DISSEMINATED INTRAVASCULAR COAGULATION SOLUBLE FIBRIN IN DIC MONKEY

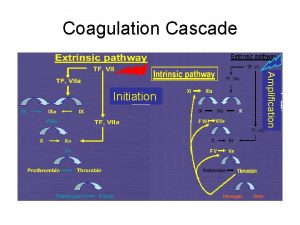
DISSEMINATED INTRAVASCULAR COAGULATION
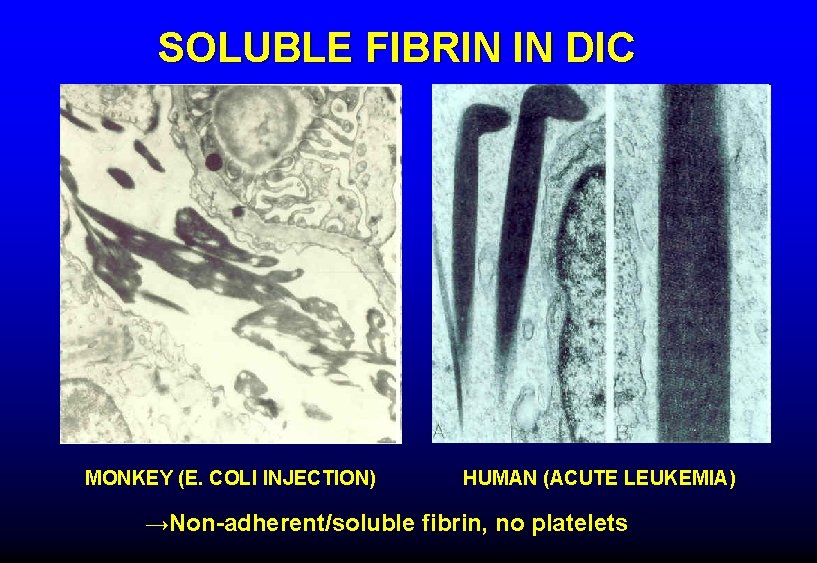
SOLUBLE FIBRIN IN DIC MONKEY (E. COLI INJECTION) HUMAN (ACUTE LEUKEMIA) →Non-adherent/soluble fibrin, no platelets

CAUSES OF DIC • Blood exposed to excess tissue factor Endothelial damage Tissue factor expression by monocytes Massive tissue/organ injury Cancer Obstetric catastrophe • • Activation of fibrinolysis Secondary to thrombin formation (t-PA) Cancer/leukemia (t-PA, u-PA, other) Cardiopulmonary bypass Other procoagulant or profibrinolytic substances Cancer cells Venoms
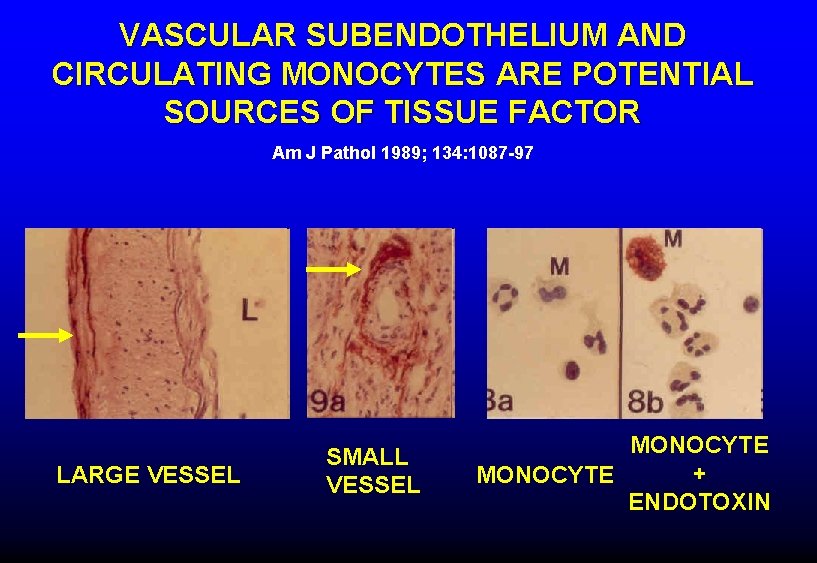
VASCULAR SUBENDOTHELIUM AND CIRCULATING MONOCYTES ARE POTENTIAL SOURCES OF TISSUE FACTOR Am J Pathol 1989; 134: 1087 -97 LARGE VESSEL SMALL VESSEL MONOCYTE + MONOCYTE ENDOTOXIN
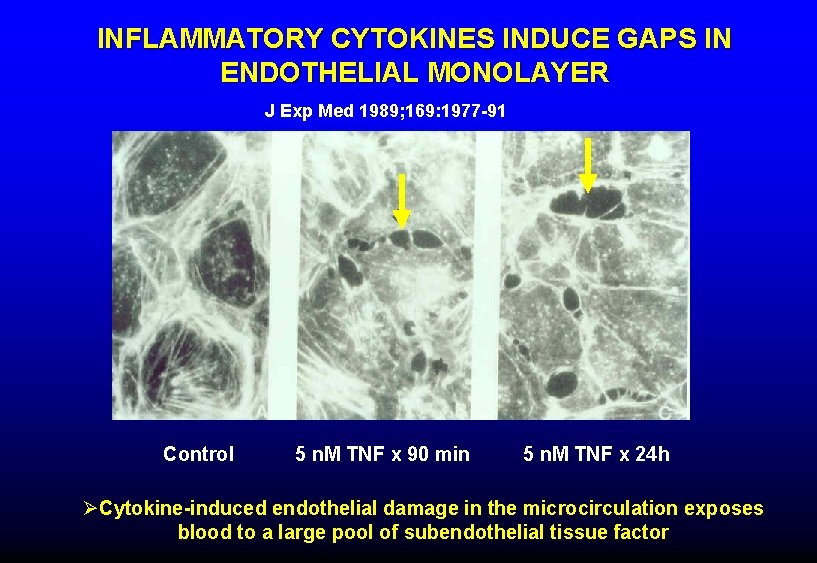
INFLAMMATORY CYTOKINES INDUCE GAPS IN ENDOTHELIAL MONOLAYER J Exp Med 1989; 169: 1977 -91 Control 5 n. M TNF x 90 min 5 n. M TNF x 24 h ØCytokine-induced endothelial damage in the microcirculation exposes blood to a large pool of subendothelial tissue factor
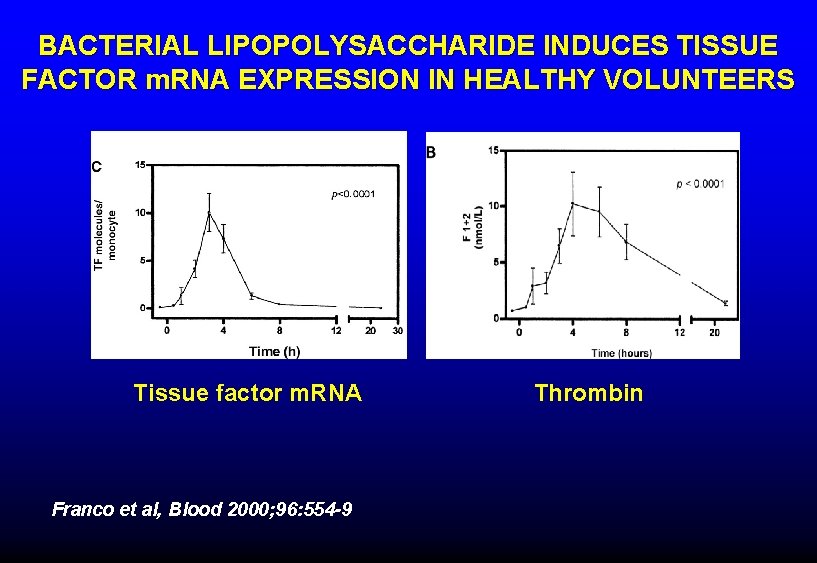
