CLINICAL TRIALS OVERVIEW PHASE 1PHASE 2PHASE 3 TRIALS















- Slides: 44

CLINICAL TRIALS OVERVIEW PHASE 1/PHASE 2/PHASE 3 TRIALS Wei Hou, Ph. D October 13, 2020

Outline Clinical Trial � Phase III Adaptive clinical trials Protocol 2

Clinical Trial NIH Definition of a Clinical Trial A research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes. Why? � Well-designed and sufficiently large randomized clinical trails are the best method. � Adverse effects or complications can be evaluated. 3

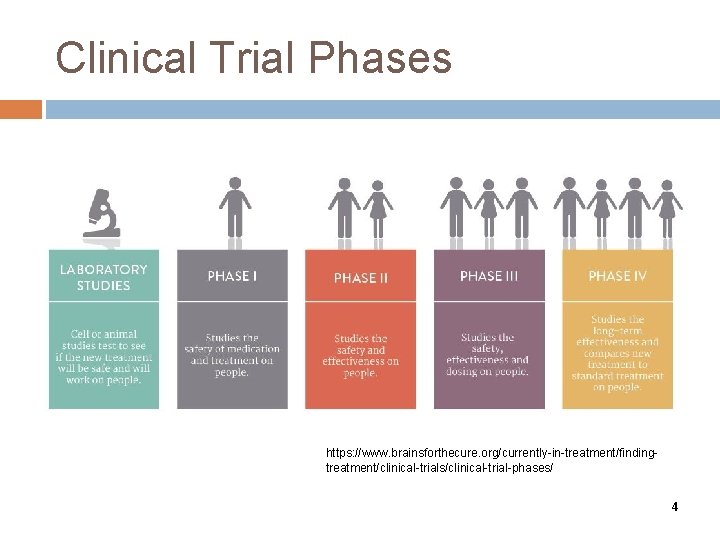
Clinical Trial Phases https: //www. brainsforthecure. org/currently-in-treatment/findingtreatment/clinical-trials/clinical-trial-phases/ 4

Phase I Primary aim is to determine the Maximum Tolerated Dose (MTD) Explore drug toxicities and characterize pharmacokinetics and pharmacodynamics Sample size is not determined based on power calculation 5

Phase I Dose usually starts at a low level for ethical reasons. Starting dose is chosen based on preclinical data (i. e. animal data). Dose escalation needs to be specified in the protocol. Dose escalation often based on Fibonacci series � 1 2 3 5 8 13. . 6

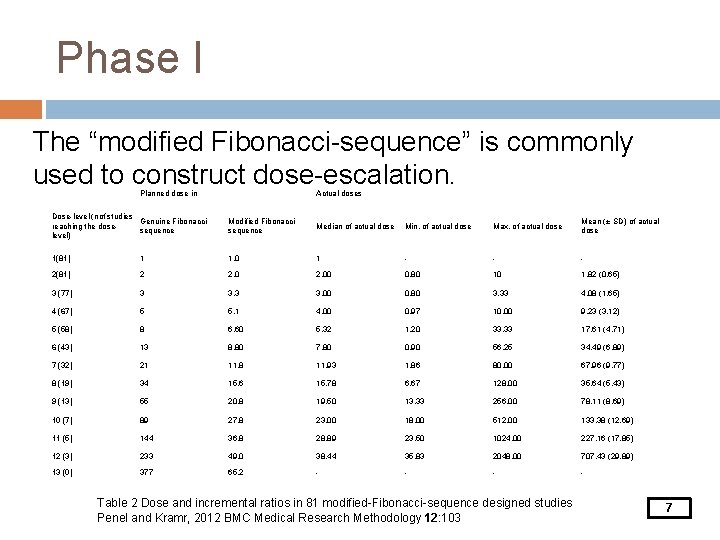
Phase I The “modified Fibonacci-sequence” is commonly used to construct dose-escalation. Planned dose in Actual doses Dose-level (nof studies Genuine Fibonacci reaching the dosesequence level) Modified Fibonacci sequence Median of actual dose Min. of actual dose Max. of actual dose Mean (± SD) of actual dose 1(81) 1 1. 0 1 - - - 2(81) 2 2. 00 0. 80 10 1. 82 (0. 65) 3 (77) 3 3. 00 0. 80 3. 33 4. 08 (1. 65) 4 (67) 5 5. 1 4. 00 0. 97 10. 00 9. 23 (3. 12) 5 (58) 8 6. 60 5. 32 1. 20 33. 33 17. 61 (4. 71) 6 (43) 13 8. 80 7. 80 0. 90 56. 25 34. 49 (6. 89) 7 (32) 21 11. 8 11. 93 1. 86 80. 00 67. 96 (9. 77) 8 (19) 34 15. 6 15. 78 6. 67 128. 00 35. 64 (5. 43) 9 (13) 55 20. 8 19. 50 13. 33 256. 00 78. 11 (8. 69) 10 (7) 89 27. 8 23. 00 18. 00 512. 00 133. 38 (12. 69) 11 (5) 144 36. 8 28. 89 23. 50 1024. 00 227. 16 (17. 85) 12 (3) 233 49. 0 38. 44 35. 83 2048. 00 707. 43 (29. 89) 13 (0) 377 65. 2 - - Table 2 Dose and incremental ratios in 81 modified-Fibonacci-sequence designed studies Penel and Kramr, 2012 BMC Medical Research Methodology 12: 103 7

