an ongoing clinical trial: a review of methodology and practice. Trials, 16(1), 179. Leeds Institute of Clinical Trials Research

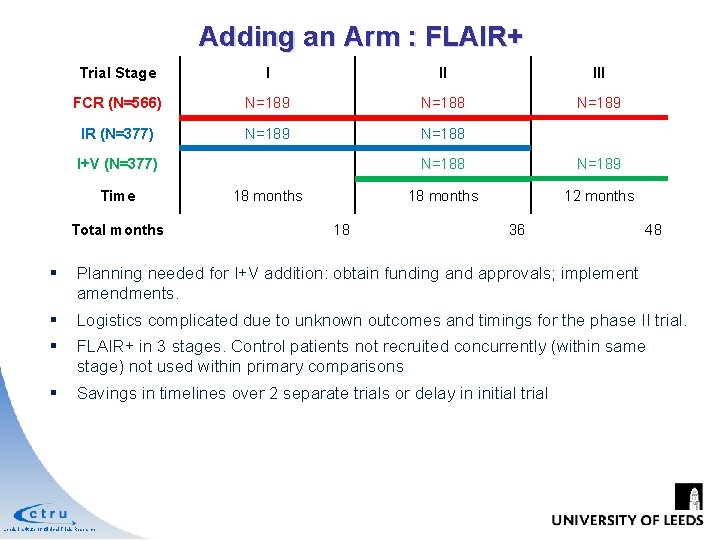
Adding an Arm : FLAIR+ Phase III Trial of IR vs FCR Phase II Trial assessing IV 4 year recruitment and 4 year follow-up Outcome expected in 2017 FCR IR I+V Randomise 1: 1 Time = 18 months Randomise 1: 1: n Survival Assessment Leeds Institute of Clinical Trials Research Response Assessment IV acceptable Should we: Ø Wait for the phase II I+V outcome, but delay the phase III trial in IR? Ø Start the trial with IR now, but deny I+V a timely investigation?

Adding an Arm : FLAIR+ Trial Stage I II III FCR (N=566) N=189 N=188 N=189 IR (N=377) N=189 N=188 I+V (N=377) Time Total months 18 N=188 N=189 18 months 12 months 36 48 § Planning needed for I+V addition: obtain funding and approvals; implement amendments. § Logistics complicated due to unknown outcomes and timings for the phase II trial. § FLAIR+ in 3 stages. Control patients not recruited concurrently (within same stage) not used within primary comparisons § Savings in timelines over 2 separate trials or delay in initial trial Leeds Institute of Clinical Trials Research

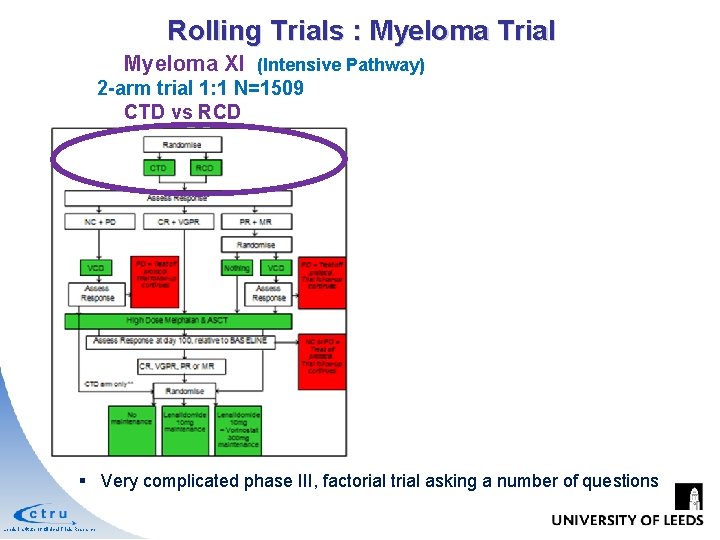
Rolling Trials : Myeloma Trial Myeloma XI (Intensive Pathway) 2 -arm trial 1: 1 N=1509 CTD vs RCD § Very complicated phase III, factorial trial asking a number of questions Leeds Institute of Clinical Trials Research

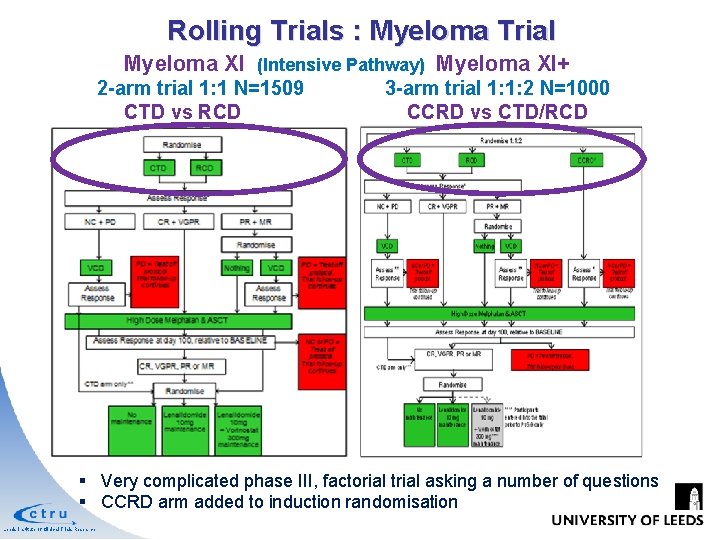
Rolling Trials : Myeloma Trial Myeloma XI (Intensive Pathway) Myeloma XI+ 2 -arm trial 1: 1 N=1509 CTD vs RCD 3 -arm trial 1: 1: 2 N=1000 CCRD vs CTD/RCD § Very complicated phase III, factorial trial asking a number of questions § CCRD arm added to induction randomisation Leeds Institute of Clinical Trials Research