BACTERIAL LIPOPOLYSACCHARIDE INDUCES TISSUE FACTOR m. RNA EXPRESSION IN HEALTHY VOLUNTEERS Tissue factor m. RNA Franco et al, Blood 2000; 96: 554 -9 Thrombin
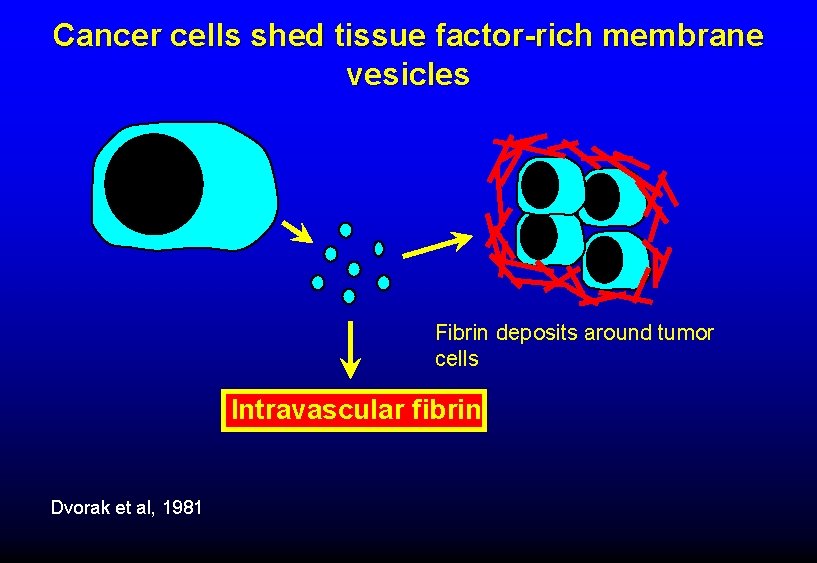
Cancer cells shed tissue factor-rich membrane vesicles Fibrin deposits around tumor cells Intravascular fibrin Dvorak et al, 1981
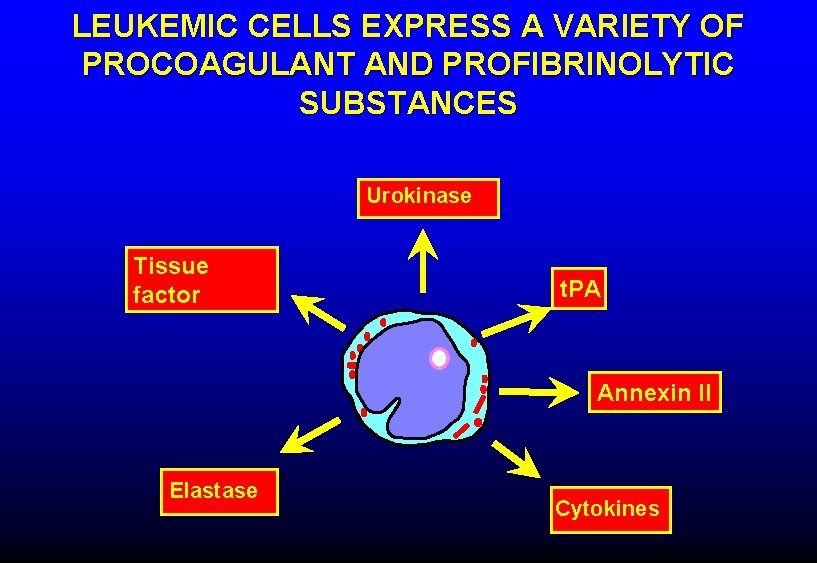
LEUKEMIC CELLS EXPRESS A VARIETY OF PROCOAGULANT AND PROFIBRINOLYTIC SUBSTANCES Urokinase Tissue factor t. PA Annexin II Elastase Cytokines

INFLAMMATION AND LIVER DISEASE PROMOTE DIC • Inflammation (TNF, IL-1, IL-6, etc) § Upregulation of procoagulant pathways § Downregulation of profibrinolytic pathways § Cytokines damage endothelium § NETosis ØIncreased risk of tissue damage/organ failure • Liver disease § Inhibitor deficiency (antithrombin, antiplasmin, protein C, etc) § Diminished clotting factor production ØIncreases severity of DIC, may increase bleeding risk
Neutrophil Extracellular Traps (NETs) • Identified in 2004 • Extracellular webs of extruded neutrophil DNA and immunoactive enzymes • Decorated with neutrophil granular proteins: neutrophil elastase, MPO, calgranulin, cathepsin G • Last-ditch effort by neutrophils to contain infections to primary site • NETs contain a variety of substances that promote clotting • They likely contribute to activation of clotting and DIC, in patients with sepsis