Phase I 3+3 design � MTD occurs when approximately 1/3 of the patients experience unacceptable toxicity Starts with 3 patients � If 0 experience toxicity escalate � If 1 experience toxicity enroll 3 more patients at the same dose level if 1/6 experience toxicity escalate If ≥ 2 of 6 experience toxicity MTD reached � If ≥ 2 of 3 experience toxicity MTD reached MTD is the dose level immediately below the level at ≥ 2 patients of 3 to 6 patients experienced toxicity 8

Phase II Primary goal is to screen for therapeutic efficacy Lead to the a recommendation whether or not the treatment is to be tested in future Phase III trials Further evaluate toxicity Dose used is the MTD determined in previous phase I trials Single-arm or multiple arms (parallel design) 9

Phase II -Single arm, single stage Evaluate the efficacy Homogeneous patients – strict inclusion/exclusion and receive same dose Shorter term endpoint, usually a surrogate Recommendation is made at the end of the trial Sample size fixed in advance 10

Surrogate Endpoints Surrogates used as alternative to desired or ideal clinical response to save time and/or resources Examples � T 4 cell counts (AIDS or ARC) � Cholesterol (heart disease) Often used in therapeutic exploratory trials Use with caution in confirmatory trials 11

Phase II -Single arm, single stage Example: A single arm, single stage phase II trial of trametinib (GSK 1120212) and GSK 2141795 in persistent or recurrent cervical cancer (NCT 01958112 Matulonis et al J Clin Oncol 33, 2015). Patients: recurrent or met CC � Method: trametinib 1. 5 mg and GSK 2141795 50 mg, both given PO and daily. � Endpoint: Cycle length is 28 days and pts are assessed for RR every 2 cycles. � RR <7% is defined as “failure” and RR>22% is defined as “success” � Sample size: 35 pts. � Power: 91% power to detect an improvement in RR from 0. 07 to 0. 22 using a one-sided 0. 09 -level exact binomial 12 test. �

Phase II – Two Stage q For ethical reasons, the trial should allow for early termination if early results are extreme. q If the response rate is low, say <5%, the trial should be terminated for futile q If the response rate is very high, say >60%, the trial should be terminated and proceed to Phase III trial q At least one interim data analysis is needed to make early decision. 13

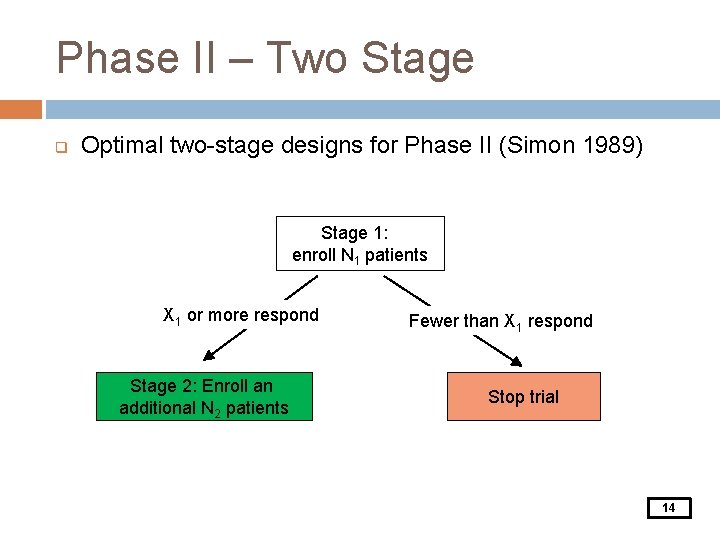
Phase II – Two Stage q Optimal two-stage designs for Phase II (Simon 1989) Stage 1: enroll N 1 patients X 1 or more respond Stage 2: Enroll an additional N 2 patients Fewer than X 1 respond Stop trial 14