Rolling Trials : Myeloma Trial § Advantages of this design amendment: § New treatment addressed without waiting for original trial outcomes: compared to both existing treatments at 2: 1 allocation § Inferior existing treatment can be dropped § Centres are in place and recruitment already has momentum § Intensive pathway recruited >40 patients/month from 100 centres; completed recruitment a year ahead of schedule. § Total planned recruitment period only extended by 1 year overall § Reduced set-up times: amendments rather than new applications; faster MHRA, ethics, R&D and endorsement approvals, industry contracts § 2½ year set-up time has been eliminated! § Cost savings over a new trial: protocol and approvals only require amendments; database exists; trial management procedures in place; staff employed and trained Leeds Institute of Clinical Trials Research

Rolling or adaptive Trials : Challenges § Can be difficult to implement: Ø Planning amendment with uncertainties: - Timing of implementation unknown - Funding applications prior to availability of early phase data - Contracts (possibly negotiating with multiple pharmaceutical companies) § Statistical considerations: Ø Type I and II error rate control Ø Multiple testing adjustments Ø Handling of non-concurrent control data Not impossible to overcome, but careful planning is needed! Adding a treatment arm to an ongoing clinical trial: a review of methodology and practice Dena R Cohen, Susan Todd, Walter M Gregory and Julia M Brown Trials (2015) 16: 179 Recommendations on multiple testing adjustment in multi-arm trials with a shared control group Dena R Howard, Julia M Brown, Susan Todd and Walter M Gregory Statistical Methods in Medical Research (on line) Leeds Institute of Clinical Trials Research

Maximise information from clinical trials • Responsible Data sharing • Enhances transparency • Fosters development and testing of new hypotheses • Avoids unnecessary repetition ‘Knowledge is power and the stakes are too high to hold back scientific information that could be used to answer new questions or guard against biased reporting of results’. Senator Elizabeth Warren Leeds Institute of Clinical Trials Research

Maximise information from clinical trials • Responsible Data sharing : Challenges • Protecting rights of patients, investigators, sponsors, funders • Requires up front planning • Logistics of long term database access Leeds Institute of Clinical Trials Research

Leeds Institute of Clinical Trials Research

Overall messages • Clinical trials continue to raise difficult design, conduct and analysis questions • Technological and clinical advances • Move towards multi arm, dynamic trial design, conduct and analysis • Increasing collaboration and changes required • Coordination of trials • Data sharing • Requires a willingness to question long held beliefs about how clinical trials are performed • Future is bright Leeds Institute of Clinical Trials Research
 Phs human subjects and clinical trials information
Phs human subjects and clinical trials information Audits and inspections of clinical trials
Audits and inspections of clinical trials Nida clinical trials network
Nida clinical trials network Site initiation visit in clinical trials ppt
Site initiation visit in clinical trials ppt Clinicaltrails.gov api
Clinicaltrails.gov api Clinical research statistician
Clinical research statistician Andrew nunn
Andrew nunn Randomization in statistics
Randomization in statistics Mpn clinical trials
Mpn clinical trials Clinical trials prs
Clinical trials prs Clinical trials
Clinical trials Clinical trials quality by design
Clinical trials quality by design Mpn clinical trials
Mpn clinical trials Dhl atyrau
Dhl atyrau Clinical hysteria salem witch trials
Clinical hysteria salem witch trials Readyset ohsu
Readyset ohsu Prs clinical trial
Prs clinical trial Clinical trials.gov login
Clinical trials.gov login Iwr clinical study
Iwr clinical study York trials unit
York trials unit Xeomin reconstitution chart
Xeomin reconstitution chart Variable costing income statement
Variable costing income statement Human vision vs computer vision
Human vision vs computer vision Fspos vägledning för kontinuitetshantering
Fspos vägledning för kontinuitetshantering Typiska novell drag
Typiska novell drag Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Vad står k.r.å.k.a.n för
Vad står k.r.å.k.a.n för Shingelfrisyren
Shingelfrisyren En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Kassaregister ideell förening
Kassaregister ideell förening Tidbok för yrkesförare
Tidbok för yrkesförare A gastrica
A gastrica Förklara densitet för barn
Förklara densitet för barn Datorkunskap för nybörjare
Datorkunskap för nybörjare Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Debatt mall
Debatt mall Delegerande ledarskap
Delegerande ledarskap Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Arkimedes princip formel
Arkimedes princip formel Publik sektor
Publik sektor Jag har nigit för nymånens skära
Jag har nigit för nymånens skära Presentera för publik crossboss
Presentera för publik crossboss Jiddisch
Jiddisch