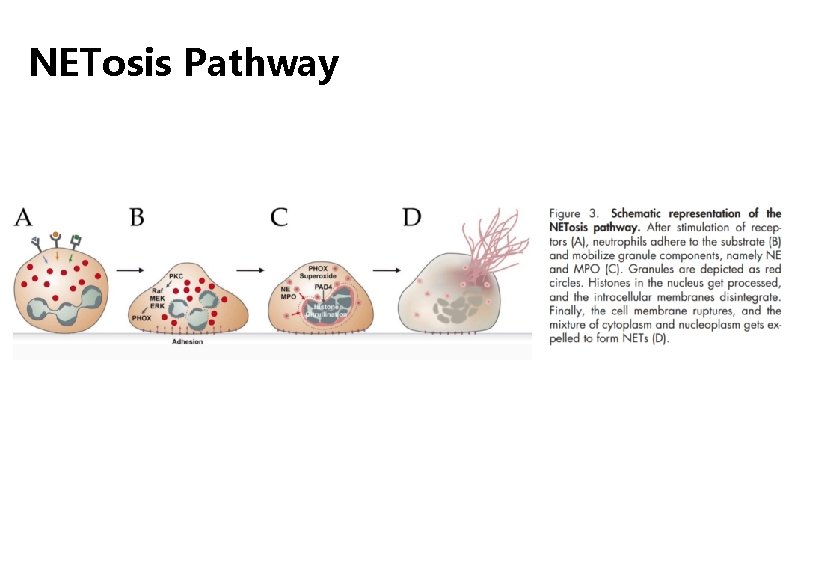
NETosis Pathway
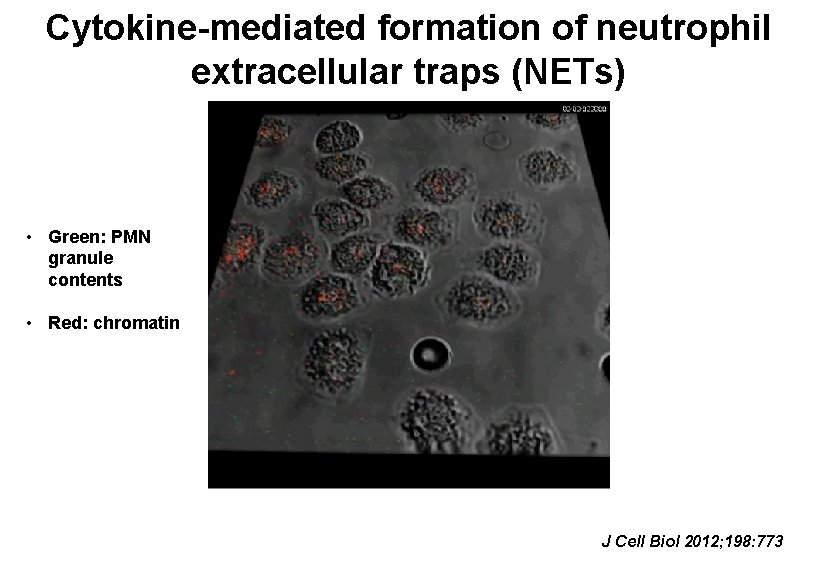
Cytokine-mediated formation of neutrophil extracellular traps (NETs) • Green: PMN granule contents • Red: chromatin J Cell Biol 2012; 198: 773
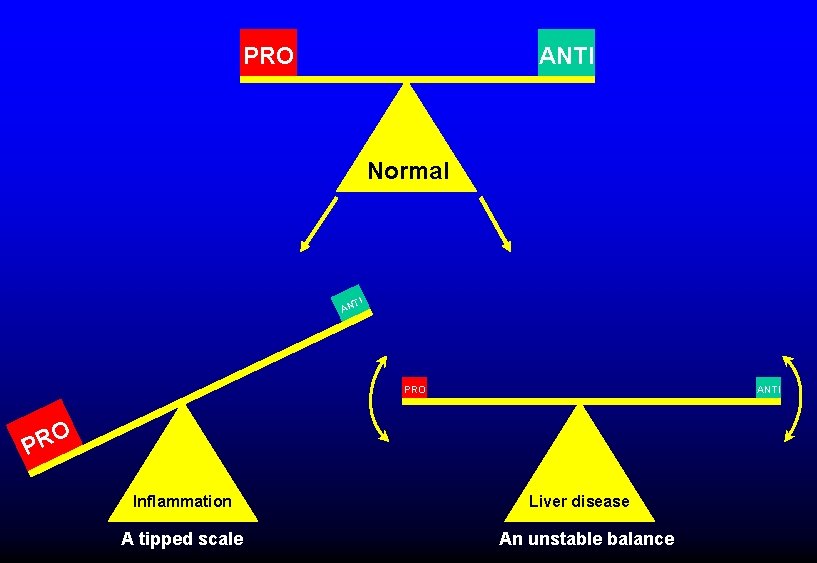
PRO ANTI Normal TI AN PRO ANTI O PR Inflammation A tipped scale Liver disease An unstable balance
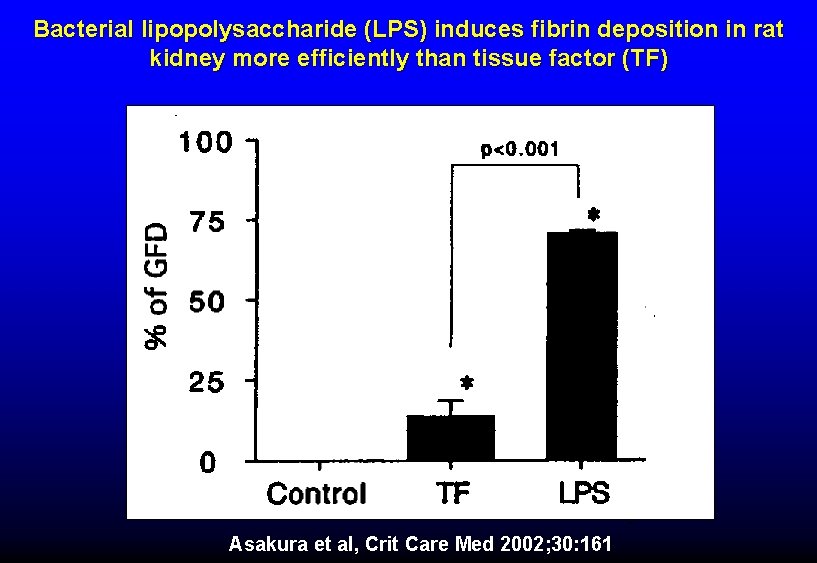
Bacterial lipopolysaccharide (LPS) induces fibrin deposition in rat kidney more efficiently than tissue factor (TF) Asakura et al, Crit Care Med 2002; 30: 161
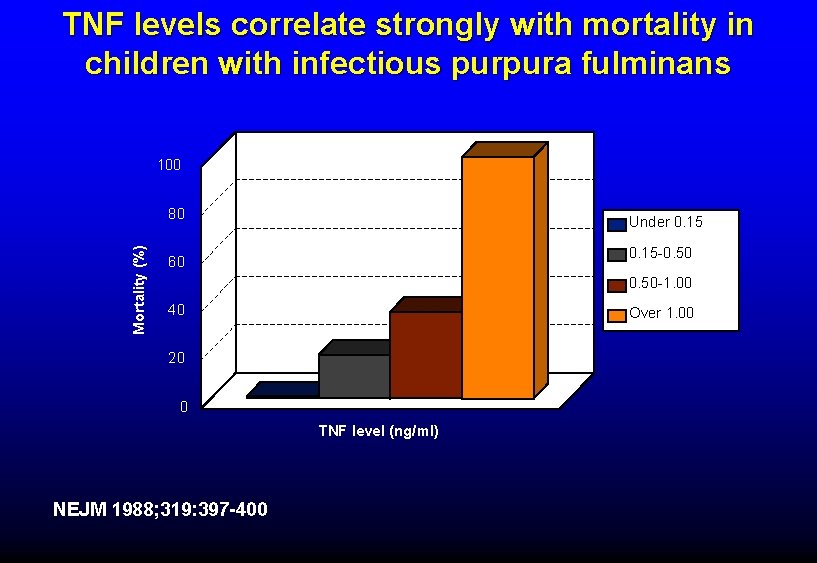
TNF levels correlate strongly with mortality in children with infectious purpura fulminans 100 Mortality (%) 80 Under 0. 15 -0. 50 60 0. 50 -1. 00 40 Over 1. 00 20 0 TNF level (ng/ml) NEJM 1988; 319: 397 -400
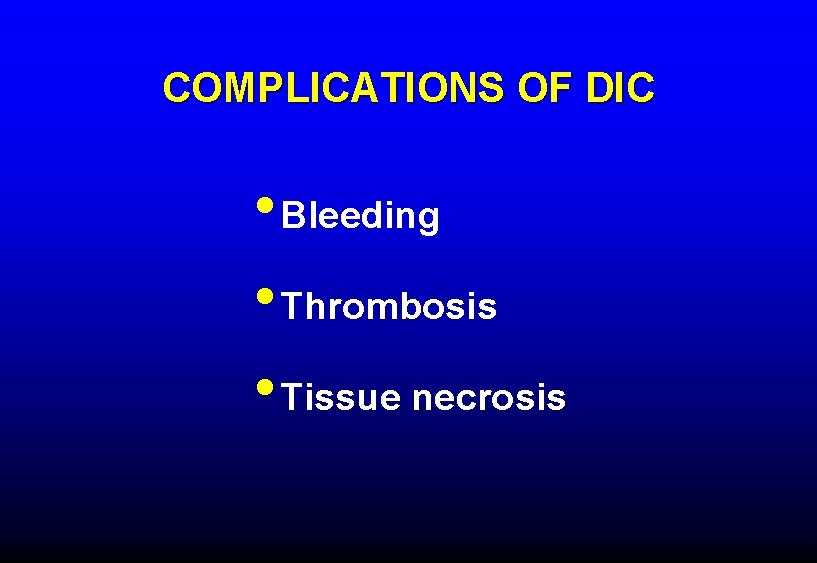
COMPLICATIONS OF DIC • Bleeding • Thrombosis • Tissue necrosis
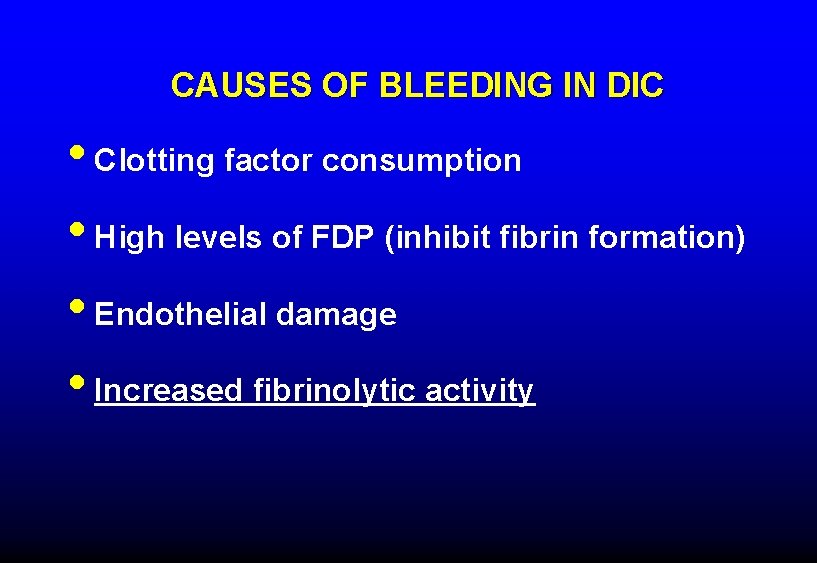
CAUSES OF BLEEDING IN DIC • Clotting factor consumption • High levels of FDP (inhibit fibrin formation) • Endothelial damage • Increased fibrinolytic activity