Phase II – Randomized Phase II Randomized to treatment and control Limited or inapplicable historical data � Increased sample size � Reduced number of new treatments � May not be efficient � Randomized to different dose levels or treatment groups Each arm is treated as a single Phase II trial (one stage or two-stage design can be used) � The best arm will be picked for further study (usually no statistical comparison between arms) � Hou et. al (2013) proposed Phase II designs with comparisons between arms � 15

Phase III Primary goal is to confirm therapeutic benefit Designed to confirm findings in Phase II Required by FDA for drug approval Large and costly Randomized controlled trial (RCT) : eliminates several sources of bias 16

Superiority trials to demonstrate that the efficacy of the test treatment is superior to the control Hypotheses: � H 0: Treatment is no better than control � H 1: Treatment is better than control Treatment effect based on estimate of the mean difference, odds ratio or hazard ratio 17

Randomized Control Clinical Trial Patients assigned at random to either treatment(s) or control “Ethical considerations suggest that randomized trials are more suitable than uncontrolled experimentation in protecting the interests of patients. Randomized clinical trials remain the most reliable method for evaluating the efficacy of therapies. ” Byar et al. (1976) New England Journal of Medicine 18

Disadvantages of RCT Generalizability – participants may not represent general population Sample size – double Acceptability of Randomization – patients suffering from life-threatening disease may refuse 19

Randomization Each patient has the same chance to be assigned to any of the treatments in the study. Neither participant nor investigators know in advance which treatment will be assigned. After randomization, the treatment groups will be balanced with equal sample sizes. Confounding factors should be alike on average between treatment groups. 20

Non-Inferiority Trials to demonstrate that the efficacy of the test treatment is similar to a known effective treatment. New treatment may not be better than the standard but may have other advantages Cost Toxicity Invasiveness 21

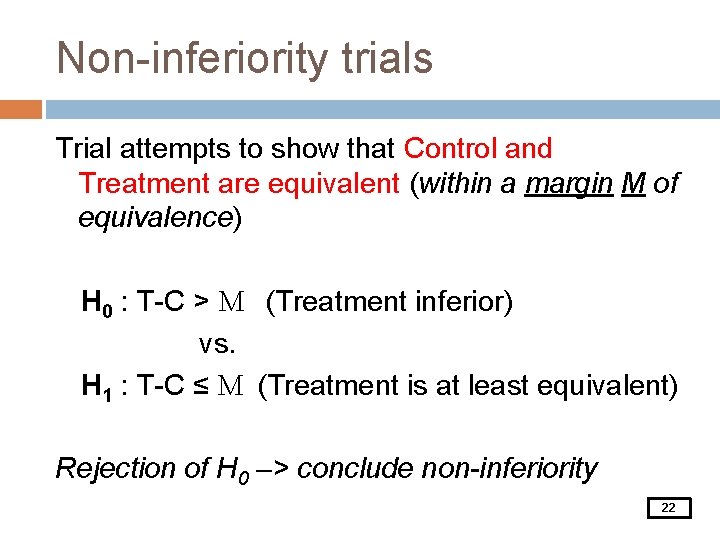
Non-inferiority trials Trial attempts to show that Control and Treatment are equivalent (within a margin M of equivalence) H 0 : T-C > M (Treatment inferior) vs. H 1 : T-C ≤ M (Treatment is at least equivalent) Rejection of H 0 –> conclude non-inferiority 22