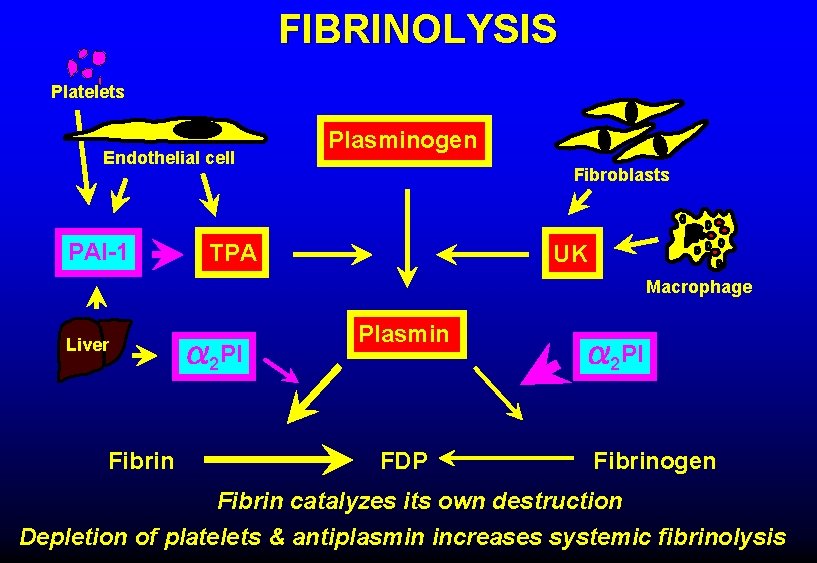
FIBRINOLYSIS Platelets Endothelial cell PAI-1 Plasminogen Fibroblasts TPA UK Macrophage Liver Fibrin 2 PI Plasmin FDP 2 PI Fibrinogen Fibrin catalyzes its own destruction Depletion of platelets & antiplasmin increases systemic fibrinolysis
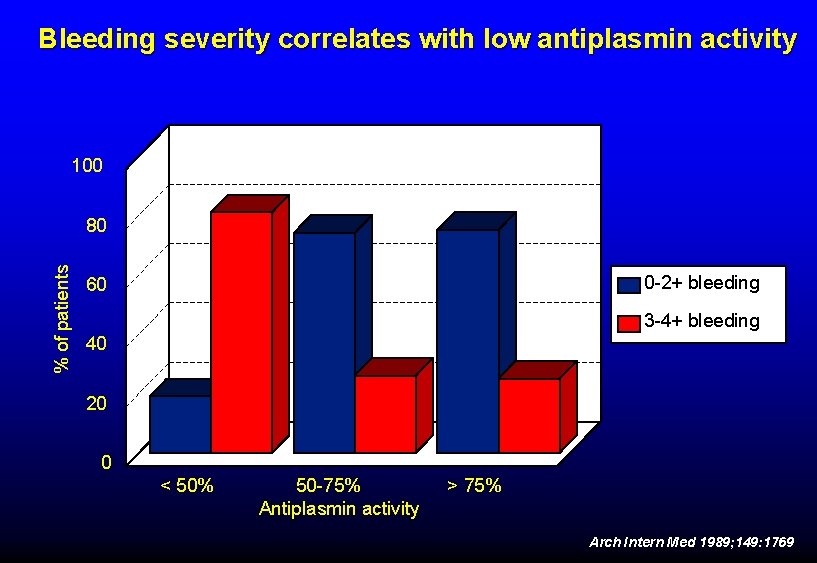
Bleeding severity correlates with low antiplasmin activity 100 % of patients 80 0 -2+ bleeding 60 3 -4+ bleeding 40 20 0 < 50% 50 -75% Antiplasmin activity > 75% Arch Intern Med 1989; 149: 1769
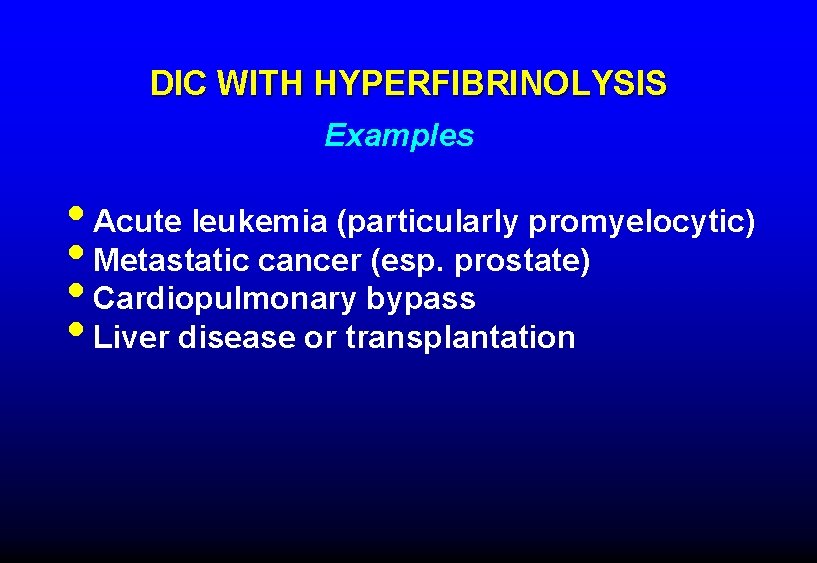
DIC WITH HYPERFIBRINOLYSIS Examples • Acute leukemia (particularly promyelocytic) • Metastatic cancer (esp. prostate) • Cardiopulmonary bypass • Liver disease or transplantation

THROMBOSIS IN DIC • Large vessel thrombosis uncommon § Disordered clotting § Increased fibrinolysis • More common in "chronic DIC" § e. g. , Trousseau syndrome • Clots may form around intravascular catheters, etc
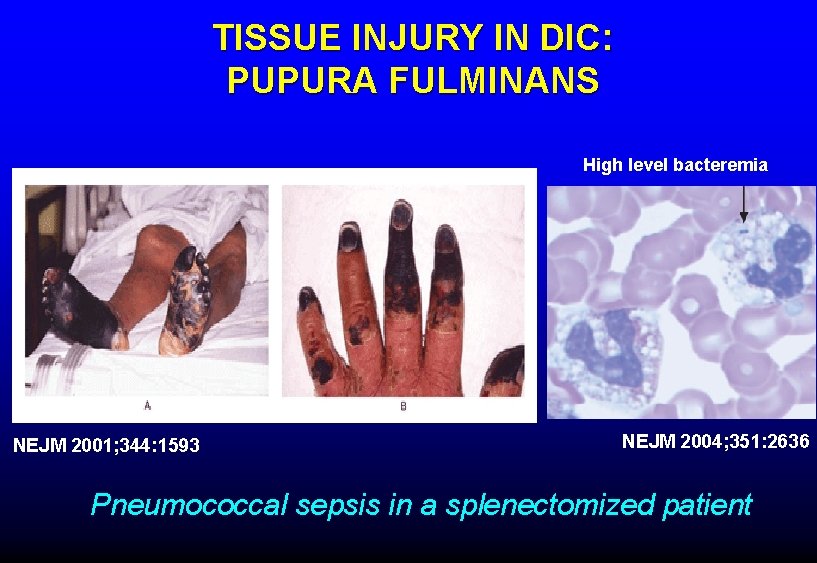
TISSUE INJURY IN DIC: PUPURA FULMINANS High level bacteremia NEJM 2001; 344: 1593 NEJM 2004; 351: 2636 Pneumococcal sepsis in a splenectomized patient
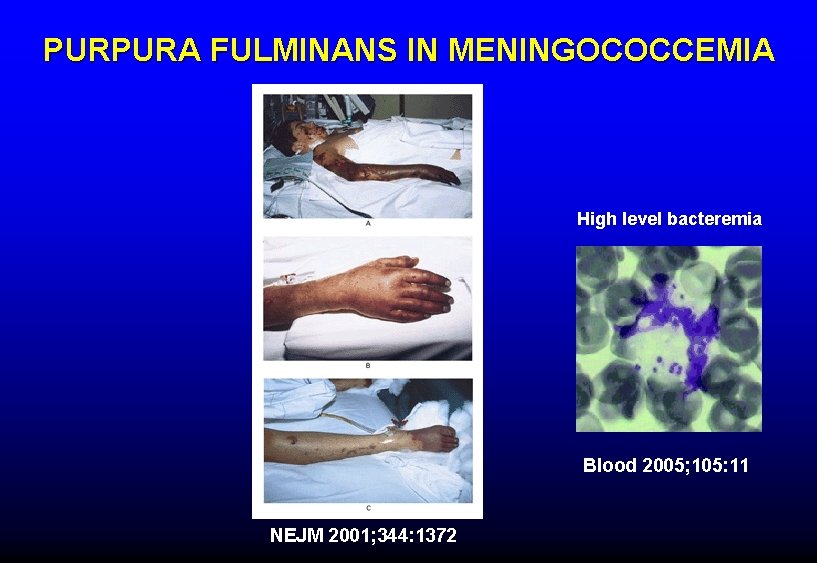