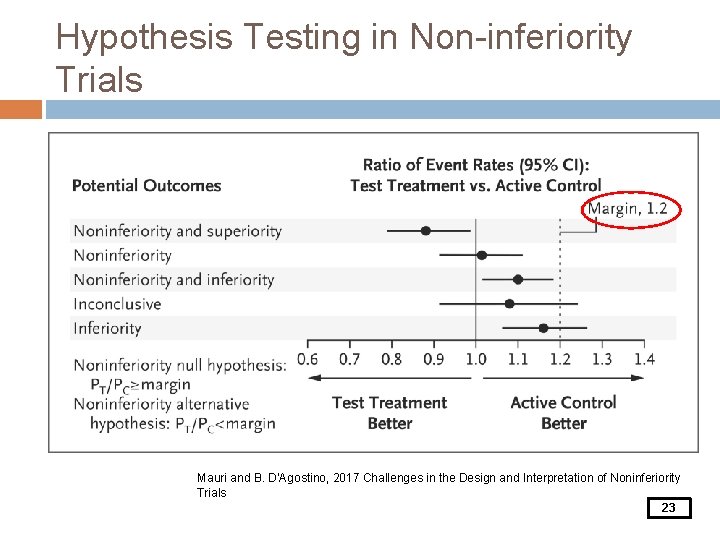
Hypothesis Testing in Non-inferiority Trials Mauri and B. D’Agostino, 2017 Challenges in the Design and Interpretation of Noninferiority Trials 23

Challenges in Non-inferiority trials Requires high quality trial Poor execution favors non-inferiority Margin selection somewhat arbitrary 24

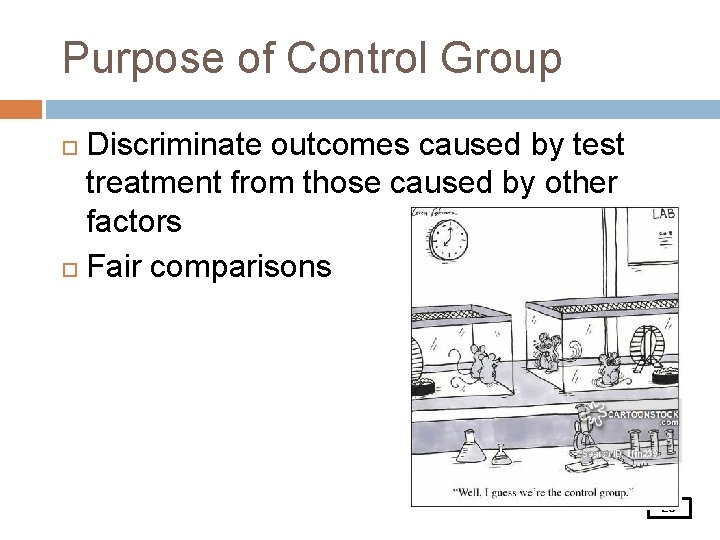
Purpose of Control Group Discriminate outcomes caused by test treatment from those caused by other factors Fair comparisons 25

Interim Analysis Comparing outcomes between treatment groups at any time before the trial end, usually before recruitment is complete. Often used with “stopping rules” Timing and frequency of interim analyses should be pre-planned in the protocol 26

Examples of Stopping a Trial Treatments found to be convincingly different Treatments found to be convincingly not different Side effects or toxicities are too severe Data quality is poor Accrual is slow Definitive information becomes available from an outside source making trial unnecessary or unethical Scientific question is no longer important Adherence to treatment is unacceptably low Resources to perform study are lost or diminished Study integrity has been undermined by fraud or misconduct 27

Early stopping for futility Stop the study if there is no hope to establish treatment effect q Purpose: q – Avoid exposing subjects to ineffective intervention – Save resources q Consequences: – It increases type II error (thus power reduced) – No effect on type I error 28

Note Interim analyses results are generally highly confidential Results from any statistical method should not be used as the sole basis in the decision to stop or continue the trial The results of interim analyses can be used to modify the sample sizes in adaptive designs 29

Ethics of Randomized Clinical Trials The patients enrolled in a RCT benefit future patients but not themselves https: //www. nytimes. com/2010/09/19/health/research/19 trial. html 30

Possible Solution -- Adaptive Design A design that allows modifications to the trial and/or statistical procedures of the trial after its initiation without undermining its validity and integrity (Chow et. al. 2015) 31