PURPURA FULMINANS IN MENINGOCOCCEMIA High level bacteremia Blood 2005; 105: 11 NEJM 2001; 344: 1372
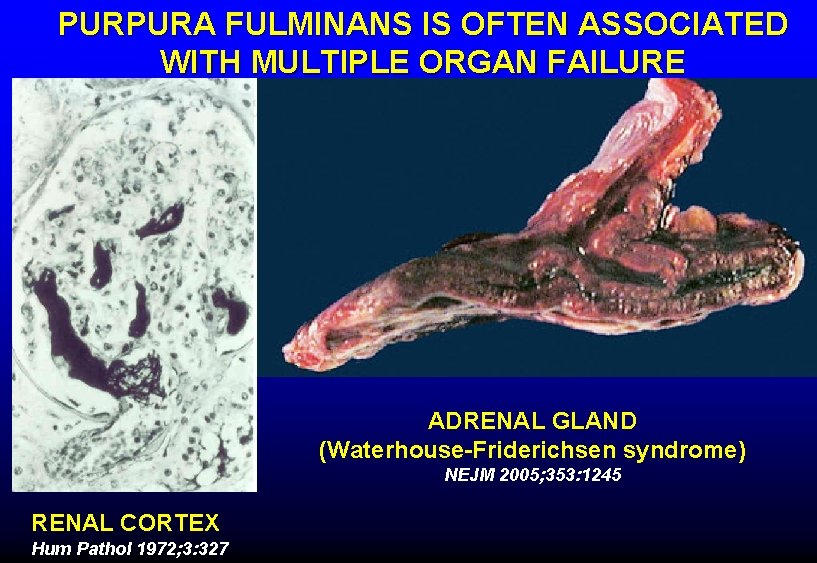
PURPURA FULMINANS IS OFTEN ASSOCIATED WITH MULTIPLE ORGAN FAILURE ADRENAL GLAND (Waterhouse-Friderichsen syndrome) NEJM 2005; 353: 1245 RENAL CORTEX Hum Pathol 1972; 3: 327
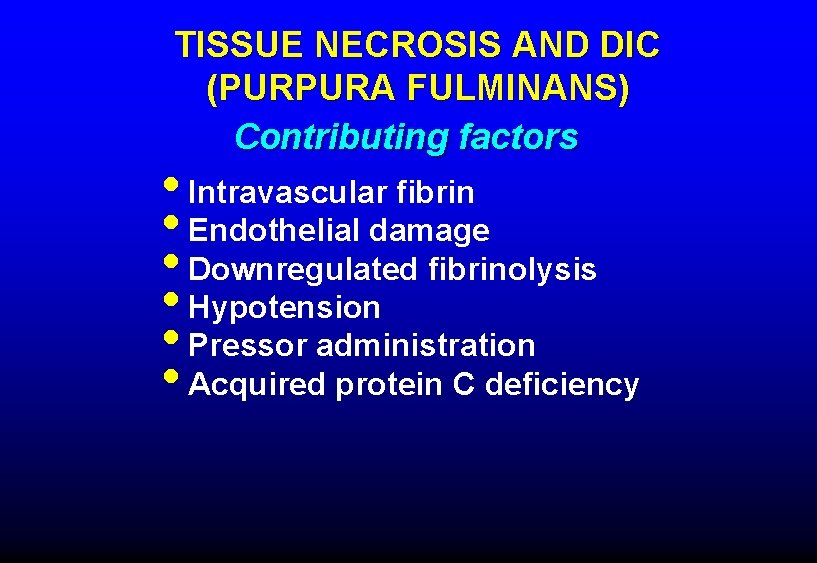
TISSUE NECROSIS AND DIC (PURPURA FULMINANS) Contributing factors • Intravascular fibrin • Endothelial damage • Downregulated fibrinolysis • Hypotension • Pressor administration • Acquired protein C deficiency
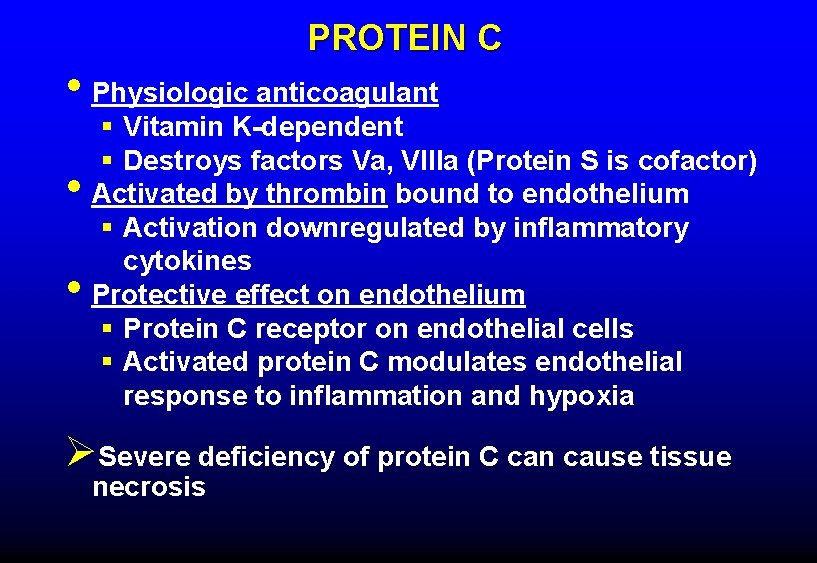
PROTEIN C • Physiologic anticoagulant • • § Vitamin K-dependent § Destroys factors Va, VIIIa (Protein S is cofactor) Activated by thrombin bound to endothelium § Activation downregulated by inflammatory cytokines Protective effect on endothelium § Protein C receptor on endothelial cells § Activated protein C modulates endothelial response to inflammation and hypoxia ØSevere deficiency of protein C can cause tissue necrosis
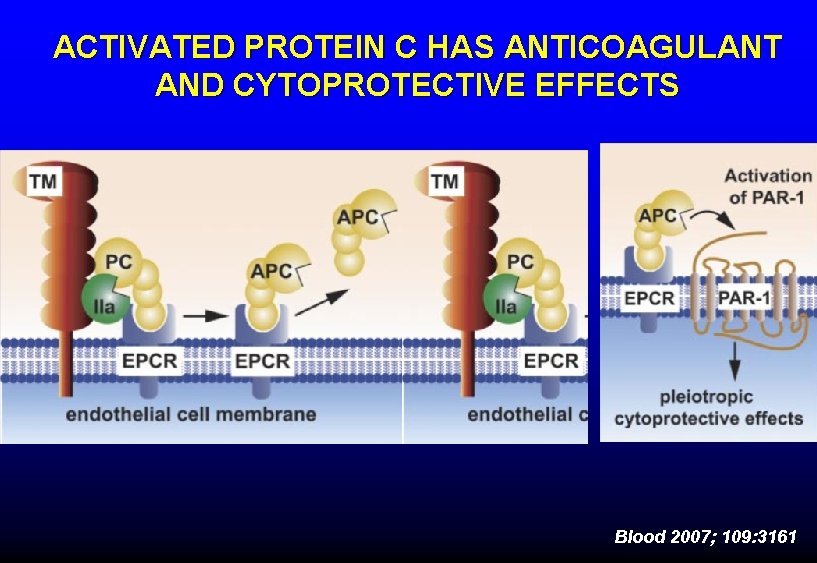
ACTIVATED PROTEIN C HAS ANTICOAGULANT AND CYTOPROTECTIVE EFFECTS Blood 2007; 109: 3161
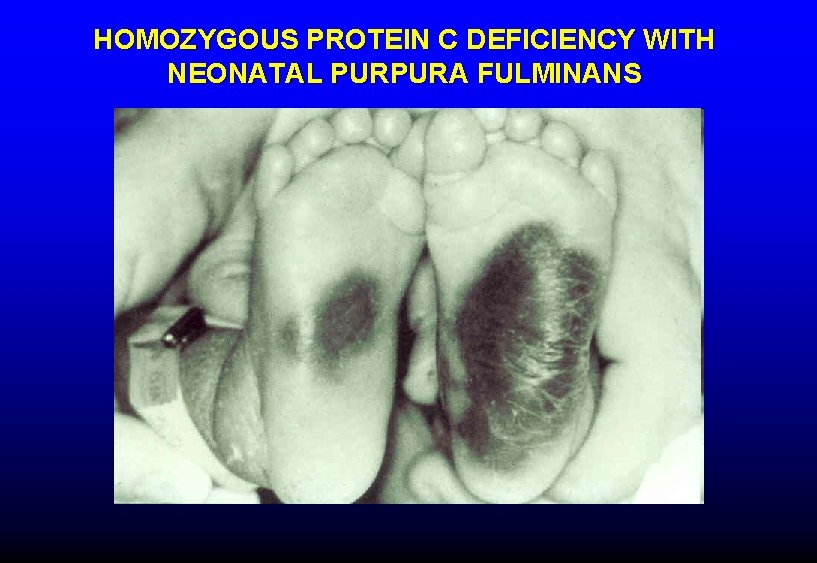
HOMOZYGOUS PROTEIN C DEFICIENCY WITH NEONATAL PURPURA FULMINANS
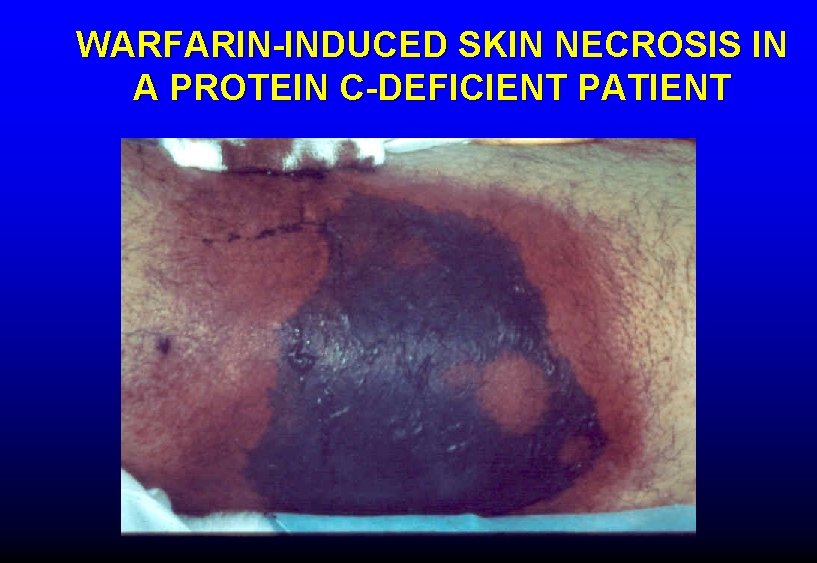