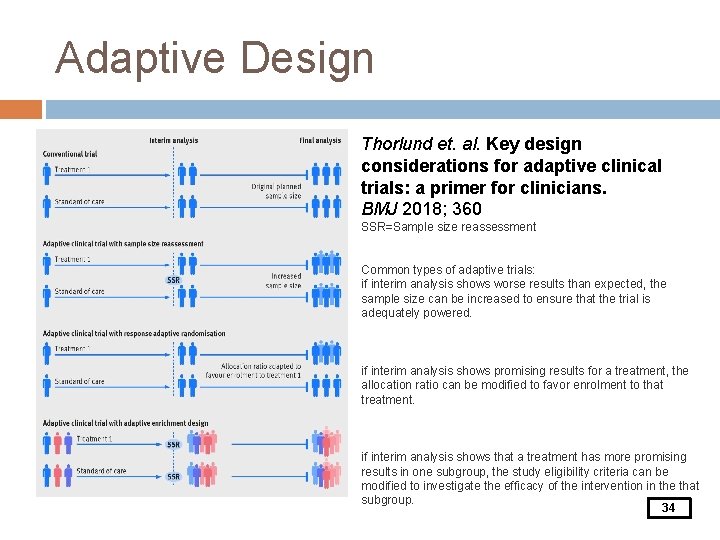
Adaptive Design Adaptive trials have the potential for decreased time to completion, reduced resource requirements and number of patients exposed to inferior treatments, and overall improved likelihood of trial success (Thorlund et. al. 2018) Adjustments can be made: � Randomization ratio � Sample size � Eligibility criteria � Treatment arms added or dropped 32

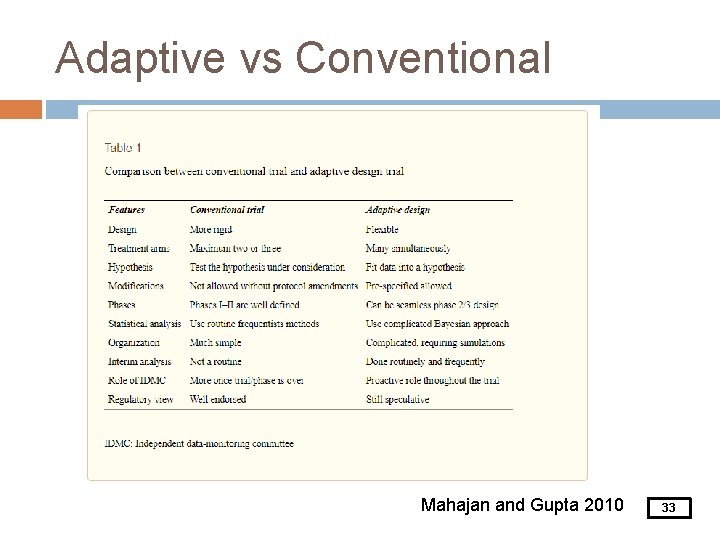
Adaptive vs Conventional Mahajan and Gupta 2010 33

Adaptive Design Thorlund et. al. Key design considerations for adaptive clinical trials: a primer for clinicians. BMJ 2018; 360 SSR=Sample size reassessment Common types of adaptive trials: if interim analysis shows worse results than expected, the sample size can be increased to ensure that the trial is adequately powered. if interim analysis shows promising results for a treatment, the allocation ratio can be modified to favor enrolment to that treatment. if interim analysis shows that a treatment has more promising results in one subgroup, the study eligibility criteria can be modified to investigate the efficacy of the intervention in the that subgroup. 34

Adaptive Design FDA Adaptive Designs for Clinical Trials of Drugs and Biologics Guidance for Industry 2018 https: //www. fda. gov/downloads/drugs/guidances/u cm 201790. pdf Disadvantage: ü More complex to conduct and analyze ü Informed consent must include the range of possible changes in the study design or be repeated ü Difficult to estimate the cost of an adaptive trial and the specific resources necessary to complete it 35

Phase III – Example The Effect of Nonsurgical Periodontal Therapy on Hemoglobin A 1 c Levels in Persons With Type 2 Diabetes and Chronic Periodontitis - A Randomized Clinical Trial (Engebretson, 2013, JAMA) OBJECTIVE: To determine if nonsurgical periodontal treatment reduces levels of glycated hemoglobin (Hb. A 1 c) in persons with type 2 diabetes and moderate to advanced chronic periodontitis. 36

Phase III - Example DESIGN: a 6 -month, single-masked, multicenter, randomized clinical trial. RANDOMIZATION: Eligible individuals were randomized using a permuted-block randomization scheme, stratified by clinical site INTERVENTIONS: The treatment group (n = 257) received scaling and root planning plus chlorhexidine oral rinse at baseline and supportive periodontal therapy at 3 and 6 months. The control group (n = 257) received no treatment for 6 months. MAIN OUTCOMES: Difference in change in Hb. A 1 c level from baseline between groups at 6 months. 37

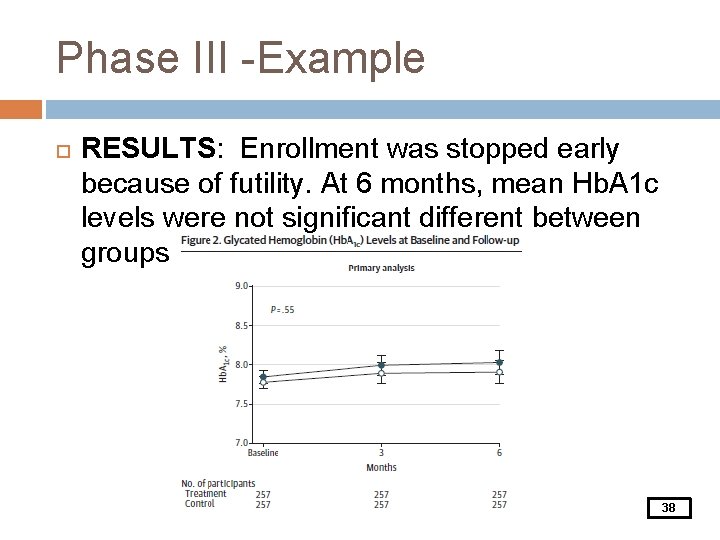
Phase III -Example RESULTS: Enrollment was stopped early because of futility. At 6 months, mean Hb. A 1 c levels were not significant different between groups 38

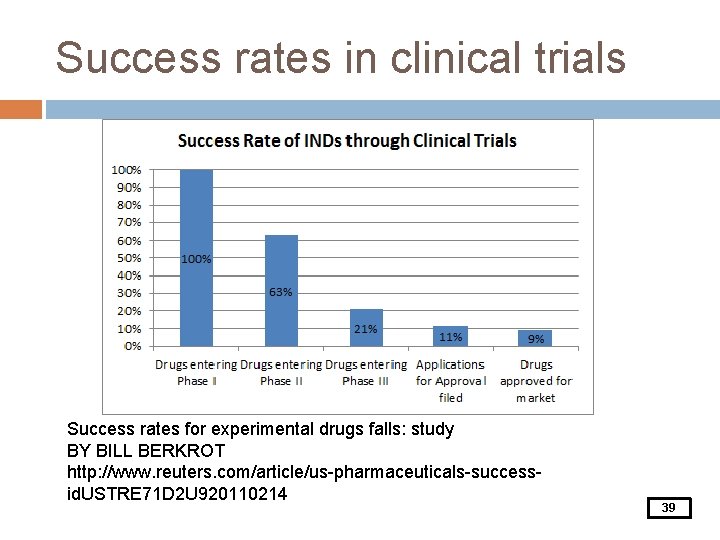
Success rates in clinical trials Success rates for experimental drugs falls: study BY BILL BERKROT http: //www. reuters. com/article/us-pharmaceuticals-successid. USTRE 71 D 2 U 920110214 39

Issues in clinical trials Many factors may influence the outcomes, e. g. nature of the disease, kind of control, sample size , duration and etc. Poorly designed, conducted and reported trials can be misleading. Results of observational studies and clinical trials could be inconsistent. Need to interpret the results in conjunction with other methodologies 40

Study protocol Should be developed before enrollment Should remain essentially unchanged Registry: Clinical. Trials. gov Recommendations for Interventional Trials (SPIRIT 2013) (Chan et al. 2013 Ann Intern Med) 41

Study protocol - Outline A. B. Background Objectives 1. 2. 3. C. Primary question and response variable Secondary question and response variable Adverse effects Design 1. 2. 3. Study population – inclusion/exclusion criteria Sample size determination Enrollment of participants 42

Study Protocol – Outline (continued) C. Design (continued): 4. Intervention 5. Follow-up visit 6. Ascertainment of response variables – training, data collection, quality control. . 7. Assessment of Adverse Events 8. Data analysis – Interim and Final 9. Termination Policy D. Organization 1. Participating investigators 2. Study administration 43

Thank you! https: //www. youtube. com/watch? v=k. MYxd 6 Qe Ass 44