WARFARIN-INDUCED SKIN NECROSIS IN A PROTEIN C-DEFICIENT PATIENT
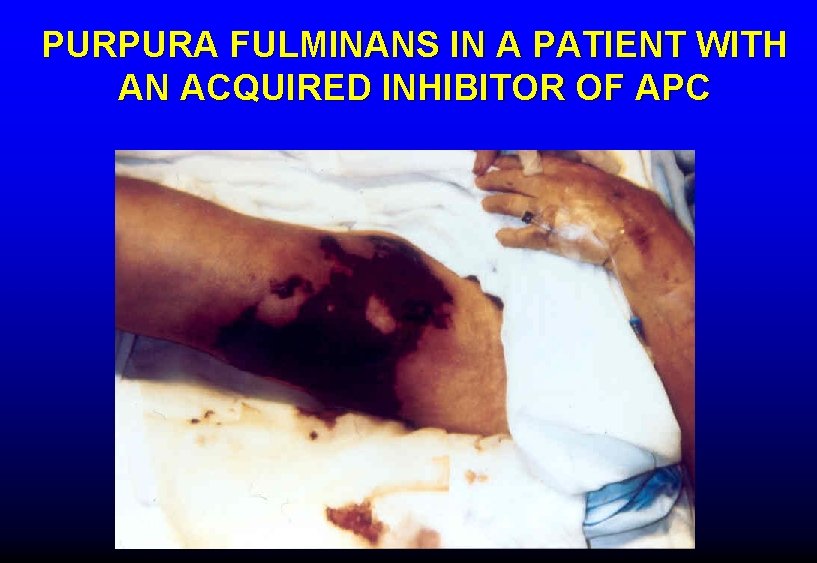
PURPURA FULMINANS IN A PATIENT WITH AN ACQUIRED INHIBITOR OF APC
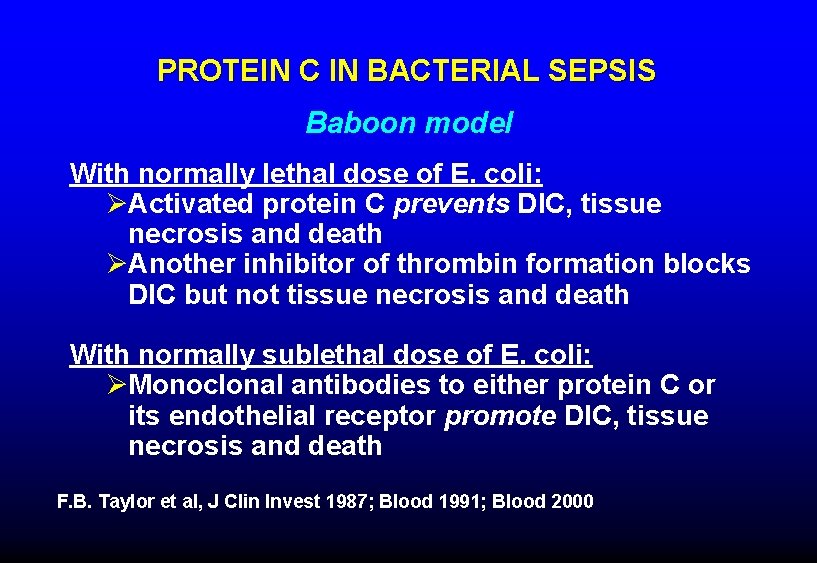
PROTEIN C IN BACTERIAL SEPSIS Baboon model With normally lethal dose of E. coli: ØActivated protein C prevents DIC, tissue necrosis and death ØAnother inhibitor of thrombin formation blocks DIC but not tissue necrosis and death With normally sublethal dose of E. coli: ØMonoclonal antibodies to either protein C or its endothelial receptor promote DIC, tissue necrosis and death F. B. Taylor et al, J Clin Invest 1987; Blood 1991; Blood 2000
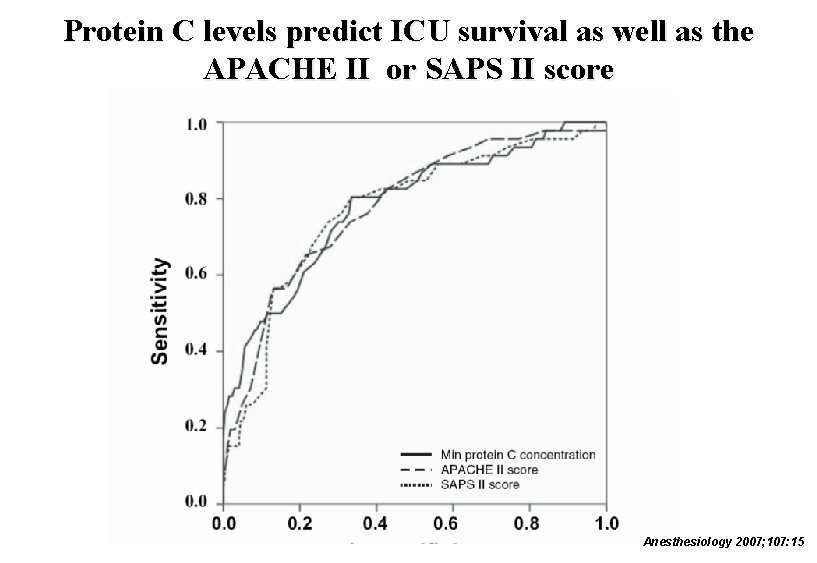
Protein C levels predict ICU survival as well as the APACHE II or SAPS II score Anesthesiology 2007; 107: 15

DIC PATHOPHYSIOLOGY Summary • Excess tissue factor + flowing blood = DIC • Inflammatory cytokines set the stage for DIC and contribute to tissue damage • Excessive fibrinolysis associated with higher bleeding risk • Acquired protein C deficiency associated with high risk of tissue necrosis/purpura fulminans

DIAGNOSIS OF DIC is likely when there is: 1. A condition known to cause DIC 2. Evidence of accelerated fibrinolysis and clotting factor consumption
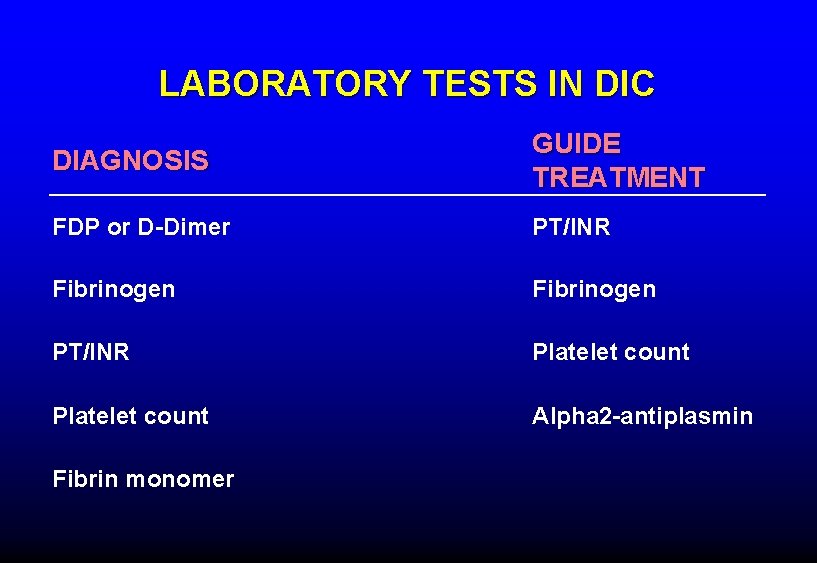
LABORATORY TESTS IN DIC DIAGNOSIS GUIDE TREATMENT FDP or D-Dimer PT/INR Fibrinogen PT/INR Platelet count Alpha 2 -antiplasmin Fibrin monomer

Death Is Coming
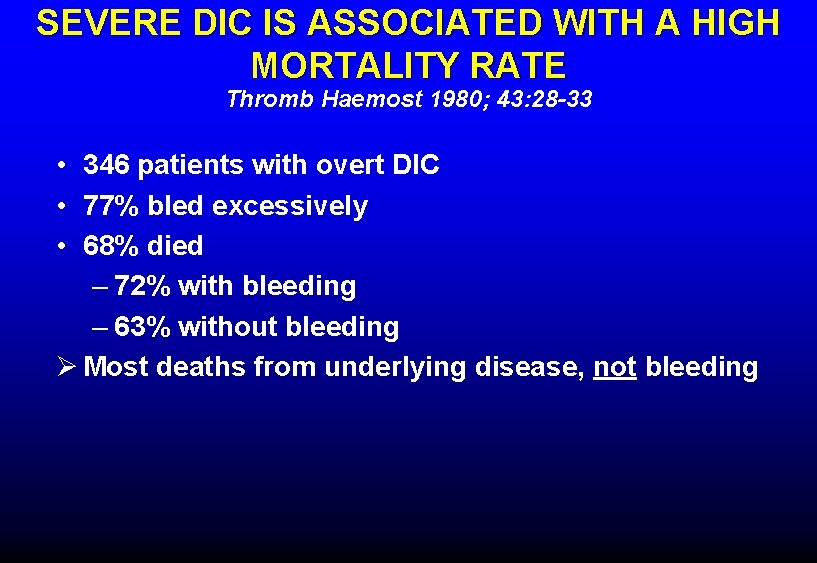
SEVERE DIC IS ASSOCIATED WITH A HIGH MORTALITY RATE Thromb Haemost 1980; 43: 28 -33 • 346 patients with overt DIC • 77% bled excessively • 68% died – 72% with bleeding – 63% without bleeding Ø Most deaths from underlying disease, not bleeding

TREATMENT OF DIC • TREAT UNDERLYING DISEASE! • Clotting factor & inhibitor replacement Fresh frozen plasma Cryoprecipitate Platelets Antithrombin III concentrate? Recombinant thrombomodulin? • Pharmacologic inhibitors Heparin Antifibrinolytics
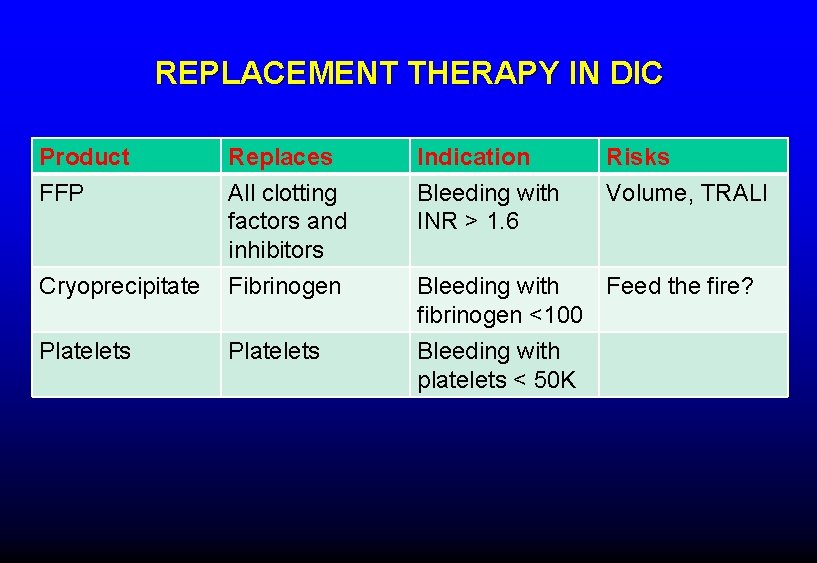
REPLACEMENT THERAPY IN DIC Product FFP Replaces All clotting factors and inhibitors Indication Bleeding with INR > 1. 6 Risks Volume, TRALI Cryoprecipitate Fibrinogen Platelets Bleeding with Feed the fire? fibrinogen <100 Bleeding with platelets < 50 K
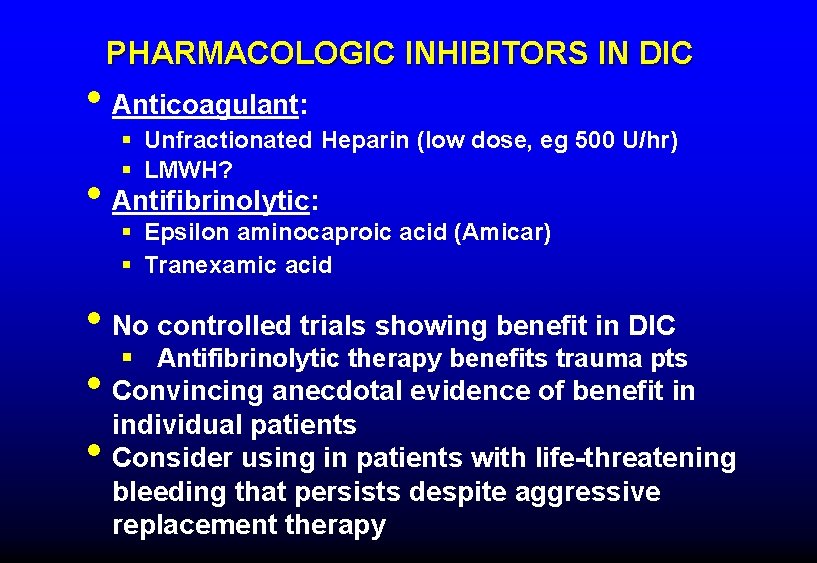
PHARMACOLOGIC INHIBITORS IN DIC • Anticoagulant: § Unfractionated Heparin (low dose, eg 500 U/hr) § LMWH? • Antifibrinolytic: § Epsilon aminocaproic acid (Amicar) § Tranexamic acid • No controlled trials showing benefit in DIC § Antifibrinolytic therapy benefits trauma pts • Convincing anecdotal evidence of benefit in individual patients • Consider using in patients with life-threatening bleeding that persists despite aggressive replacement therapy
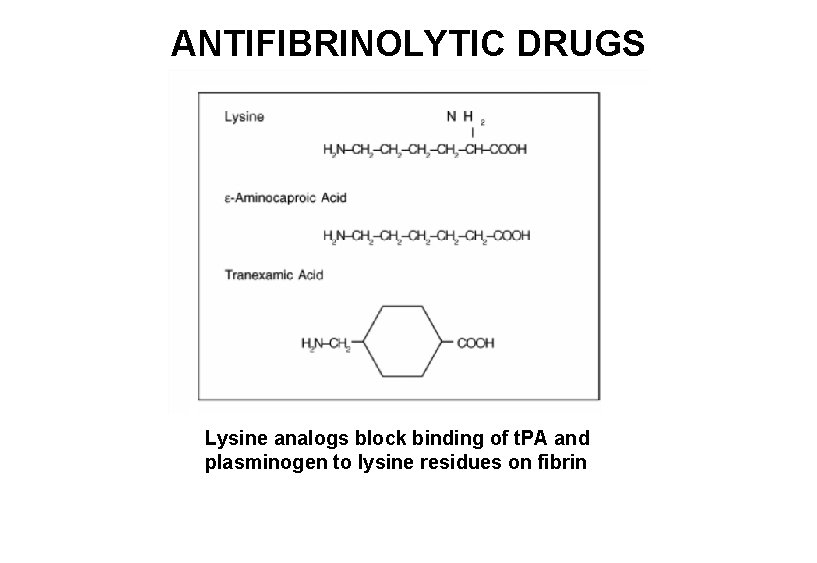
ANTIFIBRINOLYTIC DRUGS Lysine analogs block binding of t. PA and plasminogen to lysine residues on fibrin

ANTIFIBRINOLYTIC TREATMENT OF DIC ASSOCIATED WITH PROSTATE CARCINOMA 60 yo man post XRT for spine mets with diffuse bleeding
Coagulopathy in Trauma Patients • Multiple causes of bleeding after trauma – Tissue damage, hypothermia, hemodilution • Some patients become overtly coagulopathic • Contributing factors may include – Acquired protein C deficiency – Shedding of endothelial heparin-like molecules – Platelet “exhaustion” – Accelerated fibrinolysis
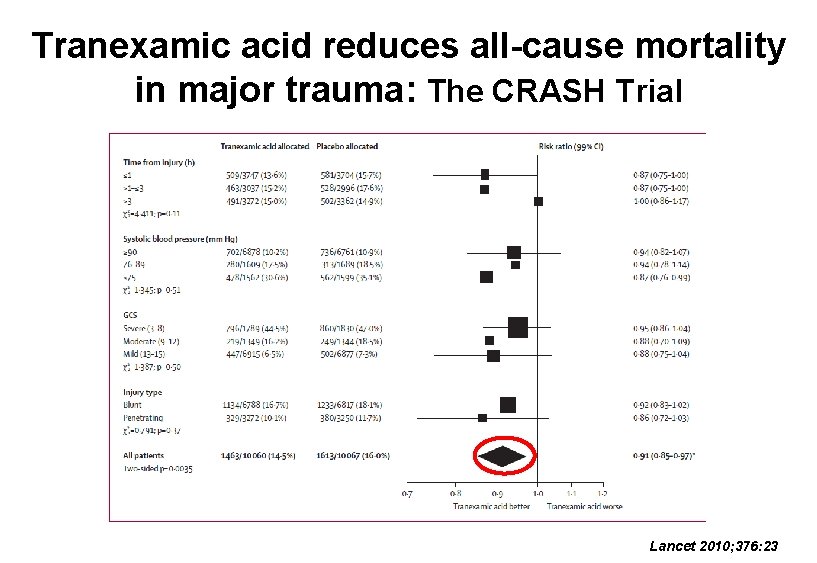
Tranexamic acid reduces all-cause mortality in major trauma: The CRASH Trial Lancet 2010; 376: 23
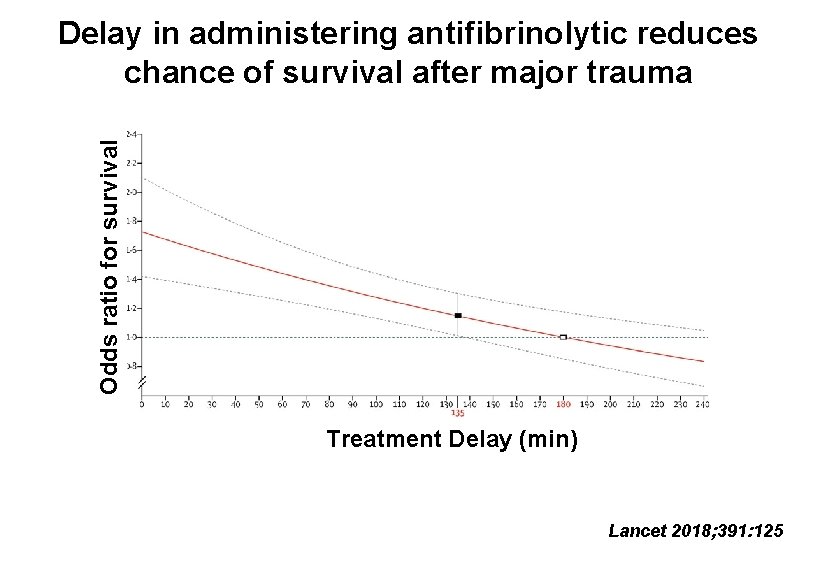
Odds ratio for survival Delay in administering antifibrinolytic reduces chance of survival after major trauma Treatment Delay (min) Lancet 2018; 391: 125
- Slides: 45